Single Photon Emission Computed Tomography
Noojan J. Kazemi
Terence J. O’Brien
Gregory D. Cascino
Elson L. So
Introduction
One of the primary goals of neuroimaging in epilepsy is to identify the epileptogenic zone. The epileptogenic zone is the focus of the brain that is necessary and sufficient to generate seizures, and the focus that, when resected, would render the patient seizure free. The phenomenon of increased blood flow at the time and location of seizure activity has long been observed and studied.13 Single photon emission computed tomography (SPECT) is a functional imaging test that can provide a semiquantitative map of cerebral blood flow changes associated with the epileptogenic zone. The SPECT radiotracer is rapidly taken up by the brain within 40 to 60 seconds after its injection.25 When the radiotracer is injected during seizure activity (ictal SPECT), it can provide a “snap shot” of the transient hyperperfusion at the region of the focal seizure activity. This is the major advantage of ictal SPECT because it can image the dynamic ictal state, unlike other imaging modalities, such as positron emission tomography (PET) and magnetoencephalography (MEG), which give information about the static interictal state. If the seizure had ended by the time the radiotracer is injected (postictal SPECT), blood flow SPECT can image the pattern of intensely reduced perfusion that is frequently present for a few minutes at the seizure.28 Furthermore, the radiotracer can also be administered during the interictal nonseizure state (interictal SPECT) to image the pattern of persistent but low-grade hypoperfusion that is detectable at some seizure foci.
An additional advantage of SPECT is that it is widely available and relatively inexpensive to perform. A cyclotron is not required for the production of SPECT radiotracers. Therefore, blood flow SPECT has been an important tool for localizing the seizure focus for epilepsy surgery. It has also been used to detect seizure spread patterns in different forms of epilepsy. This chapter discusses the principles, techniques, and clinical applications of blood flow SPECT studies and interpretation in epilepsy evaluation.
Radiotracers for Blood Flow Single Photon Emission Computed Tomography
The acquisition of images by the SPECT camera is based on the detection and localization of an internal source of γ-energy rays emitted by the radiotracer that has been administered to the patient.25 The basic images acquired by the camera when it is stationary in one position are termed planar images. Modern SPECT technique uses computer-aided mathematical reconstruction of multiple planar images taken at different rotational angles to produce a series of thin cross-sectional two-dimensional images of a three-dimensional object.
The properties of a radiotracer that is ideal for SPECT imaging in epilepsy are as follows:
Stability for at least several hours in vitro, without need for further reconstitution, so that it can be ready near the patient for immediate intravenous injection as soon as seizure activity is detected.
Rapid brain uptake that is linearly proportional to blood flow at all physiologic and pathologic blood flow rates and minimal backdiffusion after uptake. This ensures that the relative concentration of the radiotracer (and therefore the intensity of the γ radiation emitted) will closely reflect the relative regional cerebral blood flow.
Minimal extracerebral uptake and rapid blood clearance, thereby maximizing the contrast between cerebral and noncerebral structures.
Although no currently available radiotracer completely fulfils these criteria, modern blood flow SPECT radiotracers are compounds that have high first-pass brain extraction and are rapidly metabolized to hydrophilic compounds, so that back-diffusion is minimal. Therefore, the radiotracers become trapped in the brain in an amount that is closely proportional to the regional cerebral blood flow at the time of their uptake. Two technetium-based radiotracers have been in widespread clinical use in epilepsy studies: 99mTc-HMPAO (hexamethyl propylene-amine-oxime) and 99mTc-ECD (ethyl cysteinate dimer). The principal disadvantage of 99mTc-HMPAO is that it is chemically unstable in vitro and it has to be used within 30 minutes of preparation. Therefore, the radiotracer has to be reconstituted immediately prior to its injection. Preparing the radiotracer delays its injection when the onset of a seizure is recognized. Because 99mTc-HMPAO was the radiotracer used in most early SPECT studies in epilepsy, results of those studies are not comparable with those of more recent studies that used stabilized 99mTc-HMPAO or 99mTc-ECD, both of which do not require reconstitution immediately prior to their injection.16 99mTc-HMPAO has relatively high uptake in the extracerebral soft tissues (10%–30%) and a slow urinary clearance of 40% in 48 hours.42 This results in poor cerebral-to-extracerebral contrast, which makes extraction of the brain surface for surface matching coregistration algorithms particularly difficult. 99mTc-ECD has been observed to have better brain-to-background contrast. There is controversy, however, regarding the superiority of one radiotracer over the other in sensitivity for detecting the seizure focus.1,18,32
Interictal Blood Flow Single Photon Emission Computed Tomography
Earlier SPECT studies in patients with intractable partial epilepsy were interictal studies. For interictal SPECT, the radiotracer should ideally be injected following a seizure-free
period of at least 24 hours and without immediate postinjection seizure activity, including that of auras. Concurrent electroencephalographic (EEG) recording during the radiotracer injection is ideal for ensuring that the SPECT study is truly interictal, although it is not routinely practiced. The injection should be performed in a quiet room with the lights dimmed and the patient calm, to minimize the activation of cerebral regions by environmental factors.
period of at least 24 hours and without immediate postinjection seizure activity, including that of auras. Concurrent electroencephalographic (EEG) recording during the radiotracer injection is ideal for ensuring that the SPECT study is truly interictal, although it is not routinely practiced. The injection should be performed in a quiet room with the lights dimmed and the patient calm, to minimize the activation of cerebral regions by environmental factors.
Results of interictal SPECT studies varied widely among different institutions, with reported sensitivity ranging from 36% to 96% and specificity from 36% to 94%.14,30,50,53 Most studies have a small number of patients. Comparing the results of different series is difficult because of the wide variation in patient population, SPECT instrumentation, type of radiotracer used, concurrent EEG monitoring, and the criteria for determining the correct localization (i.e. EEG, magnetic resonance imaging [MRI], pathology, or surgical outcome). Spencer50 combined the results of 23 series of interictal blood flow SPECT studies in 539 patients and found sensitivity of 66% and specificity of 68% for lateralizing temporal lobe seizures to the side localized by ictal scalp EEG. Devous et al.6 performed a meta-analysis of 13 series of interictal SPECT studies in 247 patients and found a mean sensitivity of 44% and a false-positive rate of 7.4%. These results are lower than those for PET or volumetric MRI.41
Interictal SPECT is particularly disappointing in nonlesional epilepsy and extratemporal epilepsy patients. Interictal SPECT studies using 99mTc-HMPAO correctly localized the epileptogenic zone in only 20% to 47% of nonlesional temporal lobe epilepsy (TLE) patients.41,44,50 Many studies of interictal SPECT in extratemporal epilepsy found poorly localizing results.17,22,51 In a study that used 99mTc-HMPAO in pediatric frontal lobe epilepsy (FLE) patients, only 9% had focal hypoperfusion that corresponded to EEG or MRI abnormality.9 Similarly, Ho et al.11 found that interictal SPECT was localizing in only 4 of 14 parietal lobe epilepsy patients, compared with localizing ictal SPECT finding in all of the patients. It is now generally accepted that the main role of interictal SPECT images is as a baseline for comparison with ictal SPECT images.6
Peri-ictal Cerebral Blood Single Photon Emission Computed Tomography
Peri-ictal SPECT studies include both ictal and postictal SPECT studies. Attempts at ictal injection of the radiotracer might not always be successful, and the seizure might have ended by the time the radiotracer is injected. Earlier reports of peri-ictal SPECT in the literature consist mostly of postictal SPECT studies. Immediate injection of the radiotracer during seizure activity was impractical because of the need to reconstitute older radiotracers and the lack of continuous video-EEG monitoring for prompt recognition of seizure onset. The radiotracer injection technique has now been refined, however, to allow earlier injection times in the ictal or immediate postictal period, and video-EEG monitoring is now widely available. It is well known that earlier ictal injection time improves the sensitivity and the specificity of SPECT in localizing the seizure focus, particularly in extratemporal epilepsy, in which seizures are often brief in duration but rapid in their spread to other regions of the brain.7,26
Technique of Peri-ictal Single Photon Emission Computed Tomography Injection
It is essential that the peri-ictal SPECT injections be performed while the patient is undergoing video-EEG monitoring. Interpretation of the perfusion patterns on the SPECT images requires knowledge of the injection timing relative to the clinical and EEG seizure activity. The dose of 99mTc to be injected is typically 20 mCi for either HMPAO or ECD. Accurate calibration of the radioactive decay dose is needed to ensure that the correct dose is administered.24 All persons who handle the radiotracer must have formal training on safe handling of radioactive materials. If the patient has childbearing potential, a pregnancy test must be performed. An intravenous indwelling catheter is inserted to provide ready access for injecting the radiotracer. The catheter must be checked regularly to ensure patency. Ideally, the person responsible for injecting the radiotracer must be experienced in recognizing on the video-EEG screen, the clinical and the EEG manifestations of seizure activity. The radiotracer is then injected immediately into the indwelling intravenous catheter. The person injecting the radiotracer must indicate the instant when the plunger of the syringe is completely depressed and the radiotracer has been completely injected. One way to indicate this time of injection is to say “in” so that the timing of the injection relative to the time of the seizure onset can be determined accurately by reviewing the video-EEG recording. The catheter is then immediately flushed with intravenous normal saline, and the injected extremity is elevated to help clear the radiotracer from the injection site into the systemic circulation.
There are other factors that enhance the likelihood for successful ictal SPECT injection. The number of hours in a day when the injection could be performed is often determined by the availability of the radiotracer and the staff in the video-EEG, nursing, and nuclear medicine departments. Radioactivity half-life of SPECT radiotracers requires that the radiotracer be replenished every few hours. The chance of injecting during a seizure is hampered if the injection and subsequent scan can be done during only a limited of hours in the day. To increase the likelihood of seizure occurrence so that ictal SPECT can be accomplished, antiepileptic drugs (AEDs) are often withdrawn and the patient deprived of sleep. Triggers of seizures can be employed if the precipitants of habitual seizures are known.
SPECT injection is problematic in patients whose seizures occur usually during sleep at night when the SPECT procedure is typically not available. One strategy for addressing this situation is to have the patient stay up at night and sleep in the daytime or at a time when it is possible to do the SPECT study. If seizure frequency is high, as is the case of many FLE patients, arrangements can be made for the staff and the radiotracer to be at the patient’s bedside for immediate SPECT injection when a seizure occurs. This arrangement is especially important when seizures are brief, as often is the case with FLE. It is also preferable to inject during a seizure without secondary generalization because the sensitivity of ictal SPECT is reduced by seizure generalization.31,38 Because the likelihood of seizure generalization is increased by AED withdrawal, AEDs should be withdrawn more cautiously if the patient’s history suggests that habitual seizures have a tendency to generalize.
Peri-ictal Single Photon Emission Computed Tomography Perfusion Patterns
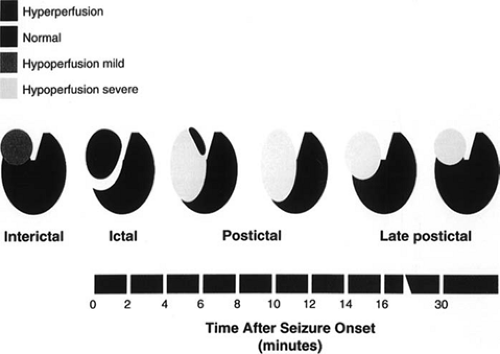
To be able to recognize focal ictal or postictal perfusion changes that are attributable to the seizure activity, ictal or postictal SPECT images have to be compared with interictal SPECT images. Peri-ictal SPECT studies in TLE have shown a characteristic pattern of evolving blood flow changes at the site of seizure activity (Fig. 1). Ictal blood flow changes consist of focal hyperperfusion at the mesial and anterolateral regions
of the temporal lobe.7,28 Within 1 to 5 minutes following the termination of seizure activity, the lateral temporal neocortical region becomes intensely hypoperfused while the mesial temporal region remains slightly hyperperfused. Over the next few minutes, the hypoperfusion becomes more diffuse in both the lateral and the mesial temporal areas. The pattern of conversion from ictal hyperperfusion to postictal hypoperfusion has been termed “postical switch.”28 The degree of this focal hypoperfusion gradually lessens over the next 15 minutes or so until it reaches the interictal state of mild hypoperfusion.
of the temporal lobe.7,28 Within 1 to 5 minutes following the termination of seizure activity, the lateral temporal neocortical region becomes intensely hypoperfused while the mesial temporal region remains slightly hyperperfused. Over the next few minutes, the hypoperfusion becomes more diffuse in both the lateral and the mesial temporal areas. The pattern of conversion from ictal hyperperfusion to postictal hypoperfusion has been termed “postical switch.”28 The degree of this focal hypoperfusion gradually lessens over the next 15 minutes or so until it reaches the interictal state of mild hypoperfusion.
The timing of the postictal switch cannot be accurately predicted. Postictal switch of perfusion may occur earlier in extratemporal seizures that are short in duration, but SPECT studies are still useful for these seizures if seizure activity persists for 10 to 15 seconds after the injection. Therefore, it is prudent to look for both focal hyperperfusion and hypoperfusion abnormalities regardless of the timing of the radiotracer injection relative to the seizure onset.
Techniques in Single Photon Emission Computed Tomography Image Analysis and Interpretation
Clinical interpretation of peri-ictal SPECT studies typically requires comparison of the peri-ictal (ictal or postictal) SPECT images with the interictal SPECT images. The comparison has conventionally been made by side-by-side visual comparison of the images, but more modern methods use computer-aided techniques for image analysis, display, and interpretation. One method subtracts the interictal from the peri-ictal images and then registers the difference image on the MRI image (subtraction SPECT). Another method statistically compares the degree of peri-ictal perfusion changes in the patient with control values derived from aggregates of interictal studies or nonepileptic volunteer studies (statistical parametric mapping [SPM]).
Conventional Single Photon Emission Computed Tomography Analysis and Interpretation
Clinical Usefulness of Conventional Ictal Single Photon Emission Computed Tomography Studies
Several studies have demonstrated the superiority of ictal SPECT studies over interictal SPECT studies in lateralizing seizure onset in patients with known temporal lobe epilepsy. Sensitivity reported for ictal SPECT in these patients ranged from 75% to 97%, whereas specificity ranged from 71% to 100%.10,50,53 Devous et al.6 conducted a meta-analysis of series of peri-ictal SPECT studies that met the following criteria: (a) localization-related epilepsy with at least interictal EEG-documented epileptiform abnormality, (b) at least six adult patients in each series, and (c) a study population that is not highly selected. Only three series with total of 51 patients met the criteria, of whom 42 were from one institution.22,27,51 The calculated sensitivity for ictal SPECT for TLE in these studies was 96% with a false-positive rate of 1.5%, but Devous et al. cautioned that the number of patients in each series is small.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree