Chapter 43 Skull Base Approaches
• Cranial base approaches are designed to provide greater tumor exposure compared to conventional cranial approaches, while avoiding retraction of normal brain structures. An understanding of the often complex anatomy is key to preserving the critical structures surrounding each approach.
• Selection of an appropriate approach for a specific tumor is determined by the cranial fossa involved and tumor extension, the ability to interrupt the tumor’s blood supply early, and relationship of the tumor with surrounding normal structures. For more extensive tumors, either combined approaches or staged operations may be required.
• Complications of skull base approaches include vascular, nerve, or brainstem injury; cosmetic deformity; and cerebrospinal fluid (CSF) leak. In many cases these complications may be avoided by mastery of the surgical anatomy, careful preoperative planning including appropriate structural and vascular imaging, intraoperative monitoring, and meticulous attention to detail during the exposure, osteotomies, and reconstruction.
• The primary indication for the transsphenoidal approach is midline sellar and suprasellar lesions such as pituitary adenomas; this surgical approach can be performed using endoscopic or microsurgical techniques.
• The extended subfrontal approach is excellent for intra- and extradural lesions of the anterior skull base, paranasal sinuses, as well as the sella, and midline clivus down to the foramen magnum. It is excellent for sinonasal malignancies, chordomas, and ethesioneuroblastomas.
• The transmaxillary and extended transmaxillary approaches are best suited for midline extradural lesions centered on the midportion of the clivus, such as chordomas and chondrosarcomas.
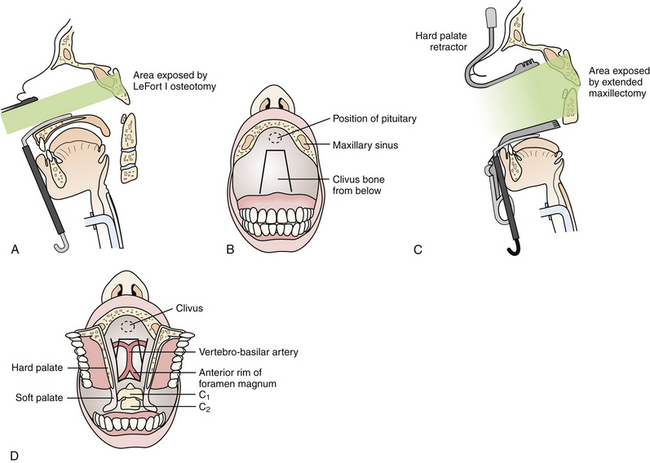
• Transoral approaches are excellent for addressing midline lesions of the lower clivus and upper cervical spine from degenerative, congenital, or rheumatic disorders.
• The frontotemporal approach with variations of the orbitozygomatic extension is among the most frequently used skull base approaches. It is applicable to vascular and neoplastic lesions involving the anterior and middle fossa, orbit, orbital apex and cavernous sinus, paraclinoid and parasellar regions, and basilar apex.
• The subtemporal transzygomatic with petrous apex resection is utilized for lesions involving the petrous apex, upper clivus, posterior cavernous sinus, Meckel’s cave, and the upper posterior fossa. Typical lesions resected via this approach include trigeminal schwannomas, petrous apex lesions such as cholesterol granulomas, cholesteatomas and chondrosarcomas, meningiomas involving the cavernous sinus and tentorium as well as smaller petroclival meningiomas, and vascular lesions in the region of the basilar artery.
• The preauricular subtemporal-infratemporal approach is an inferior extension of the subtemporal transzygomatic approach, and is better suited for lesions requiring lateral exposure of the mid and lower clivus, such as petroclival chondrosarcomas and cholesterol granulomas.
• Transpetrosal approaches provide an ideal ventral trajectory to the brainstem, particularly for posterior fossa tumors with significant extension above the tentorium, such as petroclival meningiomas.
• The extreme lateral transcondylar approach is well suited for lateral and ventral exposure of the caudal brainstem and upper cervical spinal cord at the level of the foramen magnum.
Skull base approaches were developed in response to a need to expose and maximally treat complex lesions at the base of the skull while minimizing retraction injury to normal neurological structures. As with any surgical approach, modules to the skull base are underpinned by a complete knowledge of the regional anatomy as well as appreciation of an often complex three-dimensional anatomy, supplemented by experience in cadaveric dissection. In many instances, a “skull base” approach implies an extension of classic cranial approaches, whereas in others, the approach is not implicit in typical neurosurgical training. Over the past 30 years, approaches in a circumferential fashion to the entire skull base have been developed and have been subjected to various classification schemes and terminologies. They may, however, be summarized as shown in Box 43.1.
Knowledge and expertise required in these approaches provide the fundamental knowledge for access to virtually every aspect of the base of the skull, and allow for selective implementation of part(s) of specific approaches when indicated by the respective pathology. This chapter provides an overview of approaches to the skull base, and for each module the relevant anatomy, indications, and technical principles with possible surgical pitfalls are discussed. This chapter is a summary that should not replace several extensive references on both the anatomy and surgery of the skull base,1–5 in addition to practice in the cadaveric laboratory and instruction from experts in cranial base surgery.
Anterior Skull Base Approaches
Transsphenoidal Approach
The primary indication for the transsphenoidal approach is midline sellar and suprasellar lesions, and it is the workhorse approach for most pituitary adenomas. Several authors have reported endonasal techniques of resection of other lesions, such as tuberculum sellae meningiomas and craniopharyngiomas.6 This approach is limited laterally by the internal carotid arteries; therefore, lesions extending laterally to this point cannot be resected completely. Either the microscope or the endoscope (either as the primary or assistive device) may be used for visualization. Current transsphenoidal instruments remain limited in the ability to apply standard microsurgical techniques of sharp dissection for intradural lesions, and intraoperative complications such as arterial injury are difficult to repair for this reason. Selected intradural lesions, such as tuberculum sellae meningiomas and craniopharyngiomas, may be removed via an extended transsphenoidal approach, provided an adequate arachnoid plane is visualized on preoperative imaging, and the tumor is primarily midline with no lateral extension beyond the carotid arteries or optic nerves. The caveat of an extended approach is an increased risk of postoperative CSF rhinorrhea, given the inherent difficulty of primarily repairing dural or bony openings in a watertight fashion. Dural substitutes, fibrin glue, postoperative lumbar CSF diversion, and nasoseptal mucosal flaps are useful adjuncts to prevent CSF leakage.
Extended Subfrontal Approach
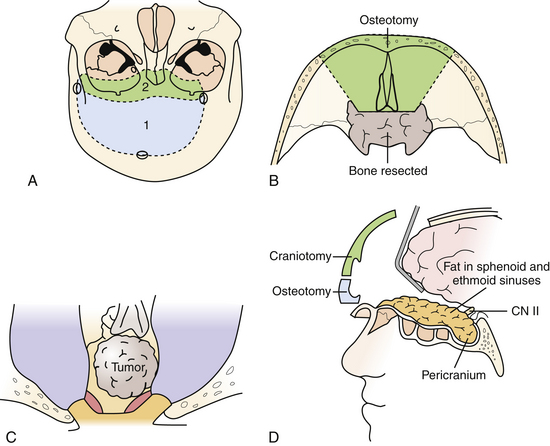
This versatile approach is useful for intra- and extradural lesions of the anterior skull base, paranasal sinuses, as well as the sella, and midline clivus down to the foramen magnum. It is also better suited than the transmaxillary approach for intradural lesions in these areas. Furthermore, it can be adapted for more lateral exposure, including the cavernous sinus and frontotemporal area as needed. As such, it is useful for purely extradural lesions, such as for craniofacial resection of sinonasal malignancies, and for mixed intra- and extradural lesions such as esthesioneuroblastomas, chordomas, and chondrosarcomas (Fig. 43.1).
The patient is positioned supine with the head neutral and the body secured for tilting as required during the procedure for contralateral exposure. Brain relaxation and CSF diversion are facilitated by intraoperative placement of a lumbar drain. A bicoronal incision is performed behind the hairline and reflected anteriorly. A pericranial incision is made from behind the skin incision to maximize the available graft and the pericranium is reflected anteriorly as a separate layer to repair the frontal and ethmoid sinuses as well as the dura at the end of the surgery. Care is taken to preserve the supratrochlear and supraorbital bundles, which may emerge either from a notch or true foramen from above the orbital rim. In the case of a foramen, they are osteotomized with a small straight osteotome and outfractured with the scalp flap. The periorbita is dissected for a distance of approximately 3 cm to allow the orbitofrontal osteotomy to be sufficiently posterior, and the frontonasal suture is also exposed. At this point a low bifrontal craniotomy is performed, taking care not to lacerate the dura or the superior sagittal sinus. The subfrontal dura is mobilized off the orbital roofs bilaterally, sparing the cribriform plate region. A bilateral orbitofrontal osteotomy is then performed using the reciprocating saw, incorporating the orbital bar and through the nasoethmoidal complex in the midline, and circumventing the cribriform plate in order to spare the olfactory dura. The remaining cribriform plate is drilled until the olfactory dural sheath is exposed. Depending on the pathology and the preoperative state of the patient’s olfaction, the olfactory nerves can be preserved during this approach, particularly for when the pathology is primarily intradural. Following an osteotomy circumventing the cribriform plate, the dura is opened, and the olfactory tract and sulcus are identified. Arachnoid dissection is used to mobilize the olfactory tracts off their sulci, allowing the basal frontal lobes to relax without transmitting traction on the olfactory tracts. In more extensive primarily extradural lesions, particularly if the patient is anosmic, the entire complex can then be suture-ligated and divided from within the nasal cavity, allowing the frontal lobes to relax superiorly. The anterior and posterior ethmoidal arteries are divided.
Transmaxillary and Extended Transmaxillary Approaches
This approach is best suited for midline extradural lesions centered on the midportion of the clivus, such as chordomas and chondrosarcomas (Fig. 43.2). The exposure is also well suited for lesions extended superiorly up to the sella turcica. The lateral limits of this exposure include the cavernous sinus and carotid arteries, and the pterygoid space. Depending on the extent of disease, however, the lower clivus, anterior cervical spine, or lateral infratemporal space may be accessed via extended maxillotomy approaches.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree