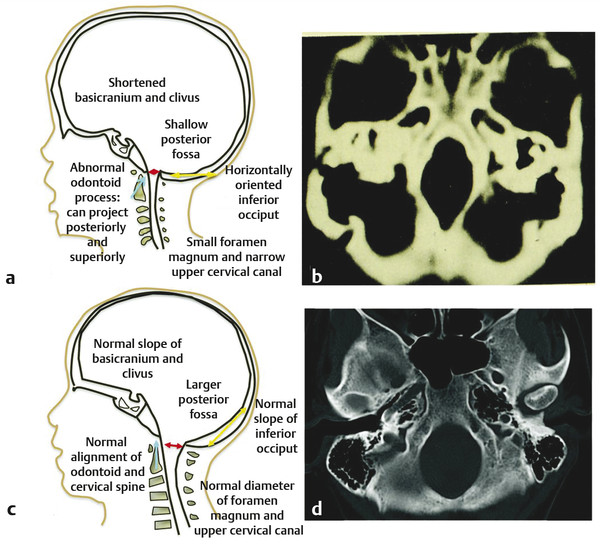
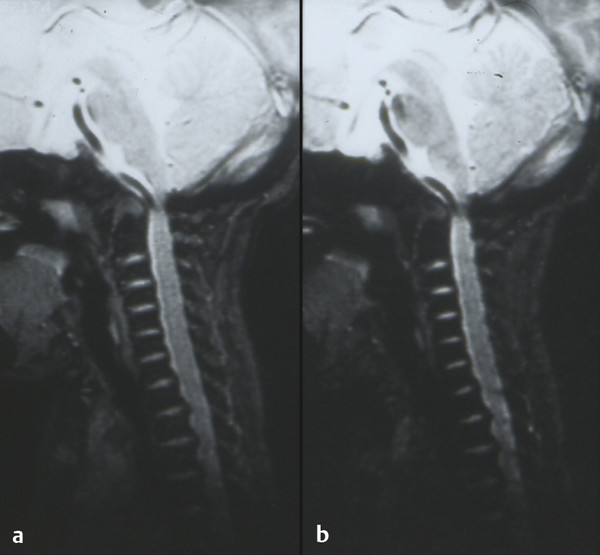
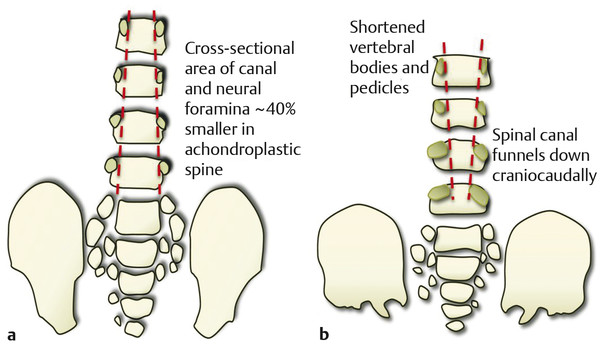
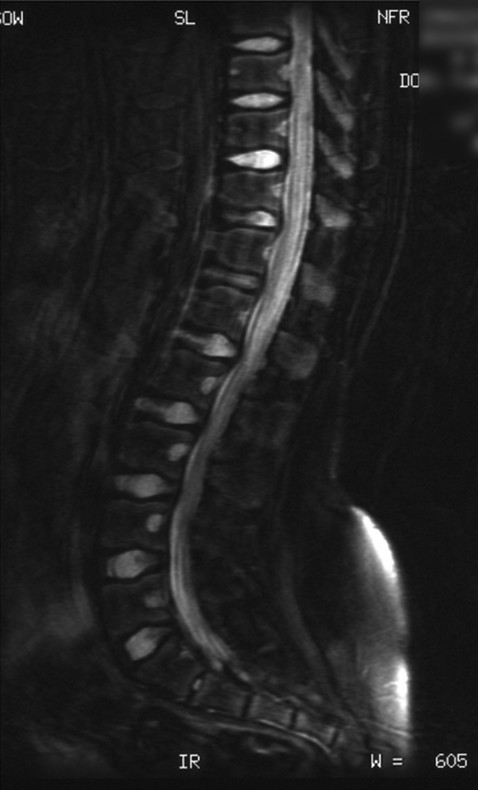
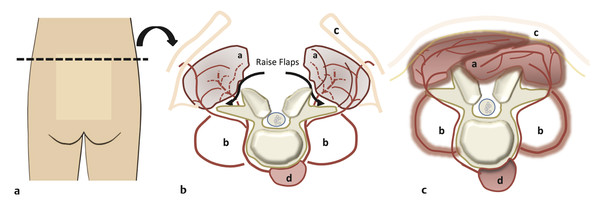
Skeletal Syndromes Skeletal dysplasias comprise a group of disorders stemming from abnormal cartilage and bone formation, growth, and remodeling. There are more than 200 types of skeletal dysplasias, and various ways exist to characterize these disorders. They range from osteochondral dysplasias, which involve the whole skeleton, to the more limited dysostoses, which involve only a single group of bones. Skeletal dysplasias can also be categorized according to their pathogenesis as idiopathic osteolytic syndromes, primary chromosomal abnormalities, or primary metabolic abnormalities. For the purposes of this review, we concentrate on achondroplasia because it is the most common skeletal dysplasia that requires neurosurgical intervention. Other skeletal dysplasias that sometimes require neurosurgical intervention are addressed at the end of the chapter. Achondroplasia is an autosomal-dominant disorder that affects the fibroblast growth factor receptor 3 (FGFR3) gene. This phenotype leads to disproportionately short stature with rhizomelic shortening of the extremities that results from the defective formation of endochondral bone.1–3 Spontaneous mutations account for 70% of cases. Rare cases of homozygous achondroplasia are uniformly fatal primarily because of chest cavity restriction. Achondroplasia occurs in 1 in every 26,000 to 28,000 births, with an incidence of 0.03 to 0.05% of all live births.1,2,4 Bony compression of the neuraxis and respiratory failure are the primary sources of morbidity in achondroplasia.5–7 Most individuals with achondroplasia are of normal intelligence; however, their motor milestones may be delayed, partly because of the mechanical limitations of their short limbs and occasionally because of generalized hypotonia.8 Patients with achondroplasia present to neurosurgeons with three varying types of symptomatology: hydrocephalus, cervicomedullary compression, and spinal stenosis. Hydrocephalus and cervicomedullary compression present in infancy and childhood9 (▶ Table 30.1). Respiratory symptoms result from both mechanical obstruction and physical restriction. Upper small airway disease results in obstructive sleep apnea and manifests as snoring.10 Many infants compensate by hyperextending their necks during sleep to overcome this obstruction. However, extension of the neck exacerbates the small foramen magnum and cervicomedullary compression.11 A smaller thoracic cavity can also result in restrictive symptoms, which may lead to respiratory compromise. This can be further compounded by gastroesophageal reflux, aspiration, and recurrent pneumonias. Spinal stenosis typically presents in young and middle adulthood. These patients present with neurogenic claudication, back pain, and occasionally urinary and bowel incontinence. We discuss further the work-up and management of patients who have achondroplasia with cervicomedullary compression and spinal stenosis. Patients with achondroplasia have neurologic manifestations in 35 to 47% of cases. They also have increased age-adjusted mortality rates at all ages, with the highest occurring in childhood. Foramen magnum stenosis is a common radiologic finding, but this does not always corroborate with the clinical symptoms. In a prospective evaluation, Pauli and colleagues studied 53 infants with achondroplasia.12 More than 70% of the children had foramen magnum stenosis or associated craniocervical abnormalities on magnetic resonance (MR) imaging. However, only 14% of them developed clinical symptoms necessitating surgical decompression.12 In an additional study, Reid and colleagues found evidence of foramen magnum stenosis in 60% of prospectively evaluated patients with achondroplasia, but only 35% demonstrated clinical symptoms of cervicomedullary compression.13 Foramen magnum stenosis results from defective endochondral bone growth and an abnormal fusion pattern of the posterior basal synchondrosis.4,14 The foramen magnum is formed by the exoccipital, supraoccipital, and basioccipital bones, which enlarge by endochondral ossification incases of achondroplasia.15 The result is a small foramen magnum, short basicranium and clivus, shallow posterior fossa, horizontally oriented inferior occiput, abnormal odontoid process, stenotic jugular foramina, and narrow upper cervical canal2,4,16 (▶ Fig. 30.1). These patients also have premature fusion and aberrant development of the two posterior synchondroses, which contribute to the stenosis and thickening of the foramen magnum rim.2,16 This can project into the brainstem and cervical canal. Further narrowing of the foramen magnum results from abnormal fusion of the posterior neural arch of the atlas with the posterior foramen magnum, as well as from dense fibrotic epidural bands found anterior to the posterior ring of the atlas.14 The odontoid process also can project posteriorly and superiorly, resulting in medullary compression.15,16 Fig. 30.1 Comparative foramen magnum anatomy in a patient with achondroplasia (a, b) and a normal person (c, d). Reduction in the sagittal and coronal dimensions of the foramen magnum leads to cervicomedullary compression (a). The cranial base is derived from endochondral ossification, which is not normal in achondroplasia. As a result, the base is stunted, shorter, and narrower than normal. The basioccipital bone is narrow and angulated, and the lateral and posterior parts have similarly abnormal characteristics that result in a diamond, triangular, or teardrop-shaped foramen magnum (b) in comparison with a normal skull base (d). The posterior fossa is small because the occipital bone is horizontally angled. This causes stenosis of the foramen magnum and cervical canal (a), which pushes the brainstem upward and applies pressure on the brainstem and upper cervical spinal cord. Compression of the cervicomedullary junction can manifest as lower brainstem and high cervical spine compression leading to myelopathy, hydrocephalus, respiratory compromise, and sudden death. Young infants may have subtle findings but can have excessive hypotonia, poor head control, feeding or sleep difficulties, and apnea. Most patients with achondroplasia exhibit hypotonia during early infancy and can exhibit developmental delay in achieving motor milestones regardless of hydrocephalus.3 However, they typically catch up to normal children by the age of 2 to 3 years. Sudden death in infants with achondroplasia is the most feared complication of cervicomedullary compression. Hecht and colleagues reviewed a cohort of 781 individuals with achondroplasia and found a 7.5% risk for sudden death within the first year and a 2.5% risk for sudden death between 1 and 4 years of age.17 A retrospective analysis by Pauli and colleagues studied 13 patients with a sudden unexplained death or apnea. Of these patients, five had evidence of acute or chronic compression of the medulla and spinal cord at autopsy.18 Other postmortem studies have found similar cystic degenerative changes in the lower brainstem and syrinx formation of the upper cervical cord, concerning for chronic compression of the cervicomedullary junction.12 Apnea may result from damage to respiratory control centers in the medulla, compression of lower motor neurons that innervate the diaphragm and accessory muscles, and nonneurologic causes including upper airway obstruction, small thoracic cage, and midface hypoplasia.14,20–22 There are two prevailing hypothesis for hydrocephalus in children with achondroplasia. The first attributes hydrocephalus to foramen magnum stenosis that leads to obstruction of the cerebrospinal fluid (CSF) outflow pathways, and the second attributes it to increased intracranial venous sinus pressure caused by compression at the jugular foramen. Yamada and colleagues favor the hypothesis of compression at the foramen magnum.15 However, patients have been noted to have relatively normal intracranial pressure (ICP) despite radiologic evidence of ventriculomegaly.16 Steinbok and colleagues found that raised intracranial venous pressure was secondary to stenosis of the jugular foramen, and occasionally of the jugular vein in the thoracic aperture.22 Treatments to consider include ventriculoperitoneal shunt and jugular foramen decompression, as these address the underlying etiology of the hydrocephalus. However, if the patient does not demonstrate signs of increased ICP, including headaches, lethargy, poor feeding, and irritability, therapy can be delayed as ventriculomegaly often abates with time.4,16 Surgical intervention is based on signs or symptoms of neurologic dysfunction, not exclusively on radiologic imaging. Clinical signs such as apnea, lower cranial nerve palsies, hyperreflexia, sustained clonus, and weakness should be considered markers of cervicomedullary compression. Imaging findings that are suggestive of foramen magnum compression include intramedullary spinal cord changes on T2-weighted magnetic resonance (MR) imaging, lack of CSF flow anteriorly or posteriorly at the foramen magnum, and the presence of a syrinx (▶ Fig. 30.2). In a prospective analysis, Pauli and colleagues determined that lower limb hyperreflexia or clonus, central hypopnea on polysomnography, and foramen magnum measurements lower than the mean for children with achondroplasia are the best signs and symptoms for predicting the need for surgical decompression.12,18 Fig. 30.2 (a) Sagittal T2-weighted magnetic resonance images showing a lack of cerebrospinal fluid flow anteriorly or posteriorly at the level of the foramen magnum. (b) The cervical canal also shows signs of congenital stenosis and a loss of normal lordosis. The abnormal anatomical architecture of the achondroplastic spine contributes to both spinal cord and nerve root compression. The vertebral bodies have a classic mushroom shape on contrast myelograms secondary to hypertrophy of the superior and inferior articular facets.23 Premature fusion of the centers of ossification of the vertebral bodies and the posterior neural arches results in laminae and pedicles that are short and thick. Posteriorly, the vertebral bodies assume a concave curvature. The consequence of this curvature can be improper projection of the inferior and superior ends into the spinal canal that further compromises the spinal subarachnoid space.24 The overall scenario results in dramatic stenosis in every anatomical dimension of the spine (▶ Fig. 30.3). Fig. 30.3 Anatomical representation of a normal (a) and achondroplastic (b) spine. The anatomical architecture of the achondroplastic spine contributes to spinal cord and nerve compression, with hypertrophy of the superior and inferior articular facets, which show a classic mushroom shape on a computed tomographic myelogram. Premature fusion of the centers of ossification of the vertebral bodies and the posterior neural arches results in shortened and thickened laminae and pedicles. Additionally, the pedicles grow larger more caudally, and as a result, the spinal canal narrows in the lumbar region. Neurologic problems located below the foramen magnum often present later in adolescence. However, in one series of patients, symptomatic stenosis was seen 35% of the time in patients before the age of 15 years.25 It is estimated that anywhere from 30 to 89% of patients with achondroplasia eventually experience symptoms associated with spinal stenosis. Children and adults typically present with multisegmental spinal stenosis involving the subaxial cervical or thoracolumbar spine. It is important to distinguish between the neurosurgical and the orthopedic characteristics for the management of the achondroplastic spine. Neurologic symptoms result from narrowing of the spinal canal due to a shortened pedicle length and small interpedicular distance. Orthopedic concerns result from patient issues such as physical limitations of the chest cavity and rotund abdomen, which tend to favor the formation of a progressive kyphosis.26,27 Some authors even discourage early ambulation and “sitting up” in these patients to minimize aggravation of their thoracolumbar kyphosis.8 In addition, hypotonia may prevent adequate protection of the skeletal structures in weight-bearing postures.9 Full-time bracing may be indicated if the kyphosis does not resolve by the age of 3 years, and if this fails to halt the progression of kyphosis, surgery can be indicated. Secondary instability can lead to worsening kyphosis or spinal deformity and is addressed in further detail below. The evaluation of these patients in more advanced cases can reveal neurologic abnormalities, such as weakness of the lower extremities and hypoesthesia. Coexistent cervical and thoracic compression presents as spasticity and hyperreflexia of the lower extremities. Imaging should include both MR imaging and computed tomography (CT) for assessment and perioperative planning. MR imaging more frequently demonstrates disk pathology and any associated soft tissue hypertrophy (▶ Fig. 30.4). CT myelography has the advantage of revealing any regions of compression due to hypertrophy and providing information distal to the level of complete spinal block. Fig. 30.4 Sagittal T2-weighted magnetic resonance (MR) image showing lumbar stenosis. MR imaging can more frequently demonstrate disk pathology and any associated soft-tissue hypertrophy. Posteriorly, the vertebral bodies assume a concave curvature that results in improper projection of the inferior and superior ends into the spinal canal. Urgent surgery is indicated for those patients who have neurologic impairments such as urinary incontinence or urgency, bowel incontinence, and worsening weakness that can be attributed to compression of the cord or cauda equina. Surgery should also be considered for patients with back pain secondary to stenosis, depending on how this affects their quality of life.27 Decompressive laminectomies are indicated for patients whose ambulation is hindered by claudication and significant weakness at rest. Preoperative urologic testing should be performed as part of the work-up for a laminectomy, given the high rate of urinary incontinence that can result from surgery. This would include a urinalysis, urine culture, and urodynamic study to assess bladder compliance. Foramen magnum decompression is recommended early in the course of symptomatic cervicomedullary compression because it has been shown to significantly improve outcome for these patients.7,28,29 An approach similar to the decompression of a Chari malformation can be utilized, which includes a suboccipital craniectomy and removal of the posterior arch of the atlas; however, the unique anatomy present in patients with achondroplasia must be taken into consideration during operative planning. Patients are positioned prone on the operative table in slight flexion to minimize significant compression of the airway as well as to limit the subarachnoid space at the foramen magnum. Preoperative steroids are given to protect the spinal cord and brainstem from local trauma. The patients are followed with somatosensory evoked potentials (SSEPs) to assess for dorsal column injury. A midline suboccipital incision is made, and the posterior ligaments and paraspinal musculature are dissected subperiosteally to expose the occiput, spinous processes, and laminae of C1 and C2. The posterior arch of C1 is then removed with a high-speed drill. If there is significant compression further caudally, then the laminae of C2 must also be decompressed. The bone of the foramen magnum is thickened and oriented more horizontally than in typical pediatric patients and can indent the underlying dura. This angle can be assessed through preoperative imaging. The posterior rim of the foramen magnum should be gradually thinned and removed with small curets. There is typically a thick, fibrous band, or pannus, extending from the foramen magnum to C1 that should be left in place during the initial bony decompression to help protect the underlying dura. The most critical part of the bony decompression is removing the lip of the foramen magnum as this can be surrounded by the dura, making it difficult to remove without a durotomy. Once the bony decompression is completed, the fibrous band can be removed. A transverse dural channel will indicate how restricted the dura was before decompression. The dura is often fused with this soft tissue band, and the annular sinus is commonly insinuated throughout the dura. If the band and dura are difficult to divide, then duraplasty should be performed in order to ensure removal of the compression. Duraplasty should also be performed if there is a persistent dural constriction after removal of the fibrous band. Intraoperative ultrasound should be used to confirm adequate cord pulsation and CSF flow. After duraplasty, a dural patch of pericranium, paraspinal fascia, or cadaveric dura is inserted to provide extra room at the foramen magnum. A watertight seal is essential as many of these patients have increased ICP and are at risk for the development of a CSF leak and possible pseudomeningocele. A ventriculostomy can be performed if there is preoperative concern for increased ICP or hydrocephalus that might complicate wound closure. Wound drains are not recommended as these can potentiate the development of a CSF fistula. Intraoperative SSEPs are critical throughout the procedure, and improvement will often be seen following decompression and restoration of CSF flow. Following the procedure, the surgeon should confirm movement in all four extremities before extubation. If there is a concern for a neurologic change, there may be damage to the underlying brainstem, and the patient should remain intubated until the respiratory drive and ability to ventilate are well established. Postoperatively, the patient should be monitored in an intensive care unit for any respiratory compromise or evidence of elevated ICP. There are several points of difference between this procedure and a traditional decompression of the posterior fossa. The foramen magnum and posterior arch of C1 provide extensive constriction and pressure over the cervicomedullary junction. Introduction of even the smallest instrument can transmit significant pressure and compression to the underlying brainstem and spinal cord, and thus the surgeon should avoid placing any instruments, curets, or Kerrison rongeurs in this area. Patients with achondroplasia also have a relatively small posterior fossa with a small underlying brainstem and spinal cord, and therefore they do not need as large a decompression. The surgeon should have an appreciation for the actual size of the underlying neural elements and tailor the decompression to the appropriate size. The decompression should encompass the dorsal surface of the cervicomedullary junction along the lateral dimensions of the medulla in order to adequately decompress the stenosis at the level of the foramen magnum. This general area can be estimated by reviewing the preoperative MR imaging. Patients with achondroplasia may also have significant engorgement of the veins lying below the ligamentum flavum both dorsally and laterally. These can be the source of significant bleeding, which must be rapidly controlled if they are entered. Air embolism can result from a tear in these venous structures, and the surgeon should ensure the patient is placed in the Trendelenburg position if this occurs and continue irrigation and packing of the area. Patients with achondroplasia typically have a significant extent and severity of spinal stenosis. Historically poor operative results were obtained before the advent of CT and MR imaging as the degree of stenosis was often underappreciated.30–32 Bulky instruments were used for decompression, and this frequently traumatized the underlying spinal cord and nerves. Postoperative instability was common, given the overly wide laminectomies performed for the size of the spinal canal.32–34 Review of the preoperative MR images and CT scans will show the degree of stenosis. If it is not clear, myelography can sometimes be used as an adjunct to show the level of stenosis, although it may not estimate the rostral to caudal length of spinal stenosis. The surgeon should be prepared with an operative plan that includes decompression of more than one or two segments above the level of the block and the same below.25 Pediatric patients are more likely to require fusion in the future because the immature spinal canal has a greater propensity toward instability and deformation,35 and thus we recommend a fusion procedure for any decompression of more than five levels and for any decompression crossing the thoracolumbar junction. The patient should be in the prone position, and perioperative steroids should be given in a similar manner to that previously described. These patients should also be monitored for SSEP changes throughout the course of the procedure. A posterior midline incision is made, and dissection of the paraspinal musculature is carried out in a subperiosteal fashion to expose the spinous processes, laminae, and facet joints over the area of decompression. The laminae medial to the facet joints should be thinned with a high-speed drill or BoneScalpel (Misonix, Farmingdale, NY) to form a trough parallel to the longitudinal axis of the spinal column. The drill head should be pointed toward the facet at approximately a 45-degree angle, which helps the surgeon maintain adequate control and avoid dural tears below. The groove is then thinned until the dura can be seen through the laminar mantle. This should be replicated on the opposite side for the length of the planned laminectomy. After the bone has been adequately thinned, a small dural patch can be inserted into the epidural space without significant compression on the spinal canal below. More recently, we have started utilizing the BoneScalpel (Misonix, Farmingdale, NY) which performs precise osteotomies through longitudinal ultrasonic oscillations. The device selectively impacts bone more than the surrounding soft tissue, and its blunt tip helps protect the underlying dura from trauma. We use this device to create bilateral troughs and can then remove the laminae en bloc without significant neural trauma. If the facet joints are violated throughout any part of this procedure, stabilization through spinal fusion becomes necessary. A retrospective analysis from our institution showed that children with achondroplasia and symptomatic stenosis were at high risk for progression to kyphotic deformity if more than five levels were removed through laminectomy. Removal of the bony architecture in addition to dissection of the paraspinal musculature creates a deep void, especially at the lumbosacral junction. To avoid pseudomeningocele formation, a multilevel closure is performed to reapproximate the muscle masses and fill the dead space.36 A paraspinal muscle encased in fascia is detached from the iliac spine and the lumbosacral laminae and reflected around its pedicle. The edge of the flap is brought down to the opposite lateral end of the laminae and is attached with resorbable sutures along the inferior part of the paraspinal muscles (▶ Fig. 30.5). The superior part of the muscle mass is then retracted over the first flap, thus completing the muscle closure and collapsing the void. Fig. 30.5 Cross section of the lumbar spine (a) that depicts elevation of a paraspinous muscle flap (b). The paraspinal musculature (a) is released from any lateral attachments and reflected to cover the resultant laminectomy defect and any associated spinal hardware (c). Attaching the paraspinal muscles to each other is important to help fill the dead space over the dura. The psoas muscle (b), skin flap (c), and medial and lateral paraspinous perforator arteries originate from the posterior intercostal artery, which in turn originates from the aorta (d). Hydrocephalus and cervicomedullary compression present earlier, in younger patients, than spinal stenosis and back pain.
Complication
< 1 y
< 5 y
< 10 y
< 20 y
> 20 y
Otitis media
60
93
96
Ventilation tubes
16
68
78
Tonsillectomy
25
33
37
39
Hearing loss
17
24
28
38
Speech delay
17
19
19
19
Orthodontics
3
22
48
Shunt
2
6
9
11
11
Apnea
8
13
16
16
Cervicomedullary compression
4
9
13
17
Cervical signs
4
7
8
15
Back pain
5
16
20
Spinal stenosis surgery
1
3
7
Source: Adapted from Hunter AGW, Banker A, Rogers JG, et al. Medical complications of achondroplasia: a multicenter patient review. J Med Genet 1998;35:705.
30.1 Indications for Surgical Treatment
30.1.1 Cervicomedullary Compression
30.1.2 Spinal Stenosis
30.2 Surgical Treatment
30.2.1 Cervicomedullary Compression
30.2.2 Spinal Stenosis
Skeletal Syndromes
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








