Skull Base Tumors
Pediatric skull base pathologies and their operative management pose unique challenges for the skull base surgeon. The entities in the differential diagnosis and the ages at presentation are diverse, and the skull base surgeon is faced with a heterogeneous patient population and a dynamic surgical anatomy characteristic of growing patients. Recent advances in imaging and treatments for skull base lesions require careful consideration when applied to the pediatric population. Indications for surgery have expanded to include both benign and malignant disease, and treatments and surgical approaches need to take into account treatment goals, potential long-term side effects of therapy, impact on craniofacial growth, cosmesis, postoperative care, and quality of life.
Pediatric skull base surgery encompasses a wide variety of surgical approaches, both open and endoscopic. There has been a dramatic increase in pediatric endoscopic approaches at our institution, which is a consequence of our experience in the adult population of patients requiring skull base surgery and an increased recognition of the benefits of endonasal surgery for the pediatric population. Decreased morbidity, faster postoperative recovery, and greater patient and family acceptance make endoscopic surgical approaches especially suitable for pediatric patients.
The primary aim of this chapter is to provide an overview of surgical approaches, both open and endoscopic, and their application to common pediatric skull base pathologies. Clinical examples of pathologies affecting different regions of the skull base are discussed, with particular reference to clinical evaluation, the relevant surgical anatomy and approaches, and reconstruction strategies. Specific planning and surgical considerations in the management of pediatric patients with skull base pathologies are highlighted.
44.1 Overview of Skull Base Anatomy
Successful cranial base surgery is based on an in-depth knowledge of the regional and embryologic anatomy of the developing skull base, an understanding of how specific pathologies affect the pediatric skull base, and an appreciation of the limitations and consequences of various surgical techniques in pediatric patients. Extensive training, complemented by dissection work in the laboratory, is crucial to achieve anatomical proficiency, a three-dimensional appreciation of the structural relationships, and a knowledge of anatomical variations. The skull base surgeon must master intracranial, extracranial, and endoscopic surgical anatomy.
The skull base can be considered in three different parts—anterior, middle, and posterior—with closely related intracranial and extracranial anatomical compartments (▶ Table 44.1). Detailed anatomy is beyond the scope of this chapter. Major considerations include a knowledge of (1) surgical anatomy for optimal access with minimum risk to critical neurovascular structures, (2) developmental anatomy of various growth stages, (3) embryologic anatomy to understand surgical pathology, (4) vascular anatomy, and (5) reconstructive anatomy.
| Anterior skull base | Middle skull base | Posterior skull base | |
| Intracranial structures | Anterior cranial fossa | Middle cranial fossa Pituitary fossa Cavernous sinus | Posterior cranial fossa |
| Osteology | Frontal bones Ethmoid bones Sphenoid bone—planum sphenoidale | Sphenoid bone Temporal bone | Sphenoid bone Occipital bone |
| Foramina | Foramen cecum Olfactory foramina Ethmoid foramina | Vidian canal Foramen rotundum Foramen ovale Foramen spinosum Foramen of Vesalius | Hypoglossal canal Foramen magnum |
| Extracranial structures | Frontal and ethmoid sinuses Nasal cavity Orbit Lacrimal apparatus | Sphenoid and maxillary sinuses Nasopharynx Fossa of Rosenmüeller Pterygopalatine fossa Infratemporal fossa Parapharyngeal space Infrapetrosal space | |
| Major vessels | Anterior and posterior ethmoid arteries Ophthalmic artery Fronto-orbital arteries Anterior cerebral arteries | Circle of Willis Internal carotid artery Superior and inferior hypophysial arteries Vidian artery Maxillary artery Sphenopalatine artery Posterior nasal artery Middle meningeal artery Accessory meningeal artery | Vertebral artery Basilar artery |
| Major nerves | Olfactory bulb Olfactory tract | Optic nerve Cavernous sinus nerves
| Abducens nerve |
44.1.1 Anterior Skull Base
This portion of the skull base extends from the internal surface of the frontal bones anteriorly to the sphenoid ridge of the sphenoid bone posteriorly. The latter is joined medially by the chiasmatic groove (▶ Fig. 44.1). The intracranial surface is formed by the frontal, ethmoid, and sphenoid bones.1 The frontal bones comprise the major part of the anterior cranial base, contributing to its lateral part. The orbital process of the frontal bone articulates posteriorly with the lesser wing of the sphenoid bone. Those two bones form the orbital roof and the optic canal, which transmit the optic nerve and ophthalmic artery. Posterolaterally, the optic canal is related to the anterior clinoid, and inferomedially to the posterior ethmoid sinus. The frontal sinus lies anteriorly between the external and internal walls (anterior and posterior tables) of the frontal bone.
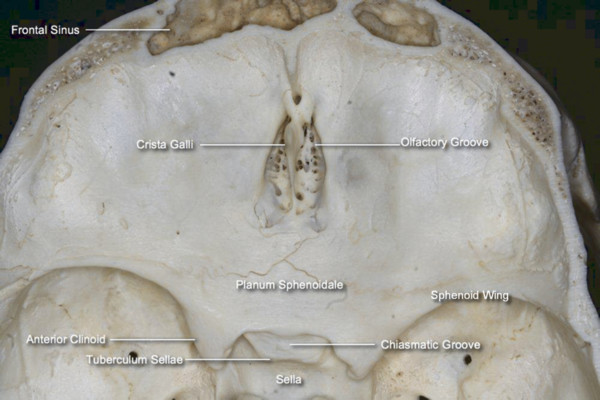
Fig. 44.1 Intracranial view of the anterior cranial base.
The anterior cranial base faces the frontal lobes, with the rectus gyrus medially and the orbital gyri laterally. In the midline, the superior sagittal sinus continues to the floor of the anterior cranial base, where it connects with a small emissary vein at the foramen cecum. The fronto-orbital artery, a branch of the anterior cerebral artery, travels along the inferior and medial surface of the frontal lobe and is therefore at risk during anterior cranial base surgeries. The olfactory bulbs lie over the cribriform plates, and the olfactory tracts continue posterolaterally, closely applied to the surface of the brain as they pass over the optic nerves.
The midline of the anterior cranial base is related to the nasal cavity, ethmoid, and sphenoid sinuses. The ethmoid bone constitutes the anterior two-thirds of the midline anterior cranial base. The regions of the ethmoid bone related to the intracranial surface from medial to lateral are the crista galli, cribriform plate, and fovea ethmoidalis. The crista galli separates the anterior half of the cribriform plate in the midline and is attached to the falx cerebri. Anterior to the crista galli, the foramen cecum transmits an emissary vein responsible for the venous drainage from the nasal cavity to the superior sagittal sinus. In addition to the potential risk for the intracranial dissemination of nasal infections, lesions like nasal dermoid, glioma, and meningocele can communicate intracranially via the foramen.2,3 The thin lateral lamella of the cribriform plate continues laterally as the fovea ethmoidalis or roof of the ethmoid sinuses. The depth and angulation of the lateral lamella affect the risk for iatrogenic cerebrospinal fluid (CSF) leak during transethmoidal procedures. The olfactory filaments pass through the cribriform plate from the nasal cavity to the intracranial olfactory bulbs and are a route for the intracranial spread of sinonasal malignancy. The posterior third of the midline anterior cranial base is formed by the planum sphenoidale, which corresponds to the roof of the sphenoid sinus.
At the junction of the ethmoid sinus and orbit, the anterior and posterior ethmoidal foramina along the frontoethmoidal suture line transmit the anterior and posterior ethmoidal arteries, respectively (▶ Fig. 44.2). These arteries are derived from the ophthalmic artery within the orbit. From the orbit, the ethmoidal arteries enter the ethmoid sinuses via their respective foramina and run in a lateral-to-medial direction in the roof of the ethmoid sinuses. In some cases, they may lie below the ethmoid roof on a bony mesentery or pedicle and are at risk during endoscopic sinus surgery. The anterior ethmoidal artery is located between the second and third ground lamellae in a coronal plane tangential to the posterior surface of the globe. The posterior ethmoidal artery is often found at the junction of the fovea ethmoidalis and planum sphenoidale. These arteries diverge as they cross the roof of the ethmoid and need to be identified and ligated or coagulated during surgical procedures involving the anterior cranial base. Medially, the arteries enter the cranial cavity through the lateral lamella and re-enter the nasal cavity to supply the upper nasal septum.
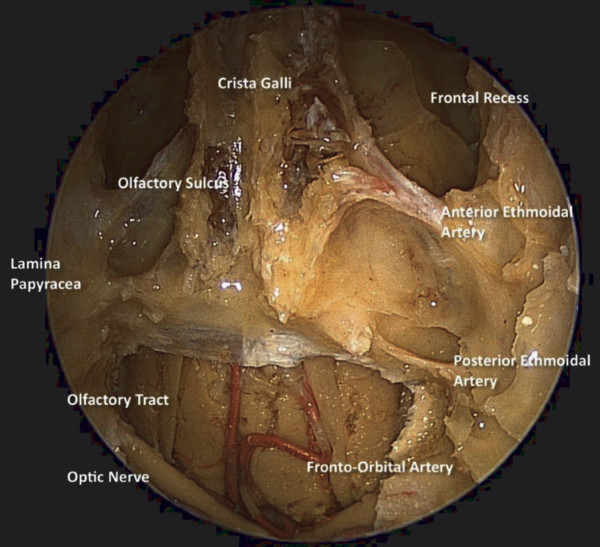
Fig. 44.2 Endonasal endoscopic view of the anterior cranial base demonstrating the relationship of the anterior and posterior ethmoid arteries to the orbit and the skull base.
44.1.2 Middle Cranial Base
The intracranial surface of the middle cranial base is formed by the sphenoid and temporal bones.1 The limit between the anterior and the middle cranial bases is the sphenoid ridge joined medially by the chiasmatic groove. The transition between the middle and the posterior cranial bases is the petrous ridge joined medially by the dorsum sellae and the posterior clinoid process.1
The intracranial surface of the middle cranial base can be divided into two regions: medial and lateral. The medial region is composed of the body of the sphenoid bone and is related to the pituitary gland and cavernous sinus, including the tuberculum sellae, pituitary fossa, middle and posterior clinoid processes, carotid sulcus, and dorsum sellae.1 The greater wing of the sphenoid bone and the temporal bone (squamosal and petrosal segments) form the lateral portion of the middle cranial base, containing the middle cranial fossa. The optic strut runs under the optic nerve and separates it from the internal carotid artery (ICA). Pneumatization of the optic strut forms the lateral opticocarotid recess (OCR), observed endonasally in the sphenoid sinus (▶ Fig. 44.3).
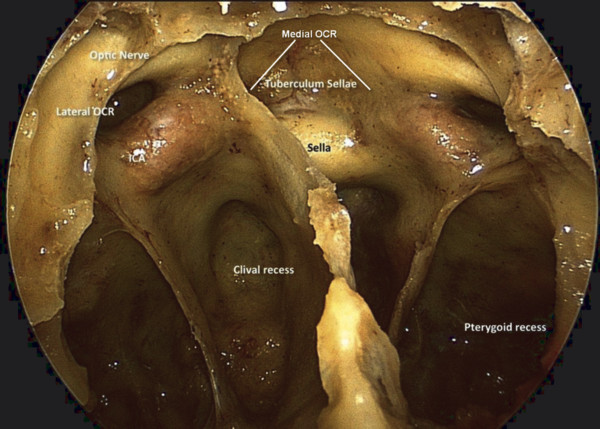
Fig. 44.3 Endoscopic view of the surface anatomy of the sphenoid sinus demonstrating the relationships of the optic canals, internal carotid arteries, and opticocarotid recesses. OCR, opticocarotid recess.
The temporal bone has a pyramidal shape, the sides of which form the middle fossa floor (superior face), the anterior limit of the posterior fossa (posterior face), the muscle attachments of the neck and infratemporal fossa (anteroinferior face), and the muscular-cutaneous–covered side of the head (lateral), which forms the base of the pyramid. The temporal bone consists of four embryologically distinct components: the squamous, mastoid, petrous, and tympanic parts.
The area below the middle cranial fossa includes the infratemporal fossa, parapharyngeal space, infrapetrosal space, and pterygopalatine fossa. The boundaries of the infratemporal fossa are the medial pterygoid muscle and the pterygoid process medially, the mandible laterally, the posterior wall of the maxillary sinus anteriorly, the greater wing of the sphenoid superiorly, and the medial pterygoid muscle joining the mandible and the pterygoid fascia posteriorly. The fossa opens into the neck below. The infratemporal fossa contains the branches of mandibular nerve, the maxillary artery, and the pterygoid muscles and venous plexus. The mandibular nerve exits the cranial base through the foramen ovale. The pterygoid venous plexus connects via the middle fossa foramina and inferior orbital fissure with the cavernous sinus and empties into the retromandibular and facial veins.1 During a lateral infratemporal approach, the following structures form a linear plane superficial to the ICA canal: the lateral pterygoid plate, foramen ovale, foramen spinosum, and spine of the sphenoid. Deep to this linear plane, the eustachian tube overlies the petrous carotid canal.
The pterygopalatine fossa is located between the maxillary sinus anteriorly, the pterygoid process posteriorly, the palatine bone medially, and the body of the sphenoid bone above. The fossa communicates laterally via the pterygomaxillary fissure with the infratemporal fossa and medially through the sphenopalatine foramen with the nasal cavity. Both the foramen rotundum (with the maxillary nerve) and the pterygoid canal (with the vidian nerve) communicate with the pterygopalatine fossa via the posterior wall. The main contents of the fossa include branches of the maxillary nerve and vidian nerve, the pterygopalatine ganglion, and the pterygopalatine segment of the maxillary artery.
The parapharyngeal space is predominantly a fat-filled space but also contains the eustachian tube, pharyngeal branches of the ascending pharyngeal and facial arteries, and branches from the glossopharyngeal nerve. It is divided into two compartments, an anterolateral pre-styloid space and a posteromedial post-styloid space, by fascia from the styloid process to the tensor veli palatini muscle. The pre-styloid space contains the deep lobe of the parotid gland, minor salivary glands, a small branch of the mandibular nerve (cranial nerve V3) to the tensor veli palatini muscle, the ascending pharyngeal artery, and the pharyngeal venous plexus, embedded in mostly fatty tissue. The post-styloid compartment contains the ICA, internal jugular vein, cranial nerves IX through XII, the cervical sympathetic chain, lymph nodes, and glomus bodies.
Endonasally, the medial aspect of the middle fossa (Meckel cave) is lateral to the paraclival ICA and superior to the petrous segment of the ICA. The second division of the trigeminal nerve (foramen rotundum) and the vidian nerve (pterygoid canal) are helpful landmarks that define the area of the pterygoid and sphenoid just anterior to this. The Meckel cave is bounded by the lateral cavernous sinus superiorly containing cranial nerves III, IV, V1, and VI. The foramen ovale is posterior to the lateral pterygoid plate, and the base of the pterygoid requires drilling to gain optimal access to this region.
44.1.3 Sphenoid Sinus
The surface anatomy of the sphenoid sinus is important for endonasal approaches to the pituitary and surrounding areas (▶ Fig. 44.3). The degree of sphenoid pneumatization and the patterns of septa vary greatly. Lateral septa almost always deviate toward the ICA, and care must be taken when they are removed. The sella is bounded by the clival recess inferiorly, cavernous sinus and ICA laterally, and optic canal superolaterally. The clival recess is bounded by the paraclival ICA and petrous apex laterally. The sixth cranial nerve courses superolaterally in the Dorello canal behind the paraclival ICA and is at risk for injury during drilling posterior to the paraclival ICA just below the level of the sellar floor (▶ Fig. 44.4).
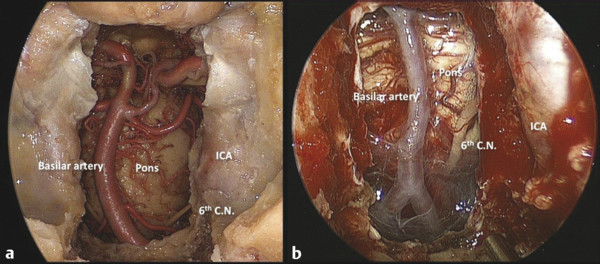
Fig. 44.4 (a) Cadaveric dissection demonstrating the course of cranial nerve VI. The nerve arises from the brainstem at the level of the vertebrobasilar junction. (b) Operative photo demonstrating the course of cranial nerve VI relative to the paraclival carotid artery. The nerve enters the Dorello canal at approximately the midpoint of the paraclival internal carotid artery. ICA, internal carotid artery; 6th C.N., sixth cranial nerve.
44.1.4 Posterior Cranial Base and Craniocervical Junction
Surgical access to the posterior cranial fossa may be posterior, inferior, and medial to the temporal bone. The sigmoid sinus defines the posterior margin of the petrous temporal bone. The infrapetrosal space contains the jugular bulb and the lower portion of the inferior petrosal sinus; branches of the ascending pharyngeal artery; the glossopharyngeal, vagus, and accessory nerves; and the opening of the carotid canal through which the ICA enters the cranial base. Endonasally, the clivus lies anterior to the brainstem and can be divided into three segments: superior, middle, and inferior. The superior segment extends from the posterior clinoid to the sella floor and intracranially is related to the oculomotor nerve and posterior cerebral and superior cerebellar arteries. The middle segment extends from the sellar floor to the floor of sphenoid sinus and is related to the abducens nerve and the basilar and anterior inferior cerebellar arteries. The inferior segment extends from the floor of the sphenoid sinus to the foramen magnum and is related to the glossopharyngeal, vagus, and hypoglossal nerves and the vertebrobasilar junction. Inferolaterally, the hypoglossal foramen is bounded superiorly by the jugular tubercle and the occipital condyle inferiorly.
44.2 Reconstructive Anatomy: Pedicled Flaps
Major reconstructive options include the nasoseptal and scalp flaps. The latter encompass the pericranial, galeopericranial, temporoparietal, and temporalis muscle flaps. Another flap that may be used is the inferior turbinate flap. The middle turbinate and palatal flaps are generally not a practical option in the pediatric population. A summary of vascularized flaps and their blood supply is provided in ▶ Table 44.2.
| Flap | Blood supply |
| Septal flap | Posterior septal artery |
| Pericranial flap, galeopericranial flap | Supraorbital and supratrochlear arteries |
| Temporoparietal flap | Superficial temporal artery |
| Temporalis muscle flap | Deep temporal arteries |
| Inferior and middle turbinate flaps | Branches of sphenopalatine artery |
| Palatal flap | Descending palatine artery |
44.2.1 Scalp
Understanding the layers of the scalp makes it possible to preserve function and plan reconstruction with pedicled scalp flaps. The scalp has five layers, designated by the acronym SCALP: skin, subcutaneous tissues, aponeurosis (galea), loose areolar layer, and periosteum. The pericranial flap comprises the loose areolar layer and periosteum, whereas the galeopericranial flap includes the galeal layer. The pericranial flap is supplied by the supraorbital and supratrochlear vessels, which exit foramina or notches along the superior orbital rim. Laterally, the galea is continuous with the superficial temporal fascia. A temporoparietal flap, derived from this layer, receives its blood supply from the superficial temporal artery. The temporalis muscle, another important reconstructive flap, is covered by the deep temporal fascia. The deep temporal arteries, terminal branches of the internal maxillary artery, supply the muscle on its deep surface.
44.2.2 Nasoseptal Flap
The nasoseptal flap is pedicled on the posterior septal branches of the sphenopalatine artery, which run across the anterior surface of the sphenoid rostrum below the level of the sphenoid ostium and immediately above the choanal margin. The superior incision extends posteriorly from the level of the sphenoid ostium and is carried forward anteriorly along the nasal septum below the skull base. The inferior incision extends from the posterior choanal margin, curving medially along the posterior margin of the vomerine septum to the nasal floor, and is carried anteriorly along the junction of the nasal septum and nasal floor. The superior and inferior septal incisions are then joined with a vertical septal incision at the squamocolumnar junction. This mucoperichondrial, mucoperiosteal nasoseptal flap is then elevated off the cartilaginous and bony nasal septum. Further surgical details of the nasoseptal flap can be found elsewhere.4 This flap can be used to reconstruct defects of the anterior (cribriform plate, fovea ethmoidalis, planum), middle (sellar, lateral sphenoid recess), and posterior (clival, craniocervical junction) cranial base.
44.3 Vascular Anatomy
In many respects, the surgical approaches to the skull base and reconstruction with vascularized flaps are determined by the vascular anatomy. Detailed vascular anatomy allows the anticipation and avoidance of injury to critical vessels, including the ICA, basilar artery, circle of Willis, perforators, superior hypophysial, fronto-orbital, and ophthalmic arteries. It also allows the anticipation and ligation of critical vessels required to devascularize the surgical field, including the anterior and posterior ethmoid, sphenopalatine, and internal maxillary arteries.
The course of the ICA has particular relevance during approaches to the middle cranial fossa. The suprapetrous and infrapetrous approaches are defined as above and below the horizontal (petrous) segment of the ICA, respectively. At least five segments of the ICA have been described: parapharyngeal (extracranial), horizontal (petrous), paraclival, cavernous, and supraclinoid.5 Surgical landmarks are described in detail elsewhere.5 The ophthalmic artery branches off the ICA as it exits the cavernous sinus and runs inferomedial to the optic nerve. The circle of Willis comprises the ICAs, anterior cerebral arteries, anterior communicating artery, posterior cerebral arteries, and posterior communicating artery. A patent circle of Willis is predictive of collateral cerebral blood flow, and anatomical variations are common.
44.4 Developmental Anatomy
Several important considerations include the development of certain pathologies, potential disruption of craniofacial growth with various surgical approaches (especially open) and anatomical limitations to surgical approaches in the growing pediatric patient. The latter is particularly important when considering transnasal approaches to the skull base, where development of the sinuses and configuration of the piriform aperture can limit access. Importantly, when considering transsphenoidal pituitary surgery, sphenoid sinus pneumatization can affect intercarotid distances and determine the amount of drilling to access the sellar. Furthermore, the development of the nasal septum relative to the cranium can limit the size of nasoseptal flaps for reconstruction.6
44.4.1 Skull Base and Facial Skeleton
There are three main components of the human skull: (1) the membranous neurocranium containing the flat bones of the skull, (2) the cartilaginous neurocranium forming the major part of the skull base, and (3) the viscerocranium or facial skeleton.7 The precursor of the cranial base is a cartilaginous plate, the chondrocranium, which subsequently undergoes endochondral ossification. The cranial base has two portions with distinct embryologic origins: the anterior part and the posterior part, derived from the neural crest and paraxial mesoderm, respectively. The anterior and posterior parts of the cranial base are separated by the sella turcica.
The anterior cranial base integrates with the mid and upper face to form a coordinated growth complex and serves as a template for facial growth. It carries the mid and upper face forward, inferiorly, and laterally. It grows at a faster rate than the posterior cranial base, and the growth occurs predominantly in the sphenoethmoidal synchondrosis and in the cartilage between the ethmoid and frontal bones. From adolescence onward, progressive pneumatization of the frontal and ethmoid bones contributes to the rest of anterior cranial growth. Thus, anomalies of anterior cranial growth (including cretinism, Turner syndrome, and Down syndrome) and surgery of the anterior cranial base that disrupts critical growth centers affect both cranial and facial growth. In such cases, the craniofacial structure is characterized by a short, retrognathic face due to reduced length of the cranial base and increased angulation between the anterior and posterior cranial base.
In contrast, the mandible articulates with the posterior cranial base, and variation of the growth and orientation of the cranial base can affect jaw position and occlusion. Mandibular prognathism has been attributed to abnormalities of posterior cranial base growth.
Skeletal maturation of the skull shape begins with the midline cranial base (7.7 years), followed by the lateral skull base (11.7 years), and finally the face (15.7 years).8 Hence, a definitive adult shape of the basicranium is obtained at approximately 12 years. The maturation of craniofacial size follows a superior–inferior gradient, beginning at the neurocranial outline in the midline (11.4 years), followed by the midline cranial base (13.6 years) and the lateral cranial floor and face (15.7 years).
During development of the primitive frontal nasofrontal process (the anterior neuropore), two openings become apparent. The fonticulus frontalis appears between the frontal and nasal bones, while the foramen cecum appears as a midline opening anterior to the crista galli. A diverticulum of dura projects through the fonticulus frontalis or through foramen cecum into the prenasal space, which is found inferior and posterior to the frontal and nasal bones but superior and anterior to the septal cartilage. The dura temporarily approximates the skin and subcutaneous tissues of the midnasal bridge at the osseous cartilaginous junction. Failure of the involution of this diverticulum can lead to the development of nasal dermoid sinus cysts, nasal gliomas, and meningoencephaloceles. All of these lesions have in common certain elements of disjunction or failed primary neurulation of the anterior neuropore. Craniofacial malformations have been reported to varying degrees in all three conditions, and magnetic resonance (MR) imaging is the imaging modality of choice for all these lesions.
44.4.2 Sella and Pituitary Gland
The pituitary gland is ectodermal in origin and has dual embryologic development. The anterior and intermediate lobes develop from oral ectodermal elements derived from the Rathke pouch, and the posterior lobe develops from neural ectoderm. An invagination (Rathke pouch) forms from the primitive stomodeum, cranial to the buccopharyngeal membrane, and extends toward the invagination derived from the neuroectoderm (infundibulum). The anterior portion of the Rathke pouch epithelium proliferates to form cells of the adenohypophysis, while the posterior portion remains largely as a cleft (intermediate lobe) between the anterior and posterior (neurohypophysis) lobes.
Neoplasms arising from the anterior lobe are classic pituitary adenomas, and their phenotype depends on the proliferating cell type. An accumulation of epithelial secretions from the intermediate lobe is thought to underlie the pathophysiology of Rathke cleft cysts. Although still debatable, the general consensus is that craniopharyngioma arises from the neoplastic transformation of cells in the Rathke cleft. Given the close relationship between the cells of origin of these pathologies, adenomas may occur simultaneously with Rathke cleft cyst.9
44.4.3 Frontal, Ethmoid, and Maxillary Sinuses
The paranasal sinuses develop at different stages, which is important to keep in mind, especially when endonasal (transnasal, transsinus) approaches to the skull base are being considered. The ethmoid and maxillary sinuses are present at birth and are well developed by 3 years of age. The frontal sinus develops at approximately 7 years of age and becomes fully pneumatized by 12 years.
44.4.4 Sphenoid Sinus
Sphenoid sinus pneumatization begins after age 2 at the anteroinferior wall of the sphenoid bone. A recent study showed that by 6 to 7 years of age, the sphenoid anterior wall is fully pneumatized in all patients, and 88% of the planum is pneumatized.10,11 The sella turcica and middle cranial fossa portion of the sphenoid bone in 6- to 7-year-old patients on average had 77% of the anterior sellar wall and 32% of the sellar floor pneumatized. There was no dorsum pneumatization in 84% of patients younger than 16 years, and the sellar floor length in patients younger than 2 years was 66% of that in adults. Pneumatization in the superior clivus and posterior cranial fossa aspect begins after the age of 10 years.
The intercarotid distance at the level of the cavernous sinus is significantly narrower in patients up to 6 to 7 years old than it is in adults, whereas there is no significant difference in patients 9 to 10 years and older.10,11 At the level of the superior clivus, the distances are similar in pediatric and adult patients.
Although incomplete sphenoid sinus pneumatization necessitates more drilling, the early maturation of the intercarotid distances and the use of intraoperative imaging are not a contraindication to endonasal approaches to the sella in pediatric patients. It is also important to consider the age-specific length of the pituitary fossa, which increases by more than 50% from age 2 to adulthood.
44.5 Pediatric Skull Base Tumors
A wide variety of skull base pathologies can affect pediatric patients (▶ Table 44.3). Tumors and other lesions may arise within the skull base, traverse the cranial base with either intracranial or extracranial origins, or present in single or multiple sites. The latter is characteristic of hereditary tumors, metastatic deposits, and developmental anomalies. These pathologies can involve the anterior, middle, and posterior skull base and the corresponding intracranial fossae and extracranial regions, which include the nasal cavity, paranasal sinuses, orbits, pterygopalatine and infratemporal fossae, pharynx, and parapharyngeal and craniocervical regions. The differential diagnosis depends on age, sex, associated developmental anomalies, location, and clinical and imaging characteristics.
| Anterior cranial base, nasal cavity, maxillary sinus, orbit | Middle cranial base, sphenoid, sella, infratemporal fossa | Posterior cranial base |
| Meningoencephalocele | Meningoencephalocele | Chordoma |
| Nasal dermoid | Pituitary adenoma | Vestibular schwannoma |
| Glioma | Craniopharyngioma | Epidermoid cyst |
| Mucocele | Cholesterol granuloma | Glomus jugulare |
| Juvenile angiofibroma | Trigeminal schwannoma | Osteomyelitis |
| Fibrous dysplasia | Angiofibroma | |
| Fibro-osseous tumors | Sarcomas | |
| Esthesioneuroblastoma |
44.5.1 Clinical Presentation and Differential Diagnosis
The clinical presentation is influenced by the age at presentation and by the nature and site of the lesion. The term congenital refers to a presentation at or within 1 month of birth and does not indicate the pathophysiology. Often, congenital lesions consist of developmental anomalies; however, hamartomas, choristomas, and teratomas may also present congenitally. The symptomatology is diverse, and in-depth knowledge of the anatomy and cranial nerve physiology is imperative for a correct clinical topographic diagnosis. An adequate clinical examination can indicate the location and estimate tumor extent. However, this may not always be possible in the very young. Endocrine symptoms such as failure to thrive, short stature, and lack of sexual development often predominate in children with pituitary pathologies. Furthermore, nonspecific symptoms, including headache, weight loss, vomiting, weakness, and loss of appetite, may also occur. Symptoms characteristic of lesions involving the anterior, middle, and posterior skull base are summarized in ▶ Table 44.4.
| Anterior skull base | Middle skull base | Posterior skull base |
Olfactory dysfunction
| Pituitary dysfunction Visual field loss Trigeminal neuralgia or numbness Facial palsy Ptosis Diplopia Trismus Eustachian tube dysfunction Temporal bone involvement (hearing loss, tinnitus, vertigo) | Tinnitus Hearing loss Balance problems Swallowing difficulties Hoarseness Speech problems Dysarthria Shoulder weakness Various syndromes |
The physical examination should include a complete assessment of cranial nerve function. In patients with nasal congestion, rhinorrhea, or airway obstruction, an evaluation by otolaryngology should be performed and should include nasal endoscopy. In patients with olfactory dysfunction, objective documentation can be performed. Visual symptoms should be evaluated further by an ophthalmologist and may include visual field testing in addition to a routine examination. Symptoms of hearing loss or vestibular dysfunction can be evaluated further with audiometric testing and vestibular tests if necessary. Lower cranial nerve dysfunction may require an evaluation of swallowing function and aspiration risk with a functional endoscopic examination of swallowing (FEES) examination or radiography (barium esophagogram). If CSF rhinorrhea is suspected, provocative tests including a Valsalva maneuver can be performed. Testing of collected fluid for β2-transferrin or beta trace protein will confirm the presence of CSF.
44.5.2 Imaging in Skull Base Surgery
Computed tomography (CT) and MR imaging provide complementary information regarding the bone and soft-tissue anatomy, respectively, for diagnosis, preoperative planning, the intraoperative period, and postoperative surveillance. Bone erosion, defects, remodeling, hyperostosis, and calcification are well recognized on CT scans. CT angiography is especially helpful to evaluate the vasculature within and surrounding a tumor and is preferred for intraoperative navigation. MR imaging is superior for delineating intracranial or intraorbital invasion and determining the nature of the lesion. Fluid collections (meningocele, obstructed sinus) appear bright on T2-weighted sequences, although chronic sinus obstruction with a high protein content may appear dark on both T1 and T2 sequences. Fat appears bright on T1 and dark on T2 images, which explains the MR imaging appearance of lesions with a high lipid content, such as cholesterol granulomas. Chondromatous neoplasms (clival chordomas, chondrosarcomas) characteristically enhance on T1-weighted MR images with contrast and exhibit a high signal on T2-weighted sequences. Special sequences, such as diffusion-weighted imaging (DWI), are helpful in confirming an epidermoid tumor.
The vascularity of tumors is demonstrated by tumor enhancement on CT with contrast and flow voids on MR imaging. Nasopharyngeal angiofibromas classically enlarge the pterygopalatine fossa and appear as an enhancing lesion with contrast on both MR imaging and CT. Angiography is used to confirm the diagnosis of highly vascular tumors (angiofibroma, paraganglioma) and for the preoperative embolization of feeding vessels.
Preoperative imaging (CT angiography and MR imaging) also provides information regarding the intracranial circulation and collateral cerebral blood flow (patency of the circle of Willis). If sacrifice of the ICA or another major vessel is anticipated or planned, a preoperative balloon test occlusion with neuromonitoring is performed. Additional measures of cerebral blood flow, including perfusion CT and perfusion MR imaging, provide further objective assessment.
Positron emission tomography (PET) shows cellular activity in the body, primarily through the detection of labeled glucose taken up by the tissues under examination. This may be combined with CT (PET-CT) to give a better anatomical definition. Although this technique eventually may be useful for the identification of primary tumors, it is most appropriate for the detection of metastasis and local recurrence of high-grade malignancies.
44.5.3 Operative Management
The choice of a surgical approach depends on the diagnosis, surgical goals, surgical access, reconstructive options, patient comorbidities, experience of the surgical team, potential complications, and available resources. Goals for surgery are outlined in the box “▶ Surgical Goals in the Pediatric Patient.” The optimal approach is one that provides adequate access and superior visualization and minimizes potential morbidity, especially that associated with manipulation of the neural and vascular structures, including retraction of the brain.
Surgical Goals in the Pediatric Patient
Diagnostic biopsy
Curative surgery
Palliation, including pain management
Salvage surgery, including after radiotherapy, chemotherapy, and chemoradiotherapy
Tumor debulking, for decompression before chemoradiotherapy
Skull base surgery is team surgery and requires the close coordination of team members. Both CT and MR imaging are used for intraoperative navigation, which aids anatomical localization during the surgery, including identification of important neural and vascular structures, determination of tumor margins, and assessment of the extent of resection. Intraoperative imaging (intraoperative CT or MR imaging) is sometimes performed to assess the extent of tumor resection, detect complications (including hemorrhage), or visualize residual tumor by updating the navigation scan following the shift associated with tumor resection.
Neurophysiologic monitoring of cortical function with somatosensory evoked potentials (SSEPs) provides an overall assessment of cerebral perfusion, which can be adversely affected by hypotension or subdural collections (air or fluid).12 Brainstem evoked response is used to monitor brainstem function during surgeries of the posterior fossa and electromyography to monitor the motor function of cranial nerves.
44.5.4 Surgical Approaches
Approaches to the Anterior Cranial Base
Surgical approaches to the cranial base can be classified based on the anatomical region (▶ Table 44.5). External approaches are classified by cranial fossa, whereas endonasal approaches are classified as surgical modules in the sagittal and coronal planes. In open approaches, craniofacial osteotomies provide access to the cranial base and help minimize brain retraction.
| Approach type | Anterior cranial base | Middle cranial base | Posterior cranial base |
| Open | Craniofacial resection Subfrontal or subcranial craniotomy | Lateral infratemporal skull base approach | Retrosigmoid craniotomy Far lateral cervical approach |
| Endoscopic | Endonasal resection of anterior cranial base Endonasal transplanum, transcribriform approach | Endonasal suprapetrous approach | Endonasal transclival and transodontoid approach Endonasal infrapetrous approach |
Anterior Cranial Base: Craniofacial Resection
Historically, the craniofacial approach pioneered by Ketcham and others has been the standard surgical option for the treatment of anterior cranial base pathology. This consists of a transcranial approach combined with a transfacial approach. A bicoronal incision is made over the vertex of the scalp from ear to ear. Laterally, the incision may be extended inferiorly in the preauricular skin crease to increase exposure. The posterior scalp flap can be elevated in a subgaleal plane to expose extra pericranium if needed for reconstruction. The anterior scalp is elevated from the underlying cranium with separation of the periosteum from the deep temporal fascia of the temporalis muscle at its margin. Laterally, the superficial layer of the deep temporal fascia is incised several centimeters above the zygomatic arch, and the interfascial fat pad is elevated with the scalp to avoid injury to the temporal branches of the facial nerve.
At the level of the superior orbital rims, the supratrochlear and supraorbital neurovascular bundles are carefully dissected free from their respective foramina to preserve the blood supply of a pericranial flap; small osteotomies may be necessary if the foramina are complete. Periosteum is elevated from the orbital roofs, glabella, and nasal bones, and the scalp is retracted inferiorly.
A bifrontal craniotomy is performed that encompasses the anterior and posterior tables of the frontal sinus. In the traditional anterior craniofacial resection craniotomy, the inferior osteotomy is placed just above the prominence of the brow. To minimize brain retraction, the supraorbital bar, including the superior orbital rims and glabella, can be removed in a single unit (subfrontal approach). Removal of the supraorbital bar requires elevation of the frontal dura from the orbital roofs. A reciprocating saw is used to transect the orbital rims at the lateral margin of the craniotomy. The orbital contents are protected while the orbital roof is transected with a drill and the bone is drilled anterior to the crista galli. For the subfrontal approach, a final transverse bone cut at the nasion transects the frontal recess and frees the bone segment.
The dura is separated from the crista galli and incised anterior to the cribriform plate and laterally along the medial margin of the orbit. The olfactory bulbs and tracts are dissected free from the frontal lobes, and the olfactory tracts and dura are incised posteriorly over the planum. The bone margins are then drilled to communicate with the sinuses, staying anterior to the optic canals.
Frequently, the craniofacial resection combines transfacial approaches with the bifrontal craniotomy. The aim of the transfacial approaches is to provide an adequate field for the dissection and resection of the lesion from the nasal cavity, paranasal sinuses, and orbit. Options include a lateral rhinotomy, midfacial degloving, and an endoscopic endonasal approach. In a lateral rhinotomy, a skin incision is started at the midpoint between the nasal dorsum and the medial canthus. The incision respects facial subunits and extends along the lateral surface of the nose to the nasal alar, then curves around the nostril to the nasal sill.13,14 The incision communicates with the nasal cavity along the piriform aperture. Additional exposure can be obtained by extending the incision with a subciliary or transconjunctival incision superiorly (Weber-Ferguson incision), or by incising the upper lip along the lateral philtrum. After exposure of the facial skeleton through these incisions, osteotomies are performed according to the location and size of the tumor. In most situations, except when subcutaneous tissue and skin are involved, in which case skin incision is required, the exposure afforded by these transfacial incisions can be easily obtained by other means (midfacial degloving or endoscopic endonasal approaches). Thus, lateral rhinotomy is rarely used.
The midfacial degloving approach avoids a facial incision and provides better bilateral exposure. A mucosal incision is made in the gingivolabial sulcus, and periosteum is elevated from the anterior maxilla. The incision communicates with the nasal cavity along the piriform aperture, and the nasal soft tissues are elevated from the anterior edge of the nasal septum following a full transfixion incision. Bilateral transmaxillary antrostomies and medial maxillectomies provide additional exposure. The midfacial degloving approach may be supplemented with endoscopy to provide better visualization.
After the appropriate transfacial or endoscopic approach, the tumor is removed en bloc, if possible, by dissecting around the periphery of the neoplasm with opening of the sinuses and transection of the nasal septum. The cranial base specimen is then mobilized through the bone cuts in the cranial base and delivered transcranially.
Reconstruction of the resultant defect is necessary to provide separation of the cranial and nasal cavities and prevent CSF leak, meningitis, and pneumocephalus. The dural defect is repaired primarily. Suitable materials include synthetic dural substitutes, fascia lata, temporalis fascia, pericranial graft, and cadaveric pericardium. An inferiorly based pericranial flap is then reflected posteriorly to cover the entire defect in the anterior cranial base. Rigid reconstruction with a bone graft or alloplastic material is not necessary. It is important to place the flap inferior to the replaced supraorbital bar, with a small gap left to prevent compression of the vascular pedicle.
Anterior Cranial Base: Endoscopic Endonasal Resection
Endoscopic endonasal techniques can be used for the resection of sinonasal malignancies. The intranasal portion of the tumor is first debulked to provide visualization of the margins and assess the extent of the tumor. Uninvolved sinuses are opened to allow visualization of the medial orbits, nasofrontal recesses, and sphenoid sinus. Bony landmarks (optic canals, carotid canals) are identified and the margins of resection are defined. In most cases, this includes the posterior wall of the frontal sinus, the medial walls of the orbit, the roof of the sphenoid sinus, and the nasal septum. The nasal septum is transected inferior to the area of tumor involvement from the frontal sinus to the rostrum of the sphenoid bone. Margins from the nasal septum mucosa are sent for frozen section analysis. If the septal mucosa is not involved by the tumor, a septal flap can be harvested for later reconstruction.
After harvesting of the septal flap, bilateral frontal sinusotomies are performed with removal of the floor bilaterally (Draf III procedure). The bone of the medial orbit is removed on the side of greatest tumor involvement. The tumor is devascularized by sacrifice of the anterior and posterior ethmoid arteries bilaterally. The arteries are identified at the junction of the orbit and skull base and are cauterized with bipolar electrocautery or ligated with hemoclips.
The bone of the anterior cranial base is thinned with a drill to facilitate the elevation and removal of bone, allowing full exposure of the dura and the area of dural invasion. Bone removal extends from the crista galli to the planum sphenoidale and to the medial orbits bilaterally. The dura is then cauterized and incised lateral to the tumor. Cortical blood vessels are identified and carefully freed from the dura. The falx is cauterized and transected anteriorly to allow mobilization of the dural specimen. The olfactory bulbs are dissected from the surface of the brain and remain attached to the dural specimen. If there is a focal area of brain invasion, the surrounding cortical tissue is removed by careful and limited suction dissection to achieve clear margins. The olfactory nerves are then transected posteriorly, and the final posterior dural incision is made to free the specimen. Additional dural margins may be excised for frozen section analysis before reconstruction.
After complete resection of the anterior cranial base, an inlay collagen or fascial graft is placed and the septal flap is positioned to cover the defect. If a septal flap is not available because of tumor involvement or insufficient dimension, a modified version of the extracranial pericranial flap is used.15 A bicoronal scalp incision is made, and the scalp is elevated to the level of the nasal bones. The bone at the level of the nasion is removed with a drill to create a window large enough to transmit the pericranial flap (approximately 1 × 2 cm). The flap is then transposed through the defect inferior to the frontal sinusotomy and positioned over the dural defect, with a drainage pathway for the frontal sinuses maintained on one side.
Approaches to the Middle Cranial Base
Open Approach: Lateral Infratemporal Skull Base Approach
The middle cranial fossa is typically accessed with a lateral transcranial approach. A bicoronal scalp incision is extended laterally in a preauricular skin crease to the inferior margin of the tragus. If transcervical exposure is required, it may be continued into the neck (parotidectomy incision). The scalp is elevated superficial to the deep temporal fascia to the level of the zygomatic arch.
The lateral approaches to the middle cranial base include the transpetrosal approaches, preauricular infratemporal approach, and frontotemporal approach. The frontotemporal (pterional) craniotomy serves as the standard approach to the middle cranial fossa. This approach can be extended with orbitozygomatic osteotomies, depending on the location of the lesion. Orbitozygomatic osteotomies incorporate the superior and lateral parts of the orbit and the zygoma to the standard pterional bone flap, enhancing the low exposure and improving access to the anterior cranial base and parasellar region. Osteotomies are placed across the superior orbital rim, body of the zygoma, and posterior attachment of the zygomatic arch. If exposure of the petrous ICA is necessary, the glenoid fossa can be included with the bone segment. With retraction of the temporalis muscle, a subtemporal craniectomy provides access to the foramen ovale, eustachian tube, and petrous segment of the ICA. This approach also can reach the sphenoid sinus, infratemporal fossa, and pterygopalatine fossa. Bony reconstruction consists of plating of the bone segments and augmentation with titanium mesh. The temporalis muscle covers the craniotomy site, and if dura or petrous ICA is exposed, temporalis muscle can be transposed to protect these tissues.16
Endoscopic Endonasal Approach: Middle Cranial Base
The endoscopic endonasal approaches permit access to different areas of the middle cranial base: the sella turcica, cavernous sinus, Meckel cave, petrous apex, and infratemporal fossa. Wide exposure of the sphenoid sinus is a common step in each endoscopic endonasal approach to the middle cranial base. Key anatomical structures (optic nerves, internal carotid arteries, cranial nerve V2, and vidian nerve) are identified within the sphenoid sinus and then followed to other areas of the skull base.
Independently of the area approached in the middle cranial base, the endoscopic endonasal exposure is initiated by lateralization of the inferior and middle turbinates to increase the space for the insertion and manipulation of instruments. The right middle turbinate may be resected to improve visualization of the surgical field. The contralateral middle turbinate is lateralized and the nasoseptal flap is harvested for reconstruction of the cranial base defect at the end of the procedure. The flap can be stored in the nasopharynx or in the ipsilateral maxillary sinus during the operation. A partial posterior nasal septectomy in addition to wide bilateral sphenoidotomies and posterior ethmoidectomies completes the nasal corridor. This corridor provides access to the sellar region and to the cavernous sinus.
The corridor can be expanded laterally by adding a transpterygoid approach to reach the lateral recess of the sphenoid sinus, Meckel cave, and petrous apex. The transpterygoid approach begins with opening of the maxillary sinus and removal of the orbital process of the palatine bone and posterior wall of the maxillary sinus, exposing the pterygopalatine fossa periosteum. Terminal branches of the internal maxillary artery (sphenopalatine, posterior septal, palatovaginal, vidian, and descending palatine arteries) are sacrificed, and the pterygopalatine contents are lateralized to expose the base of the pterygoid plates and the vidian artery in the vidian (pterygoid) canal. The descending palatine artery and the greater palatine nerve are preserved in the greater palatine canal at the inferomedial margin of the dissection. Once the vidian canal is identified, drilling can then proceed along its inferior and medial aspect. The vidian nerve dissection orients the surgeon toward the lateral portion of the foramen lacerum and the most superficial portion of the petrous ICA.
The transpterygoid approach can be extended to the pterygoid plates. In such cases, the corridor created provides access to the eustachian tube and the infrapetrous region. The infratemporal fossa is approached by removing the posterior wall of the maxillary sinus. This approach provides access to the pterygoid venous plexus, masticator muscles, and cranial nerve V3 branches.
Approaches to the Posterior Cranial Base
Open Approach: Retrosigmoid Craniotomy
A postauricular incision is made and a subperiosteal flap is elevated. The incision may be extended anteriorly into the upper cervical region for control of the proximal ICA, isolation of the lower cranial nerves, and exposure of the condyle. The upper cervical musculature (sternocleidomastoid and trapezius muscles) is detached from the mastoid and suboccipital region, and a craniotomy is performed posterior to the mastoid and sigmoid sinus. A mastoidectomy with removal of the tip provides greater access to the jugular bulb and foramen.
If a large tumor involves the temporal bone and complete access to the extracranial ICA is needed, a Fisch type C approach provides wide exposure of the middle and posterior cranial fossae. The incision extends from the temporal area to the upper cervical region. A conchal bowl incision through the skin and cartilage allows elevation of the auricle with the skin flap; the stump of the external auditory canal remains. The facial nerve can be transposed for additional exposure or remain in situ. A transtemporal approach is then performed with dissection of the ICA to the carotid canal.
The surgical defect can be reconstructed with temporalis muscle transposition or a posteriorly based pericranial flap (occipital artery). The craniotomy site is covered with titanium mesh or a plate, and the cervical musculature is reattached.
Endonasal Transclival and Transodontoid Approach
The endonasal approach provides optimal access to the clival region from the posterior clinoids to the foramen magnum. A sphenoidotomy is performed, and the mucosa of the nasopharynx and underlying musculature is resected from the floor of the sphenoid to the ring of the C1 vertebra and between the nasopharyngeal eustachian tube orifices. It is important to localize the parapharyngeal ICA with image guidance to avoid injury with dissection or electrocautery. The clivus may be anatomically considered in thirds. The upper third includes the posterior clinoids to the sella floor. Exposure of the posterior clinoids often requires pituitary gland transposition for intradural dissection. The sella is opened widely, and the lateral attachments of the pituitary gland are lysed with possible sacrifice of the inferior hypophysial arteries on one or both sides. If the posterior planum is removed, the gland can be displaced into the suprasellar space with preservation of the pituitary stalk and the superior hypophysial vessels. The middle clivus extends from the floor of the sella to the floor of the sphenoid sinus. Bone in this area is bounded by the parapharyngeal ICAs, and during drilling the sixth cranial nerve is susceptible to injury posterolateral to the vessels in the Dorello canal.17 The inferior clivus extends to the foramen magnum. Intense venous bleeding from the clival plexus is often encountered and can be controlled with application of the hemostatic materials of choice, including Surgifoam (Johnson & Johnson, New Brunswick, NJ) and FloSeal (Baxter, Deerfield, IL). The sixth cranial nerve exits the brainstem at the level of the vertebrobasilar junction and is susceptible to injury with opening of the dura (▶ Fig. 44.4).
If exposure of the upper cervical spine is necessary (basilar invagination, foramen magnum tumor), the anterior ring of C1 is exposed and removed. The odontoid and upper body of C2 can be drilled, and the ligamentous attachments are resected. Laterally, dissection is limited by the vertebral arteries.
Partial-thickness dural defects posterior to the clivus can be simply covered with fibrin glue. Septal mucosal flaps are usually inadequate in size, and reach for large and deep clival or odontoid dural defects and supplementation with fat grafts may be necessary. Pericranial flaps can be used to cover these defects if a vascularized flap is needed.
Endonasal Infrapetrous Approach
The infrapetrous approach is defined by the course of the petrous and parapharyngeal ICA. A lower transclival approach is combined with a transpterygoid approach, and the location of the petrous ICA is defined by using the vidian nerve as a landmark. Bone inferomedial to the pterygoid canal is carefully drilled, and the dense fibrocartilage of the foramen lacerum is exposed. The medial eustachian tube is resected, and the fibrocartilage is transected inferior to the foramen lacerum. This provides access to the inferior aspect of the petrous bone. Lateral dissection is limited by cranial nerve V3 and the parapharyngeal ICA.
At the level of the foramen magnum, removal of bone laterally exposes the hypoglossal canal and nerve. The bone superior to the hypoglossal nerve is the jugular tubercle and is bounded laterally by the jugular bulb. The occipital condyle is inferior to the hypoglossal nerve; excessive removal of this bone on both sides can destabilize the craniocervical junction.18
Endoscopic Endonasal Approaches in the Pediatric Population
Specific Considerations
With improvements in instrumentation and the training of skull base teams, the endoscopic endonasal approach to pediatric sinonasal and skull base lesions has become a reality19–21 (▶ Table 44.6). Although the principles of surgery are the same for adult and pediatric patients, pediatric patients may present unique challenges based on the indications for surgery, anatomical access, and skull base reconstruction. It is important to have a skull base team with adequate expertise and experience. This may include both adult and pediatric otolaryngologists and neurosurgeons.
| Disease classification | Pathology |
| Nonneoplastic | Choanal atresia Rathke cleft cyst Encephalocele Meningocele Cerebrospinal fluid leak Arteriovenous fistula Aneurysmal bone cyst |
| Neoplasm, benign | Pituitary adenoma Juvenile nasopharyngeal angiofibroma Glioma Epidermoid Olfactory groove schwannoma Teratoma Neurofibroma Ossifying fibroma Osteoma Langerhans cell histiocytosis Hemangioma Leiomyoma |
| Neoplasm, malignant | Chordoma Craniopharyngioma Germinoma Neuroendocrine tumor Rhabdomyosarcoma Lymphoma |
Indications
Applications of endonasal approaches in the pediatric population include biopsy for diagnosis, definitive treatment of benign neoplasms (including pituitary adenoma, angiofibroma, and craniopharyngioma), and downstaging of high-grade malignancies, such as rhabdomyosarcoma. The lesser morbidity and greater acceptance of an endonasal approach lower the threshold for surgical biopsy and provide greater flexibility for the treatment of benign tumors with a propensity for recurrence (craniopharyngiomas). For some benign lesions, there is a role for “wait and watch” until the child is older and more suitable for surgery.
Anatomical Access
Although similar surgical principles apply to adult and pediatric patients, access is limited in smaller patients, and the surgical approach must take into account the potential disruption of growth centers. Despite smaller nares and nasal cavities, most patients older than 4 years have sufficient room for standard endoscopic surgery. If not, a transoral or sublabial approach, such as midfacial degloving in combination with endoscopy, can be used to access the nasal cavity. Incomplete sinus pneumatization in the pediatric population can make the identification of normal anatomical structures more difficult. Cosmetic issues are a concern for both young patients and their parents, and reconstruction options are more limited because of incomplete facial growth and cosmesis. Disparate rates of development between the cranium and facial skeleton decrease the surface area of the nasoseptal flap relative to the anterior cranial base up to approximately 14 years of age.6
Blood Loss
The small size of pediatric patients is associated with smaller blood volumes. Hemostasis needs to be meticulous, and intraoperative blood loss is a particular challenge in patients with vascular tumors, bleeding disorders, or a religious aversion to blood products. Strategies to minimize blood loss include preoperative embolization, intraoperative ligation of feeding vessels, and the use of hemostatic materials and instruments. The staging of surgeries is often necessary in such patients to limit the intraoperative blood loss to less than one blood volume at each operation.
Malignant Tumors
Oncologic principles are the same in the pediatric population. The goals of surgery are complete resection of the tumor with the preservation of critical neurovascular structures. The most common malignancies in the pediatric population are sarcomas, and the role of surgery is limited to biopsy and the palliation of symptoms in most cases.
Craniofacial Disruption
A significant concern in pediatric skull base and craniofacial surgery is to avoid disruption of the permanent dentition within the maxillary complex during maxillotomy.23 Permanent tooth eruption generally occurs after 10 years of age. The Le Fort I down-fracture approach is best avoided, and a presurgical dental panoramic X-ray or coronal CT scan is helpful to plan osteotomies to avoid injury to the tooth buds. With respect to open approaches, midfacial degloving and transmaxillary approaches afford wide exposure and the resection of sinonasal tumors with preservation of normal tooth eruption.
The possibility of skull and craniofacial osteotomies having an impact on the growing head and face is of major importance. A review of patients undergoing craniofacial resection, the youngest of whom was a 2-year-old child, showed normal craniofacial development up to 36 months postoperatively.23 Longitudinal studies assessing the impact of various external and endonasal approaches on craniofacial growth are needed.
44.6 Specific Skull Base Pathology and Surgical Considerations
44.6.1 Midline Frontonasal Masses
Although they are rare, nasal dermoids, epidermoids, nasal gliomas, and meningoencephaloceles comprise the vast majority of these lesions. Of these, nasal dermoids are the most common, accounting for approximately 61% of congenital midline nasal masses.2 The main clinical entities in the differential diagnosis include nasal glioma, encephalocele, and rarely hemangioma, lymphangioma, fibroma, lipoma, lipoblastoma, hairy teratoid polyp, dacrocystocele, and dacrocystitis. A nasal glioma is heterotopic, mature glial tissue (astrocytes and connective tissue) without a true capsule, found within or around the nose. A fibrous stalk representing the intracranial connection can be found in 15% of cases. Nasal gliomas occur as an extranasal mass along the nasal dorsum in 60%, as an intranasal mass (lateral nasal wall, middle turbinate, nasal septum) in 30%, and in both locations in about 10% of patients.
Surgical resection is the treatment of nasal dermoids and is discussed elsewhere in this text. Although there is very little published literature on endoscopic techniques for the treatment of nasal dermoids, extensive endoscopic experience with lesions in the same area suggests that the same techniques can be applied. Initial experience, including our own, is supportive of endoscopic endonasal surgery for nasal dermoids with intracranial extension.2
Encephaloceles are defects in the skull and dura with herniation of intracranial contents. The most common etiology in children is a developmental anomaly causing the skull defect, and brain tissue (meningoencephalocele) is almost always present. Although rare, congenital meningoencephaloceles are more likely to contain critical vessels or specific neural structures, including the pituitary gland. Acquired defects are most commonly posttraumatic. The surgical treatment of these lesions is covered in detail elsewhere in the text.
44.6.2 Sellar and Parasellar Lesions
The differential diagnosis of pathology in this area includes numerous entities: cystic lesions (Rathke cleft cyst, arachnoid cyst), benign tumors (pituitary adenoma, craniopharyngioma, meningioma, hemangioma, ependymoma), inflammatory and metabolic lesions (hypophysitis, pituitary hyperplasia, fibrous dysplasia, sphenoid sinus mucocele, amyloidosis), and malignancy (pituitary carcinoma, germinoma, chordoma, chondrosarcoma, sinonasal malignancy, osteogenic sarcoma, fibrosarcoma, and metastasis).
Common pathology in this region includes Rathke cleft cysts, pituitary adenomas, and craniopharyngiomas. These lesions have a common embryologic development from primitive stomodeum (Rathke pouch and duct); however, they differ in pathology and cell of origin (see section on developmental anatomy). The Rathke cleft cyst is a nonneoplastic lesion, whereas pituitary adenomas and craniopharyngiomas are benign neoplasms.
Rathke Cleft Cysts
Rathke cleft cysts can occur in the pediatric population, and it is imperative to distinguish these from cystic pituitary adenomas and craniopharyngiomas. The CT and MR findings are often variable and nonspecific, and it can be difficult to distinguish these lesions. A small, nonenhancing intracystic nodule is considered pathognomonic.36 Most incidentally discovered Rathke cleft cysts are treated conservatively, and treatment is required only in patients who develop clinical symptoms (headache) or demonstrate progressive enlargement. The authors’ preferred treatment strategy is via an endoscopic transsphenoidal transsellar/infrasellar approach to fenestrate the cyst and exteriorize the cyst wall. Maximal pituitary gland is preserved by fenestrating the floor of the cyst. Removal of the cyst lining is not necessary and risks a CSF leak.
Pituitary Adenomas
Pituitary adenomas are rare in children and account for 3% of supratentorial masses. They are more likely to be functioning adenomas, in which the most common histology is prolactinoma, followed by corticotropinoma and somatotropinoma.37 Nonfunctioning adenomas, thyroid-stimulating hormone (TSH)–secreting adenomas, and gonadotropin-secreting adenomas constitute 3 to 6% of childhood pituitary adenomas.
Surgery is indicated for almost all histologic types of adenomas with the exception of prolactinoma, which can be successfully managed medically. The preoperative evaluation should include an endocrine work-up, visual field evaluation, and MR imaging with contrast. The goal of surgery is total resection with normalization of the endocrinopathy. An endoscopic endonasal transsellar approach to the pituitary gland achieves complete tumor removal in up to 100% of cases38 with minimum morbidity compared with traditional microscopic transsphenoidal approaches.38,39 Radiation is avoided in children.
Craniopharyngiomas
Craniopharyngioma is the most common benign pediatric tumor involving the sellar and parasellar region. It comprises up to 50% of sellar tumors and is of epithelial origin. The treatment challenges posed by these tumors are covered separately in the text. The endonasal approach offers a direct, anterior midline surgical pathway to the site of these tumors. Preinfundibular tumors may be removed via a suprasellar, transplanum approach with dissection in a caudal–cranial direction to the supracistern space. Transinfundibular tumors in the subchiasmatic space can be managed via a transsellar approach, and retroinfundibular tumors via a transsellar, pituitary transposition approach with dissection in an anteroposterior angle to the interpeduncular cistern.44 However, a significant concern with pituitary transposition is pituitary dysfunction.45 In addition, the endonasal approach alone is not suitable for large tumors with extensions beyond the interpeduncular cisterns, and these are best managed with a combined endonasal and open (pterional) or completely open approach.
44.6.3 Fibrous Dysplasia
Fibrous dysplasia is a nonneoplastic condition that results in the intramedullary accumulation of fibrous tissue and immature woven bone in a single (monostotic) or multiple (polyostotic) bones. It is due to an arrest of bone stromal cell differentiation, causing immature cells to proliferate and produce the masses of fibro-osseous tissue characteristic of fibrous dysplasia. McCune-Albright syndrome is a separate condition with several phenotypes and encompasses polyostotic bone involvement, endocrinopathy, and skin discoloration. The natural histories of these conditions are different. Craniofacial involvement occurs almost invariably with the polyostotic form, and the ethmoid bone is most commonly involved, followed by the sphenoid and frontal bones and the parietal, temporal, and occipital bones. Most of the common clinical problems are due to craniofacial asymmetry, pain, and cranial nerve compression. Secondary lesions, depending on the location of fibrous dysplasia, may also be symptomatic: mucoceles, cystic fibrous dysplasia, hemorrhage, and aneurysmal bone cysts.
In most cases, the diagnosis can be confirmed radiographically.46 CT demonstrates an expansile lesion with a fairly homogeneous appearance. There may be hypodense areas or cysts within the lesion. Despite a large mass, the bony growth respects cortical bone and neural foramina; the calvaria is expanded outward, and narrowing of foramina is rare. MR imaging demonstrates low-to-intermediate signal intensity on T1-weighted images and low signal intensity on T2-weighted images, although cartilaginous areas will appear very bright. A common scenario is a child who has MR imaging for unrelated reasons and the doctors are alarmed by a large skull base mass. In such cases, CT is helpful. Biopsy is not necessary to establish a diagnosis if the imaging is consistent with fibrous dysplasia.
Treatment goals depend on several factors. The natural history is an important consideration; it is now evident that fibrous dysplasia can occur after adolescence and progress into adulthood. Polyostotic disease has a higher rate of progression. The rate of malignant transformation (fibrosarcoma, osteosarcoma) is reported to be 0.4%, especially with radiation exposure.46 Generally, treatment is reserved for symptomatic patients. Clinical and radiologic follow-up is sufficient, and surgery is considered when disfigurement, cranial neuropathies (including visual loss resulting from optic nerve compression), and pain occur. Although medical treatments with bisphosphonates have been used, the response rate is mixed because of selection bias, and long-term data are lacking. Corticosteroid treatment is reserved for patients with acute visual symptoms before surgery. Headaches do not typically resolve with surgery, and medical therapy should be pursued first.
Surgical intervention is the mainstay of symptomatic fibrous dysplasia. Overall, reports indicate a lower recurrence rate with the complete removal of fibrous dysplasia; however, the extent of surgery depends on the predicted morbidity due to surgery. A useful algorithm for deciding on the extent of surgery, proposed by Chen et al, classifies patients into four major groups according to the area affected: zone 1—fronto-orbital, zygomatic, upper maxillary; zone 2—hair-bearing cranium; zone 3—central cranial base; zone 4—teeth-bearing regions of the maxilla and mandible.47 Total resection is suggested for zone 1 and partial resection for zones 2 through 4. Most sinonasal lesions can be completely removed with endoscopic sinus surgery, and skull base lesions are treated with either endoscopic or open approaches (depending on the location and extent of the lesion).
The optic canal is involved in up to 90% of sphenoid bone lesions. Available data indicate that asymptomatic individuals with optic nerve involvement are best managed conservatively, as up to 97% patients had stable vision without surgery.46 Surgery carries its own risk for visual deterioration; 87% of asymptomatic patients who underwent prophylactic decompression and 65% of symptomatic patients who underwent surgery had stable long-term vision. If needed, optic nerve decompression can be performed safely with an endoscopic endonasal approach, and partial decompression is often sufficient. Any surgical intervention in patients with fibrous dysplasia is difficult because of the loss of normal anatomical landmarks and risk for neurovascular injury. Surgery should be undertaken by an experienced team with neurophysiologic monitoring and radiologic image guidance.
44.6.4 Paranasal Sinus Mucoceles
A paranasal sinus mucocele is an epithelium-lined sinus cavity filled with mucus secretions that results from obstructed sinus outflow. Its ability to expand by resorption and remodeling of the adjacent bone distinguishes it from a blocked sinus filled with trapped mucus. As it expands, it can erode into the adjacent orbit and skull base. It is rare in children, and predisposing factors include trauma, acute and chronic sinusitis, nasal polyps, cystic fibrosis (with nasal polyps), fibrous dysplasia, and iatrogenic causes (postoperative).49 It is important to exclude underlying benign and malignant nasal and sinus lesions, including meningocele, neuroblastoma, lymphoma, and rhabdomyosarcoma. Mucoceles in children are often unilateral and affect the frontoethmoid region, followed by the maxillary sinus, sphenoid sinus, and posterior ethmoid sinus.
The preferred treatment option is intranasal endoscopic surgery and drainage. The wall of the mucocele is removed widely with preservation of the lining mucosa. Recurrence is uncommon and can be further managed endoscopically. Complex cases may have to be managed with a combination of open and endoscopic approaches.
44.6.5 Juvenile Nasopharyngeal Angiofibroma
Juvenile nasopharyngeal angiofibroma (JNA) is a highly vascular, benign lesion found almost exclusively in males between the ages of 12 and 21 years. JNAs have extensive vascular and fibrous stromal components, and the cells of origin of these components are still debated. Recent evidence indicates that JNA is likely to be a vascular malformation or a hamartoma, derived from incomplete regression of the artery of the first branchial arch, rather than a true neoplasm.50 JNA is more prevalent in patients with familial adenomatous polyposis. A hormonal pathogenesis is suggested; however, this remains controversial.
Anatomically, the site of origin lies in an area superior to the sphenopalatine foramen, which some authors consider to be in the pterygopalatine fossa at the level of the vidian canal aperture. The tumor is locally aggressive and from its origin enlarges in a submucosal plane into adjacent anatomical sites and involves the basisphenoid. From the pterygopalatine fossa, it may grow medially (nasal cavity, nasopharynx, and opposite side), anteriorly (toward the maxilla), anterosuperiorly through the inferior orbital fissure (orbital apex), laterally (pterygopalatine fossa to the infratemporal fossa), or posteriorly (root of the pterygoid canal). Intracranial extension is seen in 10 to 20% of patients, and this may occur through direct bone erosion or extension along the pterygoid canal, foramen rotundum, or superior orbital fissure. True dural invasion and intradural disease are rare.
Nasal obstruction and recurrent epistaxis are common presenting symptoms, and in advanced disease, facial deformity can be appreciated. Surgery is the treatment of choice, and CT and MR imaging are useful to delineate the extent of disease for surgical planning. Tumor vascularity poses a significant surgical challenge, and the degree of intraoperative blood loss may influence how much tumor can be resected at any one time. Blood supply is mainly from the internal maxillary artery branches, which can be embolized successfully; however, many intermediate and large tumors derive a significant blood supply from the ICA (vidian artery and cavernous branches), which precludes effective embolization. Residual vascularity from the ICA is correlated with increased intraoperative blood loss, difficult surgical resection, and the need for multiple surgeries to achieve complete resection. A new staging system based on residual vascular supply has been proposed that provides better prognostication and facilitates preoperative planning.51
Surgical resection has traditionally been performed via transfacial (lateral rhinotomy, Weber-Ferguson), midface degloving incisions, and, for intracranial components, lateral (pterional craniotomy) approaches. In the past decade, endoscopic transnasal ± transmaxillary (via a Caldwell-Luc) approaches have been used to achieve complete resection of extensive, multicompartmental, and intracranial lesions. An anterior endoscopic endonasal or endoscopically assisted approach provides access to all routes of tumor extension, both medial and lateral to the cavernous ICA. Incomplete resection of areas of skull base erosion or intracranial extension can be observed for regrowth. Most small residual tumors do not progress and involute over time. In our opinion, there is no role for radiation therapy. Regrowth of residual tumor can be treated endoscopically in most cases. Currently, many authors agree that the vast majority of JNAs, including those with intracranial involvement and significant lateral extension, can be surgically removed without an open approach. ▶ Fig. 44.5 shows a large JNA with intracranial extension that was resected in two stages, including a combined endoscopic and open approach.
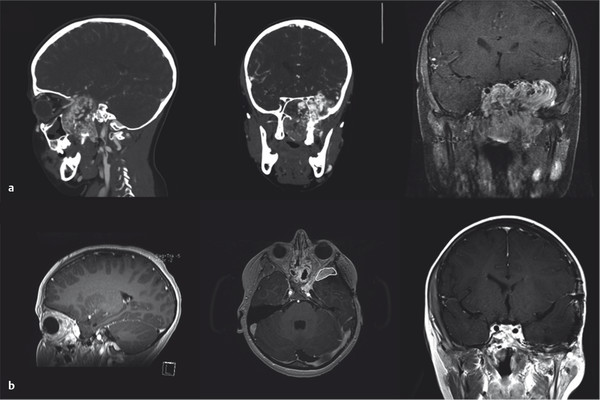
Fig. 44.5 (a) Coronal and sagittal reconstructions from pre-embolization CT angiography and coronal postcontrast T1-weighted imaging of a juvenile nasopharyngeal angiofibroma with intracranial extension that was resected in three stages: a combined pterional and endoscopic endonasal approach and two endoscopic endonasal approaches. (b) Sagittal, axial, and coronal postcontrast T1-weighted images 1 year after resection. On the axial image, a small residual superolateral to the orbit is circled in white. This has remained stable.









