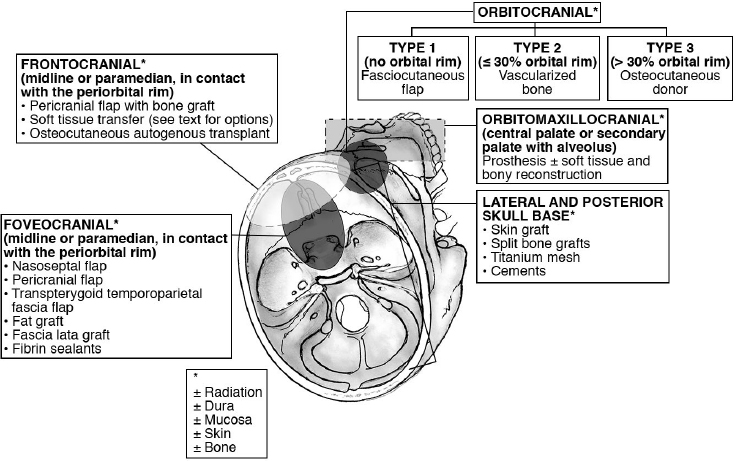
27 Skull Base Reconstruction Techniques Surgical resection of skull base tumors has improved because of our expanded ability to reconstruct cranial base defects. The basic principle of skull base reconstruction is the separation of the cranial contents from the sinonasal, orbital, or oral compartments. The treatment of cranial base tumors is most often multimodal, including radiation and chemotherapy; the principles, therefore, require the surgeon to consider the possibility and implications of adjuvant treatment, including delayed wound healing, infection, and resorption of nonvascularized tissues. More recent advances in the endoscopic approach to the skull base have been paired with novel techniques in endoscopic reconstruction and may lead to an inside-out consideration of reconstruction rather than the traditional out-side-in approach. The ideal classification system would categorize defects in a manner that would relate to a specific set of reconstructive approaches. Such a classification system has not yet been developed because of the anatomic complexity of the skull base and the complexity and variability of the defects. There needs to be a balance between making the system simple enough for effective communication among clinicians but complex enough to be useful as a defect-based decision-making tool. There are several classification systems that categorize skull base defects. Irish and colleagues1 classified tumors into skull base regions (I, II, and III) based on anatomic boundaries and tumor growth patterns (see Chapter 23, Fig. 23.1, page 647). This classification system is useful for describing the specific area of the skull base that requires reconstruction. A more sophisticated description of skull base defects has been developed, with the aim of comparing end results using different reconstructive techniques.2 This classification takes into account the following anatomic components: (1) dura, (2) bone, (3) skin, (4) mucosa, (5) cavity, (6) neurologic status, and (7) carotid artery. The classification system is very useful for describing the defect, determining the treatment plan, predicting potential postreconstructive complications, and counseling patients. A classification system involving subdivisions of the anterior cranial fossa defects into compartments that direct the reconstructive algorithm is described here. In the anterior skull base, the regions are foveocranial, frontocranial, orbitocranial, and orbitomaxillocranial (Fig. 27.1). Foveocranial defects are anterior cranial fossa defects that are in the median or paramedian location and that involve the ethmoid bone or the inner table of the frontal bone. They do not involve the orbit or the outer table of the frontal bone. Because the frontal and orbital rim bony structural supports remain intact, reconstruction of this defect is focused on separation of the cranial compartment from the nasal cavity with a watertight dural seal and use of vascularized tissue to prevent cerebrospinal fluid (CSF) leak and intracranial infectious complications. The foveocranial defect is well suited for endoscopic approaches to skull base lesions. Recent innovations with local pedicled flaps, such as the nasoseptal flap, have facilitated more extensive exposures and reliable reconstruction. • The vascularized pedicled nasoseptal flap significantly enhanced the reconstruction of skull base defects following both standard and extended endoscopic approaches to the skull base.3–5 • The risk of postoperative CSF leak with the nasoseptal flap is 5%, and is comparable to that with open reconstructive techniques.4 • The pericranial flap is one of the most commonly used flaps in skull base surgery6,7 (see Chapter 14, Fig. 14.3, page 342). • For open skull base resections, the pericranial flap is the best option for supporting a dural closure and separating the cranial and nasal cavities for foveocranial defects. • Studies utilizing injection data and blood vessel patterns show that the blood supply to pericranial flaps almost entirely depends on the deep branches and a variable component of the superficial branches of the supraorbital and the supratrochlear vessels.6 • The risk of CSF leak after reconstruction using this flap has been consistently reported to be approximately 5%. • The flap is thin, pliable, and reported to be well vascularized even after regional irradiation. • A minimally invasive endoscopic pericranial flap has been described for use in conjunction with an expanded endoscopic approach.8 This approach uses an incision along the bicoronal line and the removal of bone in the region of the nasion to deliver the flap to the foveocranial defect. • The temporoparietal fascial flap can be passed through a pterional bur hole to the foveocranial defect. • This is an excellent alternative if a pericranial flap has already been harvested, particularly for patients who have had endoscopic surgery and have not had an incision crossing the territory of the superficial temporal artery. Frontocranial defects involve the frontal bone and the anterior cranial fossa, but do not entail orbital exenteration. Frontocranial defects without orbital exenteration require attention to repair of the periorbita, prevention of transmission of the cerebral pulse to the globe, proper position and function of the lids, proper attachment of the canthi, and patency of the lacrimal system. Because of the projection of the frontal bone, reconstruction must be performed to avoid major deformity. To optimize the cosmetic result and adequately support the orbital contents, anterior frontal bone defects greater than 30% of the length of the orbital rim should be reconstructed with bone. There are two options: nonvascularized calvarial bone grafts, which are most suited to the nonradiated patient, and vascularized bone transfers, which are appropriate for radiated or chemoradiated patients. The literature would suggest lower rates of surgical complications in radiated patients when a vascularized autogenous flap is utilized.9 • The pericranial flap with calvarial bone graft is the reconstruction of choice if the patient has not been previously irradiated. • The split calvarial bone can be harvested from the inner table of the craniotomy bone flap. • Some authors have reported excellent results using pericranium to wrap nonvascularized bone grafts when used in an irradiated field.10 The use of calvarial bone graft in a radiated field can result in healing, but the associated complication rate is unacceptably high compared with the results of using vascularized tissue transplantation. • Soft tissue reconstruction of the frontocranial defect is a good alternative for patients with frontal bar defects 30% or less of the orbital rim diameter, particularly in a radiated field. • In addition, if cosmesis is not a priority, then these donor sites can be used for frontal bar defects greater than 30% of the orbital rim diameter. • Radial forearm: • Anterolateral thigh: • Rectus abdominis:
 Classification of Skull Base Defects
Classification of Skull Base Defects
 Anterior Defects: Foveocranial
Anterior Defects: Foveocranial
Nasoseptal Flap
 Surgical Steps
Surgical Steps
 The pedicled nasoseptal flap utilizes blood supply from the posterior nasoseptal artery and is designed by making two parallel incisions, with the inferior one placed along the septum over the maxillary crest.
The pedicled nasoseptal flap utilizes blood supply from the posterior nasoseptal artery and is designed by making two parallel incisions, with the inferior one placed along the septum over the maxillary crest.
 When planning the flap, if the ipsilateral olfactory bulb is not involved in the resection, then the superior incision should be planned 1 cm below the most superior aspect of the septum in order to preserve the olfactory epithelium.
When planning the flap, if the ipsilateral olfactory bulb is not involved in the resection, then the superior incision should be planned 1 cm below the most superior aspect of the septum in order to preserve the olfactory epithelium.
 See Chapter 16 for further details (page 420).
See Chapter 16 for further details (page 420).
Pericranial Flap
 Surgical Steps
Surgical Steps
 Harvest of the pericranial flap begins near the bicoronal incision and involves elevation in the subgaleal plane down to the level of the supraorbital rims; in the supraorbital region, dissection is done so as to identify the supraorbital notch and free the supraorbital vascular bundle.
Harvest of the pericranial flap begins near the bicoronal incision and involves elevation in the subgaleal plane down to the level of the supraorbital rims; in the supraorbital region, dissection is done so as to identify the supraorbital notch and free the supraorbital vascular bundle.
 If a foramen is present, a 4-mm osteotome can be used to preserve the neurovascular bundle and maintain the arterial supply to the flap.
If a foramen is present, a 4-mm osteotome can be used to preserve the neurovascular bundle and maintain the arterial supply to the flap.
 The pericranial flap is placed intracranially before the supraorbital bone is re-secured to enable coverage of the frontal bar.
The pericranial flap is placed intracranially before the supraorbital bone is re-secured to enable coverage of the frontal bar.
Transpterygoid Temporoparietal Fascia Flap
 Surgical Steps
Surgical Steps
 The temporoparietal fascia flap is supplied by the superficial temporal artery and vein, and has been utilized extensively in head and neck reconstruction.
The temporoparietal fascia flap is supplied by the superficial temporal artery and vein, and has been utilized extensively in head and neck reconstruction.
 The pedicle is located just deep to the bicoronal incision and can be injured during the opening of the skin.
The pedicle is located just deep to the bicoronal incision and can be injured during the opening of the skin.
 An elevation just deep to the hair follicles is performed and can result in skin loss overlying the donor site.
An elevation just deep to the hair follicles is performed and can result in skin loss overlying the donor site.
 The deep layer is harvested after the superficial layer and includes the pericranium above the temporal line and the superficial layer of temporal fascia below the temporal line.
The deep layer is harvested after the superficial layer and includes the pericranium above the temporal line and the superficial layer of temporal fascia below the temporal line.
 This site is most useful in revision cases; unfortunately, the bicoronal incision has frequently damaged the pedicle.
This site is most useful in revision cases; unfortunately, the bicoronal incision has frequently damaged the pedicle.
 Anterior Defects: Frontocranial
Anterior Defects: Frontocranial
Pericranial Flap with Bone Graft
 Surgical Steps
Surgical Steps
 The pericranial flap harvest was described earlier in the chapter.
The pericranial flap harvest was described earlier in the chapter.
 Split calvarial bone grafts are harvested from the outer table of the cranium after raising a bicoronal skin flap and pericranial flap, or by harvesting the inner table of the craniotomy bone flap.
Split calvarial bone grafts are harvested from the outer table of the cranium after raising a bicoronal skin flap and pericranial flap, or by harvesting the inner table of the craniotomy bone flap.
 When harvesting a cranial bone graft, care must be taken to avoid full-thickness bone cuts and dural injury.
When harvesting a cranial bone graft, care must be taken to avoid full-thickness bone cuts and dural injury.
 Troughs are cut around the calvarial bone graft with a fissure bur.
Troughs are cut around the calvarial bone graft with a fissure bur.
 Then an oscillating offset saw blade or a curved osteotome is used to undercut the graft in the cancellous space and to free it from the underlying inner table.
Then an oscillating offset saw blade or a curved osteotome is used to undercut the graft in the cancellous space and to free it from the underlying inner table.
 After inset of the bone graft, the pericranium should be closely applied to the graft to separate it from contaminated fields.
After inset of the bone graft, the pericranium should be closely applied to the graft to separate it from contaminated fields.
Soft Tissue Transfer: Radial Forearm, Anterolateral Thigh, Rectus Abdominis, Latissimus Dorsi
 The radial forearm autogenous transplant is an excellent option for low-volume reconstructions if local tissue, such as the pericranial flap, is absent. This donor site is reliable, has a long vascular pedicle, provides thin pliable tissue, and has been shown to be effective for closing CSF leaks.11
The radial forearm autogenous transplant is an excellent option for low-volume reconstructions if local tissue, such as the pericranial flap, is absent. This donor site is reliable, has a long vascular pedicle, provides thin pliable tissue, and has been shown to be effective for closing CSF leaks.11
 Potential disadvantages of the radial forearm transplant over local flaps include a longer operative time and an additional, although low, risk of hand morbidity.
Potential disadvantages of the radial forearm transplant over local flaps include a longer operative time and an additional, although low, risk of hand morbidity.
 The anterolateral thigh autogenous transplant based on the lateral femoral circumflex artery is a good option when the volume of the defect is greater than the volume that the radial forearm donor site can obliterate.
The anterolateral thigh autogenous transplant based on the lateral femoral circumflex artery is a good option when the volume of the defect is greater than the volume that the radial forearm donor site can obliterate.
 This donor site also contains ample fascia, which can be used for dural repair. The fascia can remain vascularized for dural closure. The authors of a series of seven patients reconstructed with anterolateral thigh free tissue reported no CSF leaks or meningitis after a mean follow-up of 10 months.12
This donor site also contains ample fascia, which can be used for dural repair. The fascia can remain vascularized for dural closure. The authors of a series of seven patients reconstructed with anterolateral thigh free tissue reported no CSF leaks or meningitis after a mean follow-up of 10 months.12
 The donor site of the anterolateral thigh flap can be closed primarily. If a perforator-based harvest is chosen, the volume of the reconstruction is easier to control because there is no muscle in the transplant that will atrophy and result in volume loss.
The donor site of the anterolateral thigh flap can be closed primarily. If a perforator-based harvest is chosen, the volume of the reconstruction is easier to control because there is no muscle in the transplant that will atrophy and result in volume loss.
 The rectus abdominis autogenous transplant is the historical donor site of first choice in skull base reconstruction.13
The rectus abdominis autogenous transplant is the historical donor site of first choice in skull base reconstruction.13
 The vascular pedicle anatomy is long and reliable, with the ability to transfer large amounts of skin, fat, muscle, and fascia.
The vascular pedicle anatomy is long and reliable, with the ability to transfer large amounts of skin, fat, muscle, and fascia.
 If long-term precise volume contouring is desired, then the donor site can be harvested as a perforator-based transplant so that muscle atrophy will not adversely affect the reconstructed contour.
If long-term precise volume contouring is desired, then the donor site can be harvested as a perforator-based transplant so that muscle atrophy will not adversely affect the reconstructed contour.
 When harvested as a myocutaneous skin paddle, the rectus has a tendency to become ptotic if the volume of the flap exceeds the volume of the defect.
When harvested as a myocutaneous skin paddle, the rectus has a tendency to become ptotic if the volume of the flap exceeds the volume of the defect.
 The pale color of the donor site is a poor match for Caucasian patients.
The pale color of the donor site is a poor match for Caucasian patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree