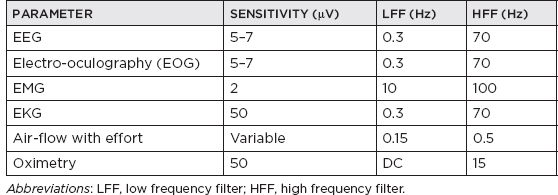
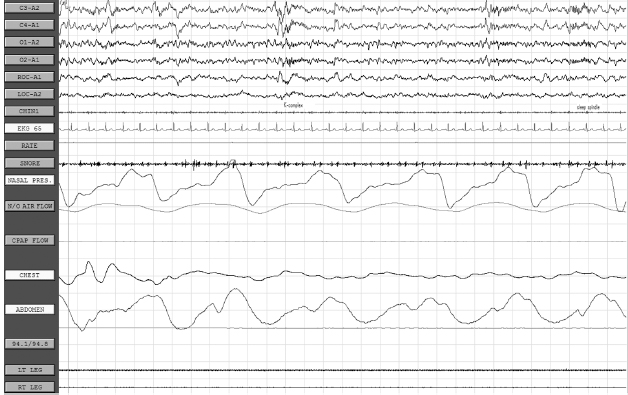
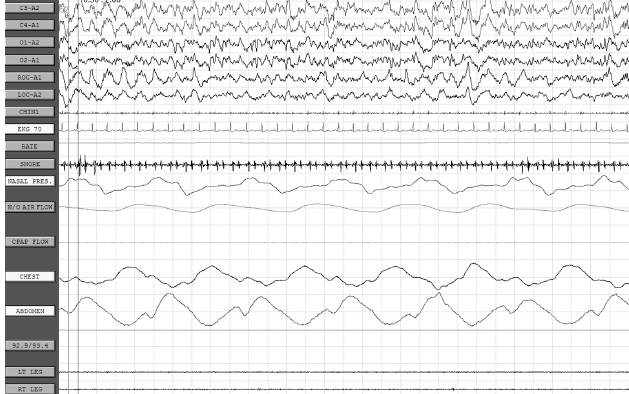
CHAPTER 20 Sleep Neurology I. Polysomnography (PSG) A. Recording 1. To visually stage sleep adequately, the basic monitoring must be at least: a. Central and occipital referential (usually to an earlobe or mastoid) electroencephalogram (EEG) linkages b. An oculogram for rapid eye movement (REM) c. Submental electromyogram (EMG) for axial muscle tone 2. May also place additional EEG electrodes for superficial EMG of upper and lower extremities for evaluation of restless legs syndrome; intercostal EMG for respiratory status; upper airway exchange (thermistors or thermocouplers); monitors of important chest or abdominal patterns, arterial blood gases, or O2 saturation; EKG; and nocturnal penile tumescence 3. Standard PSG recording parameters B. EEG monitoring 1. Colloid provides better contact for long-term monitoring. 2. Resistance/impedance should be kept <5,000 Ω. 3. Basic 10 to 20 international electrode placement. 4. Only C3 and C4 are used to record sleep in adults (with referential to ear); in infants, O1 and O2 are frequently added. 5. A single central channel (either C3-A1 or C4-A2) is necessary to stage sleep, but six or more channels are recommended, including a combo of any of the following electrodes: FP1, FP2, C3, C4, O1, O2, T3, T4; O1 and O2 provide analysis of prominent waking rhythms, whereas central channels are best for V waves, spindles, and/or K complexes. 6. Electrodes to measure eye movement (EOG) are placed at the outer canthus of both eyes; there is a small electrical dipole of the eye with the cornea positive in relation to the retina. 7. EMG from chin also recorded 8. Standard paper speed is 10 seconds with 30-second epochs, which results in “compression” of cerebral activity. C. EOG 1. It is commonly recommended to have the referential electrode recording from the lateral canthus to the ipsilateral (IL) ear (provides out-of-phase recording for horizontal eye movements); the disadvantage is marked artifact, especially during slow-wave sleep (SWS) when the EEG reaches max amplitude; this also applies to the referential supra- and infraorbital electrodes for evaluation of vertical eye movements. 2. REMs usually last approximately 50 to 200 milliseconds and have a frequency of >1 Hz. 3. Slow rolling eye movements usually have a frequency between 0.25 and 0.50 Hz, with the duration of the sharpest slope >0.5 seconds. D. EMG monitoring 1. The first electrode is placed submentally on the skin over the mylohyoid muscle, and the second electrode is usually placed 3 cm posterior and lateral (in case first electrode is defective). 2. Tonic EMG activity usually decreases from stage 1 through stage 4 non-REM (NREM) sleep and is absent in REM. 3. A limb EMG is used to evaluate periodic leg movements of sleep (PLMS)/restless legs syndrome, and electrodes are placed over the anterior tibialis muscle (identified by having the patient dorsiflex against resistance); a bipolar derivation is obtained by recording from one electrode on each leg. 4. An intercostal EMG may assist in respiratory monitoring. E. Respiratory monitoring 1. Useful in determining between central, obstructive, and mixed apnea 2. By definition, apnea is a lack of upper airway exchange that must last >10 seconds with >4% oxygen desaturation. 3. All measures of upper airway airflow and of chest/abdominal movement use a band-pass of DC to 0.5 Hz. 4. Monitoring of oxygen saturation/oxygen tension and systemic pulmonary artery or other pressure may also be done, if indicated. 5. Upper airway breathing: a. Thermistor i. Thermistor resistor fluctuations are induced by temperature changes in air passing in and out of the mouth/nostrils. ii. Useful only for evaluation of respiratory rate b. Thermocouple i. Thermoelectric generators constructed of dissimilar metals (e.g., constantan and copper) ii. Generate a potential in response to temperature change iii. Usually, two thermocouplers are attached to the nostrils. c. Capnography: uses carbon dioxide monitor to document CO2 retention d. Pneumotachography i. Only technique that allows direct quantification of ventilation during sleep ii. Can measure flow rate, tidal volume, and other respiratory variables iii. Disadvantage: uses uncomfortable airtight mask and is therefore rarely used 6. Thoracoabdominal movement: a. Strain gauge i. Most consist of a silicone tube filled with a conductor (e.g., mercury or packed graphite), the resistance of which varies with core diameter. ii. Inspiration: stretches tube → decreases the core diameter → increases resistance (vice versa for expiration) iii. Piezoelectric crystals of quartz or sapphire strain gauges: distortion by inspiration or expiration creates a current; these are more sensitive to movement artifact. b. Inductive plethysmography i. It is essentially an improved method of spirometry that separates chest and abdominal movement and adds them together, thus mimicking total spirometric volume. ii. The sensors are two wire coils (one placed around the chest and the other around the abdomen). iii. A change in mean cross-sectional coil area produces a proportional variation in coil conductance, which is converted into a voltage change by a variable-frequency oscillator. iv. Three output channels: rib cage movement, abdominal movement, and total volume c. Impedance plethysmography: rarely used method involving changes in impedance based on abdominal/chest movement 7. Snoring monitors: a. Snoring suggests reduced upper airway diameter and/or hypotonia. b. Bursts of loud guttural inspiratory snorts after quiescent periods are characteristic of obstructive sleep apnea syndrome. 8. Arterial oxygen: transcutaneous oxygen tension for measurement of desaturation with respiratory distress events. F. EKG 1. Obstructive sleep apnea syndrome patients often may have sinus arrhythmias or extra asystoles, and may have more serious disorders, such as prolonged asystole, atrial fibrillation, or ventricular fibrillation. G. Esophageal pH: patients may have insomnia due to esophageal reflux from a hiatal hernia or other conditions. H. Penile tumescence 1. Strain gauges are placed at the tip and base. 2. Buckling resistance (rigidity) is measured by a technician during the maximal penile circumference during an erection by applying a force gauge to the tip of the penis; force is gradually increased until the penis buckles (or a force of 1,000 g is reached); buckling pressure of >500 g is considered normal (because this has been determined to be the minimal force required to achieve penetration during intercourse). I. Technical parameters 1. Must be performed under conditions conducive to natural sleep 2. A nocturnal sleeper must be tested at night (note that a shift worker must be tested during the period of his or her longest sleep time); for a nocturnal sleeper, daytime testing is not acceptable because there is a circadian distribution of REM and SWS, with REM sleep peaking between 3 a.m. and 6 a.m. and SWS peaking between 11 p.m. and 2 a.m. 3. Must avoid prior sleep deprivation (alters arousal threshold) and pharmacologic medications for sleep J. Sleep staging 1. Basic sleep staging a. Most labs use the guidelines set by Rechtschaffen and Kales in 1968. b. Usually done at a paper speed of 10 mm per second c. Sleep is divided into epochs of 60, 30, or 20 seconds; each epoch is scored as the stage that occupies >50% of the epoch. d. A minimum of 6 to 8 recording hours is recommended. e. Sleep architecture is commonly altered in patients undergoing initial PSG owing to unusual environment/conditions (“first-night effect”); findings associated with the first-night effect include prolonged sleep latency and REM latency, reduction in sleep efficiency, increased unexplained arousals and awakenings, and reduced or absent stage 3/stage 4 and REM sleep (often accompanied by an increase in stage 1 sleep). 2. Sleep parameters and scoring Stage 1 May be subdivided into stage 1A (α rhythm diffuses to anterior head regions, often slows by 0.5–1.0 Hz, and then fragments before disappearing) and stage 1B (when EEG contains <20% diffuse slow α [>40 µV] and the EEG consists of medium-amplitude mixed-frequency [mostly θ] activity with occasional vertex waves); scored when >50% of epoch consists of relatively low-voltage mixed frequencies (mainly 2–7 Hz) with relative reduction in EMG activity; other features include slow rolling eye movements and vertex waves (vertex waves may persist into stage 2 and SWS; diphasic sharp transients having initial surface negativity followed by a low-voltage positive phase that is maximum at C3 and/or C4 and with phase reversal over the midline; present by 8 wks postterm) Stage 2 Characterized by ≥1 sleep spindle, K complexes, and <20% of the epoch containing Δ; spindles are 11.5- to 15.0-Hz central bursts that must last >0.5 secs and have an amplitude >15 µV to be scored, and appear as rhythmic sinusoidal waves of progressively increasing amplitude followed by progressively decreasing amplitude; K complexes are diphasic waves that must contain two of three features (negative vertex sharp wave maximal over central regions, a following negative slow wave maximally frontal, and/or a sleep spindle maximal centrally); K complexes usually occur in trains either spontaneously or after a stimulus; K complexes may appear in infants as early as 5 mos old; may also have vertex waves (see Figure 20.1) Stage 3 Scored when 20%–50% of epoch contains Δ waves of 0.5–2.5 Hz and >75 µV; sleep spindles may be present but are less frequent than in stage 2 and of lower frequency (10–12 Hz) Stage 4 Scored when >50% of epoch contains Δ of <2 Hz and amplitude >75 µV; spindles may be present but are rare (note: stages 3 and 4 are collectively also known as SWS; predominates in the first third of night) (see Figure 20.2) REM Contains medium-amplitude, mixed-frequency (mainly θ and Δ), low-voltage activity associated with REM and relative absence of EMG; bursts of sawtooth waves at 2–6 Hz may appear in frontal or midline regions (generally just before REM bursts); initial REM period may contain some low-voltage spindles, but generally sleep spindles and K complexes are absent; may also demonstrate α frequencies at rate 1–2 Hz slower than patient’s waking background rhythm; also may have autonomic instability; although there is relative muscle atonia, bursts of phasic EMG activity may be noted in conjunction with REM; REM stage predominates in the last third of night (see Figure 20.3) Sleep latency Time from lights out to the first epoch of sleep (in minutes) REM latency Time from sleep onset to the first epoch of REM sleep (in minutes); significantly reduced in certain sleep disorders, sleep deprivation, drug withdrawal; REM latency is usually 60–120 mins Time in bed Total time in bed; from time of lights out to lights on Total sleep Total time from sleep onset to final awakening (note: some authors do not include stage 1 sleep) Sleep Percentage of time spent in bed asleep (i.e., total sleep time/time efficiency in bed) Arousals Defined as an abrupt shift in EEG frequency, including θ, α, and/or frequencies >16 Hz (but not spindles) that meet the following criteria: (1) at least 10 secs of sleep of any stage must precede an arousal and must be present between arousals; (2) at least 3 secs of EEG frequency shift must be present; (3) arousals in REM also necessitate a concurrent increase in chin EMG amplitude, because bursts of θ and α are found intrinsically during REM sleep; (4) arousals are not scored based on chin EMG alone; (5) artifacts, K complexes, and △ are not scored as arousals unless accompanied by EEG frequency shift of >3 secs; (6) pen-blocking artifact is only considered an arousal when contiguous with an arousal pattern and then may be included toward the 3-sec duration criteria; (7) nonconcurrent but contiguous EEG and EMG changes that are <3 secs but together are >3 secs are not scored as arousals; (8) intrusion of α in NREM sleep is scored as arousal only if >3 secs in duration and preceded by >10 secs of α-free sleep; and (9) transitions between sleep stages are not scored as arousals unless they meet the previous criteria; arousals are scored using EEG alone with the exception of REM sleep arousals that also require simultaneous increase in chin EMG amplitude 3. Sleep onset and sleep cycles a. Sleep onset i. No definitive parameters signifying sleep onset ii. Three basic PSG assessments (A) EMG: gradual diminution but without discrete change (B) EOG: slow asynchronous rolling eye movement (C) EEG: change from normal background α to low-voltage mixed-frequency pattern (stage 1 sleep), which usually occurs within seconds to minutes of rolling eye movements; patients, if aroused during stage 1 sleep, typically state they were awake, and therefore sleep onset recognized by EEG is taken at stage 2 (presence of K complexes and sleep spindles) Figure 20.1 Stage 2 sleep. Figure 20.2 Stage 4 slow-wave sleep. Figure 20.3 REM sleep. b. The first sleep cycle i. Stage 1: in normal adults, the first sleep cycle begins with stage 1 NREM sleep, lasting only a few minutes (1–7 minutes on average). ii. Stage 2: follows stage 1 and usually lasts 10–25 minutes; progressive increase in frequency of SWS is appreciated, denoting evolution to stage 3. iii. Stage 3: SWS (20–50% of EEG) that usually persists normally for a few minutes and evolves into stage 4 iv. Stage 4: SWS (>50% of EEG) with higher voltage; lasts 20 to 45 minutes during first cycle; if body movements occur, there is transient return to lighter sleep (stages 1 or 2); often there is transition from stage 4 to stage 2 sleep just before the patient enters REM sleep. v. REM: the transition from NREM to REM is not abrupt; REM sleep cannot be identified until the first REM; the REM period during the first cycle is short (between 2 and 6 minutes); REM sleep often ends with a brief body movement, and a new cycle begins. vi. The first NREM–REM cycle usually lasts approximately 70 to 100 minutes. c. Later sleep cycles i. The average length for later sleep cycles is 100 to 120 minutes; the last sleep cycle is usually the longest. ii. As the night progresses, REM sleep generally becomes longer; stages 3 and 4 occupy less time in the second cycle and may nearly disappear in later cycles, with stage 2 expanding to make up the majority of NREM sleep. d. Dissociated or otherwise atypical sleep patterns i. α-Δ Sleep (A) Characterized by presence of α and Δ waves in stages 3 and 4 SWS (B) May be induced in healthy individuals without awakening them by using auditory stimuli (C) Associated with a number of nonrestorative sleep disorders, especially fibromyalgia ii. REM-spindle sleep (A) Due to breakdown of barriers between NREM and REM sleep (B) May occur in up to 8% of normal patients (C) Increases in a number of sleep disorders, including increased frequency in the daytime sleep of hypersomniacs and the nocturnal sleep of schizophrenics and narcoleptics iii. REM sleep without atonia (A) Common in patients taking tricyclic antidepressants, monoamine oxidase inhibitors, and phenothiazines (B) Disorders include REM behavior disorder. iv. REM burst during NREM sleep: exhibited by patients being treated with clomipramine (depression, narcolepsy), which is a medication that suppresses REM-based activity v. Isolated REM atonia (A) Cataplexy represents the selective triggering, during wakefulness and by emotional stimuli, of REM sleep atonia. (B) Sleep paralysis is the isolated appearance of REM sleep atonia associated with full wakefulness either before entry into REM sleep or during awakenings from REM sleep. vi. Sleep-onset REM periods (A) The sleep-onset REM period is usually defined as entry into REM sleep within 10 minutes of sleep onset. (B) Its presence is highly suggestive of diagnosis of narcolepsy-cataplexy and characterizes approximately 50% of onsets of night sleep in these patients (but sleep deprivation, alcoholism, drug withdrawal, irregular sleep-waking habits, and/or severe depression must be ruled out). 4. Respiratory parameters and scoring a. Apneas and hypopneas represent decrements in airflow that may or may not be associated with arousals and/or oxygen desaturation. b. Apnea: cessation or >90% reduction of nasal/oral airflow with >4% oxygen desaturation c. Brief central apneas that occur during transitional periods from wakefulness to sleep are believed to have no clinical significance. d. Hypopnea: i. No consensus agreement of what constitutes a hypopnea ii. Defined as >50% reduction of airflow lasting >10 seconds and also reductions of airflow between 30% and 50%, which are associated with arousal or desaturation of at least 4% e. Hypopneas and apneas have the same clinical significance as apneas (i.e., apneas and hypopneas are combined to give a total number divided by number of hours of sleep = apnea-hypopnea index or respiratory distress index). 5. Leg movement (LM) parameters and scoring a. LMs can be periodic (PLMS) or aperiodic/random (after arousals, respiratory events, snoring, etc.). b. An LM is defined as a burst of anterior tibialis muscle activity with a duration from onset to resolution of 0.5 to 5.0 seconds, and an amplitude of >25% of the bursts recorded during calibration. i. LMs do not include hypnic jerks that occur on transition from wake to sleep, aperiodic activity during REM, phasic EMG during REM sleep, other forms of myoclonus, or restless legs. c. When scoring LMs, must document whether there are arousals, awakenings, or respiratory events; arousals attributed to LMs should occur no >3 seconds after termination of LM. d. PLMS: i. Characterized by rhythmic extension of the great toe and dorsiflexion of the ankle with occasional flexion of the knee and hip (similar to triple flexor response). ii. Lasting from 0.5 to 5.0 seconds iii. Occur at intervals of 20 to 40 seconds iv. Occur intermittently in clusters lasting minutes to hours throughout the night v. Typically, the total number of LMs is reported with a breakdown of the number associated with arousals or awakenings and respiratory events. vi. PLMS arousal index of >5 is important in middle-aged adults (but a cutoff of 10–15 should be used in elderly patients). vii. If associated with arousals, patient may present with hypersomnia/excessive daytime sleepiness. viii. Patients also commonly will have restless legs syndrome (may be associated with anemia due to iron deficiency, renal failure, and a variety of neurologic disorders). ix. A PLMS sequence or epoch is a sequence of four or more LMs separated by at least 5 seconds and not by >90 seconds (measured from LM onset to LM offset). x. PLMS are more abundant in stages 1 and 2 and less frequent in stages 3 and 4 and REM sleep. II. The Epworth Sleepiness Scale (ESS) The ESS: self-administered questionnaire with 8 items; it provides an index of the general level of daytime sleepiness, or the average sleep propensity in daily life. The ESS requests respondents to rate, on a 4-point scale (0–3), their usual chances of dozing off or falling asleep in eight different situations or activities that most people engage in as part of their daily lives, although not necessarily every day. Interpretation: 0 to 7: It is unlikely that you are abnormally sleepy. 8 to 9: You have an average amount of daytime sleepiness. 10 to 15: You may be excessively sleepy depending on the situation. You may want to consider seeking medical attention. 16 to 24: You are excessively sleepy and should consider seeking medical attention. III. Multiple Sleep Latency Test (MSLT) and Maintenance of Wakefulness Test A. MSLT 1. Use a. Developed by Carskadon and Dement (1977) and first tested on excessive daytime sleepiness patients by Richardson (1978) b. Used to evaluate i. Excessive daytime sleepiness (by quantifying the time required to fall asleep) ii. REM latency (to evaluate specific disorders, e.g., narcolepsy) c. Must perform urine toxicology screen for narcotics, psychotropics, stimulants, hypnotics, and so forth 2. General procedures a. Standard montage using the Rechtschaffen and Kales (1968) guidelines b. Monitored for five 20-minute nap periods with 2 hours between each period; the first is standard setup for between 9:30 a.m. and 10:00 a.m.; first nap is performed at least 90 minutes after wake-up time. c. MSLT is usually performed on the night after PSG so that sleep disorders that might artifactually produce short daytime sleep latencies are ruled out (important note: nocturnal PSG will negate the usefulness of MSLT); patient must have at least 360 minutes of sleep on night before MSLT. d. General considerations for MSLT i. 2 weeks of sleep diaries preceding MSLT ii. PSG on night before MSLT to evaluate habitual sleep and quantitate possible sleep-confounding deprivation before MSLT iii. Consideration of drug schedule (both prescribed and illicit drugs) with stable regimen for at least 2 weeks before testing (especially benzodiazepines, barbiturates, etc.) iv. Minimum of four tests at 2-hour intervals beginning 1.5 to 3.0 hours after waking v. Quiet, dark, controlled-temperature room vi. No alcohol or caffeine for at least 2 weeks before MSLT 3. Scoring a. Sleep onset is defined by any of the following parameters: i. The first three consecutive epochs of stage 1 NREM sleep ii. A single epoch of stage 2, 3, or 4 NREM sleep iii. REM sleep b. Sleep offset is defined as two consecutive epochs of wakefulness after sleep onset c. A nap is terminated after one of the following: i. No sleep has occurred after 20 minutes ii. After 10 minutes of continuous sleep as long as sleep criteria are met (if sleep onset is at 20 minutes, the sleep is allowed to continue until 30 minutes, etc.) iii. After 20 minutes or any point thereafter if the patient is awake d. Sleep latency is measured from the time of lights out to first sleep epoch; usually an average sleep latency of four or five naps is calculated. e. REM latency: time of sleep onset to first epoch of REM sleep 4. Interpretation of MSLT a. Normal sleep latency on MSLT AGE SLEEP LATENCY Young adult (21–35 y/o) 10 mins Middle-aged adult (30–49 y/o) 11–12 mins Older adults (50–59 y/o) 9 mins b. Decreased REM-onset latencies during MSLTs can occur with: i. Sleep pathology (e.g., narcolepsy, severe obstructive sleep apnea) ii. Sleep deprivation c. MSLT interpretation of sleep-onset latency SEVERITY OF SLEEPINESS SLEEP LATENCY ON MSLT CLINICAL CORRELATION Severe <5 mins Presence of pathologic or significant sleepiness; sleep episodes are present daily during times that require moderate attentiveness, such as eating, driving, and so forth, resulting in impairment of normal daily function Moderate 5–10 mins Excessively sleepy; sleep episodes occur daily during times that require moderate attentiveness, such as watching a movie/performance or attending a meeting Mild 10–15 mins Sleep episodes occur normally during times of relaxation, requiring little attentiveness, such as a passenger in a car or watching television d. Eighty-five percent of narcoleptics have a mean sleep latency of <5 minutes. e. Patients with mild to moderate obstructive sleep apnea syndrome or sleep deprivation may have borderline sleep latency between 5 and 10 minutes. f. Sleep latency may be affected by several factors, including: i. Sleep deprivation: causing a shortened sleep-onset latency ii. Sleep–wake schedule: must assess during patients’ normal sleep schedule (e.g., shift worker) iii. Medications iv. Environment: environmental factors (e.g., noise) may cause prolonged sleep latency. B. Maintenance of wakefulness test 1. An alternative to MSLT 2. Requires subject to sit in dark room with eyes closed reclining at a 45-degree angle 3. Required to attempt to stay awake for 20 minutes 4. Three to four testing periods every 2 hours
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





