Spinal cord
The spinal cord and its associated spinal nerves are of immense functional importance. These structures act to:
▪ receive afferent fibres from sensory receptors of the trunk and limbs
▪ control movements of the trunk and limbs
The internal organisation of the cord permits many functions to operate in an automatic or reflex fashion. In addition, extensive connections with the brain, through ascending and descending nerve fibre tracts, both convey afferent information to higher centres and mediate their controlling influence over spinal mechanisms.
External features of the spinal cord
Topographical anatomy
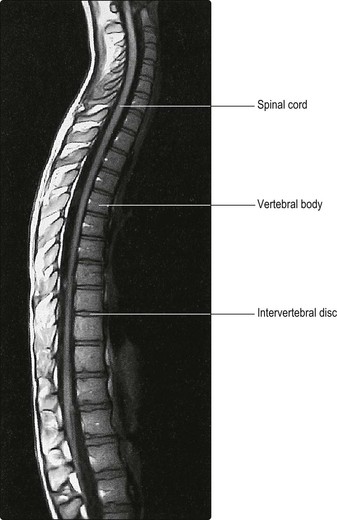
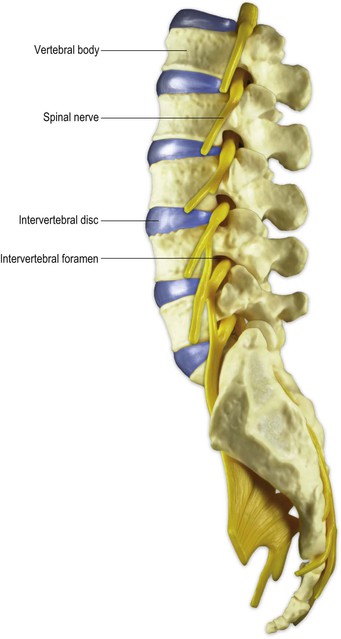
The spinal cord occupies the vertebral (or spinal) canal within the vertebral column, which provides support and protection (Figs 8.1, 8.2). Rostrally, the cord is continuous with the medulla oblongata of the brainstem.
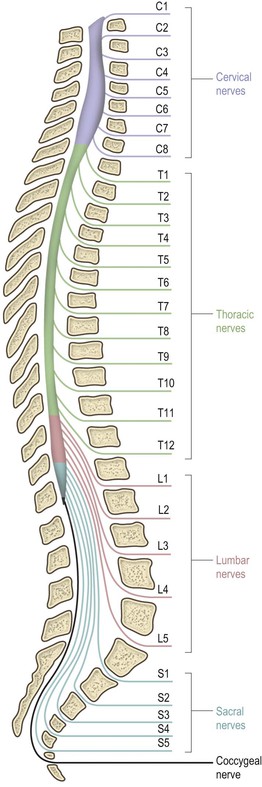
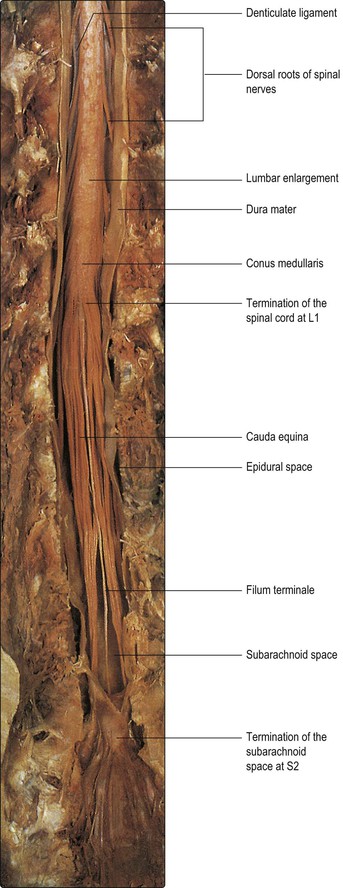
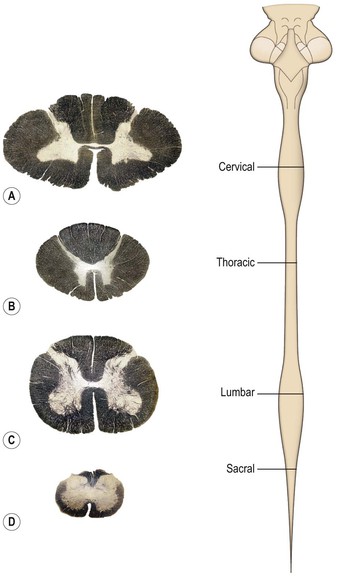
The spinal cord is essentially a segmental structure; from rostral to caudal, it consists of 8 cervical (C), 12 thoracic (T), 5 lumbar (L), 5 sacral (S) and 1 coccygeal segments (Fig. 8.3). The cord is approximately cylindrical in shape but its diameter varies considerably at different levels. It bears two enlargements, cervical and lumbar. The cervical enlargement consists of cord segments C4–T1 and provides innervation for the upper limb via the brachial plexus (see Fig. 3.11). The lumbar enlargement is made up of segments L1–S3 and is associated with innervation of the lower limb via the lumbosacral plexus (see Fig. 3.13). Caudal to the lumbar enlargement, the cord tapers abruptly to form a conical termination, the conus medullaris. From the tip of the conus, a strand of connective tissue, the filum terminale (Fig. 8.4), extends caudally and is attached to the dorsal surface of the first coccygeal vertebra.
Until the third month of fetal life, the spinal cord occupies the entire length of the vertebral canal. Thereafter, the rate of elongation of the vertebral column exceeds that of the spinal cord; as a result, at birth the cord terminates at the level of the third lumbar vertebra and in adult life, it terminates at about the level of the intervertebral disc between L1 and L2 (Figs 8.3, 8.4).
The approximate location of spinal cord segments relative to the bony vertebrae may be identified in the living adult subject by reference to the posterior spinous processes of the vertebrae. As a rule-of-thumb, cervical cord segments lie approximately one spine higher than their corresponding vertebrae (e.g. C7 cord segment lies adjacent to C6 vertebra), thoracic segments lie approximately two spines higher, and lumbar segments three to four spines higher than their corresponding vertebrae (Fig. 8.3).
Spinal nerves
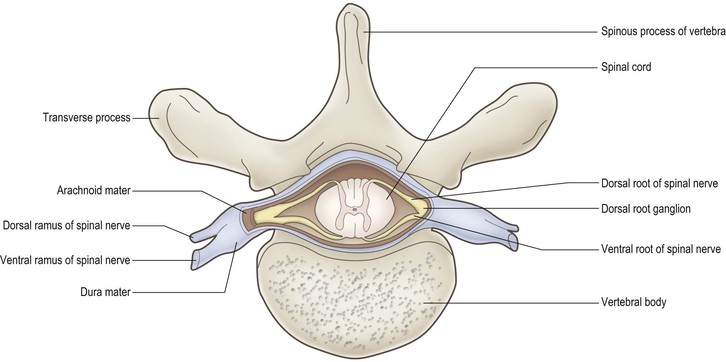
The spinal cord bears 31 bilaterally paired spinal nerves, each pair being associated with its corresponding cord segment. The spinal nerves originate as two linear series of nerve fascicles, or rootlets, attached to the dorsolateral and ventrolateral aspects of the cord (Fig. 8.5). Groups of six to eight contiguous fascicles that are attached to each cord segment coalesce to form dorsal and ventral nerve roots. The dorsal and ventral roots of each cord segment then pass to their corresponding intervertebral foramen (Fig. 8.6), in or near which they join to form the spinal nerve proper. While the spinal nerves are mixed nerves, containing both afferent and efferent neurones, the dorsal and ventral roots are functionally distinct. The dorsal roots contain primary afferent neurones, running from peripheral sensory receptors to the spinal cord and brainstem. The nerve cell bodies of these neurones are located in dorsal root ganglia (Fig. 8.5; see also Fig. 1.10), which appear as small enlargements on the dorsal roots near their convergence with the ventral roots at the entrance to the intervertebral foramina. The ventral roots of the spinal nerves carry efferent neurones, the cell bodies of which are located in the spinal grey matter. These are comprised of motor neurones, which innervate skeletal muscle, and preganglionic neurones of the autonomic nervous system.
The C1–C7 spinal nerves exit from the vertebral canal above the first seven cervical vertebrae; C8 spinal nerve exits below the seventh cervical vertebra and the remainder leave below their corresponding vertebrae (Fig. 8.3). Because of the different lengths of the spinal cord and the vertebral canal, only in the cervical region do the spinal cord segments lie adjacent to their corresponding vertebral bodies. Below this level, successive spinal nerve roots follow an increasingly oblique and long downwards course to reach their respective intervertebral foramina. This is most marked for the lumbar and sacral roots, which descend below the termination of the cord in a leash-like arrangement, the cauda equina (Figs 8.3, 8.4).
Immediately after leaving the intervertebral foramina, spinal nerves divide to produce a thin dorsal (posterior) ramus and a much larger ventral (anterior) ramus (Fig. 8.1). The dorsal ramus supplies the muscles and skin of the back region. The ventral ramus supplies the muscles and skin of the front of the body and also the limbs.
The peripheral distribution of spinal nerves, including their pattern of cutaneous innervation (dermatomes) and innervation of muscle groups (myotomes), is described in Chapter 3.
Spinal meninges
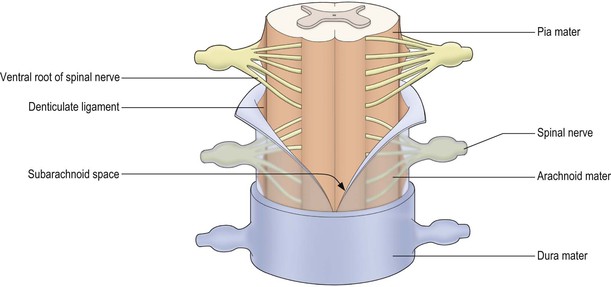
The spinal cord, like the brain, is invested by three concentric meningeal coverings: the pia mater, arachnoid mater and dura mater (see Figs. 1.17, 8.4, 8.5).
The innermost covering, the pia mater, is a delicate, vascular membrane that is closely applied to the surface of the cord and nerve roots. Along a line midway between the dorsal and ventral roots of the spinal nerves is attached a flat, membranous continuation of the pia, called the denticulate ligament. The ligament has a free lateral border for much of its length but, intermittently, lateral projections tether the spinal cord to the arachnoid, and through it to the dura (Figs 8.4, 8.5).
The arachnoid mater lies between the pia and dura. It is a translucent membrane that invests the cord like a loose-fitting bag. Between the pia and arachnoid lies the subarachnoid space. This contains CSF, which is produced in the cerebral ventricular system (Ch. 6).
The outer covering of the cord, the dura mater, is a tough, fibrous membrane. It envelops the cord loosely, as does the arachnoid with which it is in contact, though separated by a theoretical plane, the subdural space. The dura is separated from the bony wall of the vertebral canal by the epidural space.
Although the spinal cord terminates at vertebral level L1–L2, the arachnoid and dural sheaths and, therefore, the subarachnoid space continue caudally to S2. As the spinal nerve roots pass towards their intervertebral foramina they evaginate the arachnoid and dura, forming meningeal root sleeves that extend as far as the fusion of dorsal and ventral roots. Thereafter, the arachnoid and dura become continuous with the epineurium ensheathing the spinal nerve.
Internal structure of the spinal cord
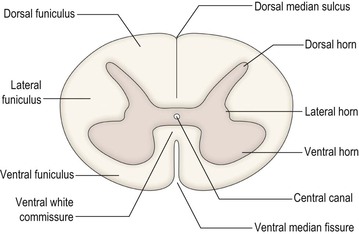
The spinal cord is incompletely divided into two symmetrical halves by a dorsal median sulcus and a ventral median fissure (Fig. 8.8). In the centre of the cord is the small central canal, which is continuous rostrally with the cerebral ventricular system. Surrounding the central canal is the spinal grey matter, consisting of nerve cell bodies, their dendrites and synaptic contacts. The outer part of the cord consists of white matter, which contains ascending and descending nerve fibres. Some of these serve to join neighbouring and distant cord segments for the integration of their functions, while others run between the cord and the brain. Many of the fibres that share a common origin, course and termination are grouped together in fascicles, forming the long tracts of the spinal cord.
Different cord levels vary in the relative amounts and configuration of grey and white matter (Fig. 8.9). Higher levels contain greater amounts of white matter. This is because ascending tracts gain fibres at each successive level, whereas the opposite is true of descending tracts.
Grey matter of the spinal cord
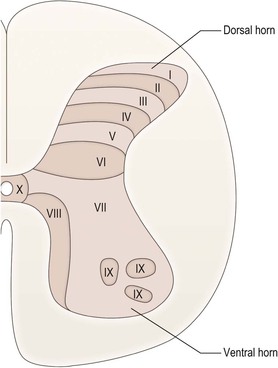
The grey matter is approximately H-shaped, or butterfly-shaped, with four protrusions, the dorsal (posterior) and ventral (anterior) horns, extending dorso- and ventrolaterally towards the attachment zones of the dorsal and ventral root fascicles, respectively. The size and shape of the dorsal and ventral horns varies according to the level (Fig. 8.9). Many afferent nerve fibres entering in the dorsal roots terminate in the dorsal horn, while the ventral horn contains the cell bodies of motor neurones that exit through the ventral nerve roots and innervate skeletal muscle. Both dorsal and ventral horns are, therefore, particularly well developed at cervical and lumbar levels, in association with innervation of the upper and lower limbs. Thoracic and upper lumbar segments additionally possess a small lateral or intermediolateral horn, located between the dorsal and ventral horns, that contains the cell bodies of preganglionic sympathetic neurones (Figs 8.8, 8.9B).
The grey matter of the spinal cord may be divided, on the basis of its cytoarchitecture, into ten zones, known as Rexed’s laminae, which are numbered sequentially from dorsal to ventral (Fig. 8.10). Some of these laminae are equated with cell groupings of particular functional types.
Dorsal horn
Afferent fibres entering through the dorsal roots divide into ascending and descending branches. They mostly terminate near their point of entry but may travel for varying distances in either direction, running in the dorsolateral fasciculus or Lissauer’s tract, which is located superficial to the tip of the dorsal horn (see Fig. 8.14). Dorsal root afferents may, therefore, establish synaptic contacts over several segments of spinal grey matter. Dorsal root fibres terminate extensively within the grey matter but most densely in the dorsal horn. Cutaneous afferents tend to terminate in superficial (dorsal) laminae, while proprioceptive and muscle afferents project mostly to deeper laminae.
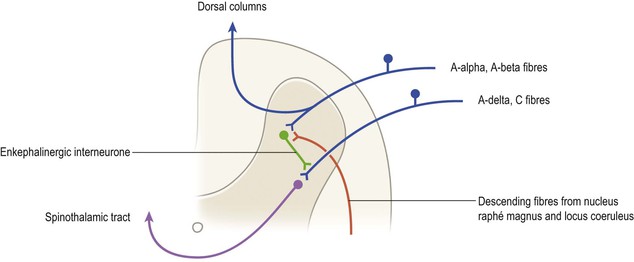
The tip of the dorsal horn, approximating to Rexed’s laminae I–III, is also known as the substantia gelatinosa. This region receives collaterals of the smallest-diameter myelinated (group A delta) and unmyelinated (group C) afferents that are associated with nociception. These neurones are excitatory and use glutamic acid and the peptide substance P as neurotransmitters. In the substantia gelatinosa, complex interactions occur with other types of afferent terminal, interneurones, and with descending pathways from the brain, which control the transmission of pain information to ascending spinothalamic and spinoreticular tract neurones distributed throughout the dorsal horn (Fig. 8.11). These mechanisms are often referred to as the ‘gate control theory’ of pain. For example, input from large-diameter afferents (groups A alpha and A beta) carrying tactile information inhibits transmission of nociceptive impulses to ascending tract neurones, which is thought to explain why rubbing a sore spot can relieve pain. The basic mechanism underlying this phenomenon is that the large-diameter afferents excite dorsal horn interneurones which use the endogenous opioid peptide encephalin as a neurotransmitter. These interneurones in turn establish presynaptic contacts with the terminals of nociceptive primary afferent neurones. By the process of presynaptic inhibition, they decrease the release of transmitter from these afferent terminals and, thus, decrease the excitation of ascending tract neurones. Certain descending pathways from the brain can also profoundly influence the transmission of nociceptive information at this level. Thus, descending serotonergic fibres originating from the nucleus raphé magnus of the medulla and noradrenergic fibres from the locus coeruleus of the upper pons can excite the enkephalinergic dorsal horn interneurones, thereby inhibiting the onward transmission of nociceptive input. The action of endogenous encephalin is via opiate receptors located on the terminals of nociceptive primary afferents. The analgesic properties of opiates such as morphine are partly a result of their action at this site.
Deeper in the dorsal horn, lamina VII contains a number of important cell groups. At cord levels C8–L3 lie the cells of Clarke’s column (thoracic nucleus, nucleus dorsalis), which are the origin of ascending fibres of the dorsal spinocerebellar tract. The cells of Clarke’s column receive afferent input from muscle spindles, Golgi tendon organs, tactile and pressure receptors. At thoracic and upper lumbar levels, the lateral part of lamina VII also contains preganglionic sympathetic neurones that constitute the lateral horn, while at sacral levels (S2–S4), it contains preganglionic parasympathetic neurones.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree