CHAPTER 14
Somatosensory Systems
Input from the somatosensory systems informs the organism about events impinging on it. Sensation can be divided into four types: superficial, deep, visceral, and special. Superficial sensation is concerned with touch, pain, temperature, and two-point discrimination. Deep sensation includes muscle and joint position sense (proprioception), deep muscle pain, and vibration sense. Visceral sensations are relayed by autonomic afferent fibers and include hunger, nausea, and visceral pain (see Chapter 20). The special senses—smell, vision, hearing, taste, and equilibrium—are conveyed by cranial nerves (see Chapters 8, 15, 16, and 17). In addition, nociceptive sensation or pain-signaling serves to warn the organism when there is contact with noxious or potentially damaging elements in the environment, or when tissue is damaged.
RECEPTORS
Receptors are specialized cells for detecting particular changes in the environment. Exteroceptors include receptors affected mainly by the external environment: Meissner’s corpuscles, Merkel’s corpuscles, and hair cells for touch; Krause’s end-bulbs for cold; Ruffini’s corpuscles for warmth; and free nerve endings for pain (Fig 14–1). Receptors are not absolutely specific for a given sensation; strong stimuli can cause various sensations, even pain, even though the inciting stimuli are not necessarily painful. Proprioceptors receive impulses mainly from pacinian corpuscles, joint receptors, muscle spindles, and Golgi tendon organs. Painful stimuli are detected at the free endings of nerve fibers.
Each efferent fiber from a receptor relays stimuli that originate in a receptive field and gives rise to a component of an afferent sensory system. Each individual receptor fires either completely or not at all when stimulated. The greater the intensity of a stimulus, the more end-organs that are stimulated, the higher the rate of discharge is, and the longer the duration of effect is. Adaptation denotes the diminution in rate of discharge of some receptors on repeated or continuous stimulation of constant intensity; the sensation of sitting in a chair or walking on even ground is suppressed.
CONNECTIONS
A chain of three long neurons and a number of interneurons conducts stimuli from the receptor or free ending to the somatosensory cortex (Figs 14–1 to 14–3).
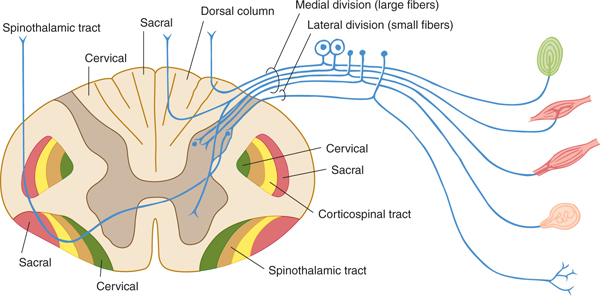
FIGURE 14–1 Schematic illustration of a spinal cord segment with its dorsal root, ganglion cells, and sensory organs.
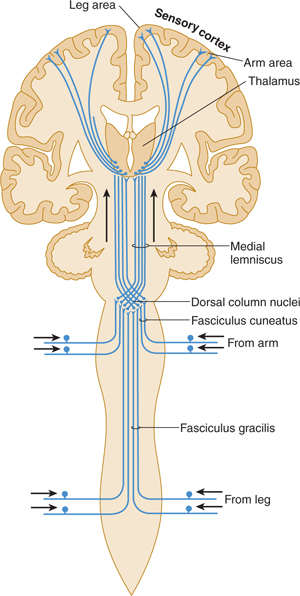
FIGURE 14–2 Dorsal column system for discriminative touch and position sense (lemniscus system).
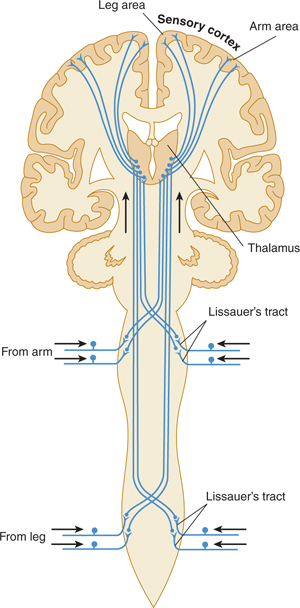
FIGURE 14–3 Spinothalamic tracts for pain and temperature (ventrolateral system).
First-Order Neuron
The cell body of a first-order neuron lies in a dorsal root ganglion or a somatic afferent ganglion (eg, trigeminal ganglion) of cranial nerves.
Second-Order Neuron
The cell body of a second-order neuron lies within the neuraxis (spinal cord or brain stem; examples are provided by the dorsal column nuclei, ie, the gracile and cuneate nuclei, and by neurons within the dorsal horn of the spinal cord). Axons of these cells usually decussate and terminate in the thalamus.
Third-Order Neuron
The cell body of a third-order neuron, which lies in the thalamus, projects rostrally to the sensory cortex. The networks of neurons within the cortex, in turn, process information relayed by this type of neuron; they interpret its location, quality, and intensity and make appropriate responses.
SENSORY PATHWAYS
Multiple neurons from the same type of receptor often form a bundle (tract), creating a sensory pathway. Sensory pathways ascending in the spinal cord are described in Chapter 5; their continuation within the brain stem is discussed in Chapter 7. The main sensory areas in the cortex are described in Chapter 10.
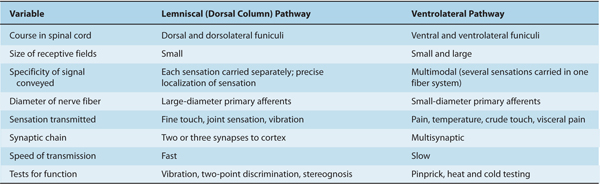
One major system—the lemniscal (dorsal column) system (see Fig 14–2)—carries touch, joint sensation, two-point discrimination, and vibratory sense from receptors to the cortex. The other important system—the ventrolateral system—relays impulses concerning nociceptive stimuli (pain, crude touch) or changes in skin temperature (see Fig 14–3). Significant anatomic and functional differences characterize these two pathways: the size of the receptive field, nerve fiber diameter, course in the spinal cord, and function (Table 14–1). Each system is characterized by somatotopic distribution, with convergence in the thalamus (ventroposterior complex) and cerebral cortex (the sensory projection areas; see Figs 10–13 and 10–15), where there is a map-like representation of the body surface. The sensory trigeminal fibers contribute to both the lemniscal and the ventrolateral systems and provide the input from the face and mucosal membranes (see Figs 7–8 and 8–12).
TABLE 14–1 Differences between Lemniscal and Ventrolateral Systems.
CORTICAL AREAS
The primary somatosensory cortex (areas 3, 1, and 2) is organized in functional somatotopic columns that represent points in the receptive field. Within each column are inputs from thalamic, commissural, and associational fibers, all of which end in layers IV, III, and II (see Fig 10–10). The output is from cells in layers V and VI; however, the details of the processing occurring in each column and its functional significance (how it is felt) are largely unknown.
Additional cortical areas—secondary projection areas—also receive input from receptive fields in the columns. The somatotopic maps in these areas are more diffuse, however.
PAIN
Pathways
The free nerve endings that emanate from peripheral and cranial nerves are receptors, or nociceptors, for pain (see Figs 14–1 and 14–3). Nociceptors are sensitive to mechanical, thermal, or chemical stimuli. (Polymodal nociceptors are sensitive to several of these types of stimuli.) The pain fibers in peripheral nerves are of small diameter and are readily affected by local anesthetic. The thinly myelinated A-delta fibers convey discrete, sharp, short-lasting pain. The unmyelinated C fibers transmit chronic, burning pain. These nociceptive axons arise from small neurons located within the dorsal root ganglia and trigeminal ganglia.
Cells within injured tissue may release inflammatory molecules such as prostaglandins or other neuroactive molecules (such as histamine, serotonin, and bradykinin, in the aggregate comprising an “inflammatory soup”), which lower the threshold of peripheral nociceptors and thereby increase the sensibility to pain (hyperalgesia). Aspirin and other nonsteroidal anti-inflammatory drugs inhibit the action of prostaglandins and act to relieve pain (hypalgesia or analgesia).
Pain Systems
The central projections of nociceptive primary sensory neurons impinge on second-order neurons within superficial layers of the dorsal horns of the spinal cord. According to the gate theory of pain, the strength of synaptic transmission at these junctions is decreased (probably by presynaptic inhibition) when large (non-pain-signaling) axons within the nerve are excited (the gate “closes”). Conversely, the strength of synaptic transmission along the pain-signaling pathway is increased when there is no large-fiber input.
CLINICAL CORRELATIONS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








