Spinal Extradural Neoplasms and Intradural Extramedullary Neoplasms
Central nervous system (CNS) tumors are responsible for 24% of cancer-related pediatric fatalities, and 5 to 10% of these tumors arise in the spinal axis.1–3 The ratio of intraspinal tumors to intracranial tumors is smaller in the pediatric than in the adult population, with estimates ranging from 1:5 to 1:20.4–9 Spinal neoplasms may be primary lesions arising within the spinal axis, secondary lesions resulting from extraspinal contiguous spread, or rarely, metastatic lesions.4 Neoplasms are categorized according to the space they occupy: extradural, intradural extramedullary, or intramedullary.1,2,4
This chapter features a discussion of the epidemiology, pathology, presentation, and treatment for pediatric extradural and intradural extramedullary neoplasms.
45.1 Epidemiology
Whereas several series show an equal distribution of spinal lesions in boys and girls, others report a predominance in boys.5–13 An increased rate among boys may relate to the inclusion of congenital lesions, which predominate in boys.6 Up to 12% of spinal tumors have been reported to arise in the first year of life; these tumors include lipomas, teratomas, and neurenteric cysts.4 Neuroblastomas commonly extend to the epidural compartment and cause a mass effect in early childhood, resulting in 12 to 30% of cases of neoplastic cord compression in this age group. Intradural extramedullary spread may occur at any age, although the metastasis of intracranial lesions like medulloblastoma, ependymoma, choroid plexus carcinoma, and retinoblastoma usually occurs within the first decade.4 Excluding congenital lesions, tumor occurrence is evenly distributed throughout the first 15 years of life.14
▶ Table 45.1 summarizes the various types of neoplasms, both intramedullary and extramedullary, found in the spinal canals of 649 pediatric patients in 10 large series published between 1953 and 1990.5–8,10–12,15–17 ▶ Table 45.2 reviews the anatomical distribution of intraspinal tumors in 413 pediatric patients, with the predominance of tumors arising in the thoracic and lumbar region.68The statistics reported in these large series must be examined carefully, as they often include developmental abnormalities that are not true neoplasms.
| Locationa | Tumor type | No. of patients | Total (%) |
| Intramedullary | 189 (29.1) | ||
| Astrocytoma | 114 | ||
| Ependymoma | 50 | ||
| Lipoma | 25 | ||
| Intradural extramedullary | 156 (24.0) | ||
| Dermoid | 39 | ||
| Neurofibroma | 28 | ||
| Schwannoma | 20 | ||
| Meningioma | 17 | ||
| Epidermoid | 14 | ||
| Primitive neuroectodermal tumor | 30 | ||
| Hemangioepithelioma | 8 | ||
| Extradural | 233 (35.9) | ||
| Sarcoma | 67 | ||
| Neuroblastoma | 64 | ||
| Teratoma | 35 | ||
| Metastasis | 29 | ||
| Ganglioglioma | 19 | ||
| Lymphoma | 19 | ||
| Others | 71 (10.9) | ||
| Source: Adapted from references5–8,10–12,15–17 . aSome of the teratomas, neurofibromas, and dermoid tumors arose from both intradural extramedullary and extradural compartments. | |||
▶ Table 45.2 shows the distribution of tumors, both intramedullary and extramedullary, along the spine. Tumors are distributed fairly evenly throughout the cervical, thoracic, and lumbar spinal regions. The thoracic spine and lumbar spine are the sites of 20.5% and 23%, respectively, of pediatric spinal tumors. This distribution is in contrast to the thoracic predominance of spinal neoplasms seen in adults.4 Sacral tumors are less common, and many of the tumors reported to arise in the lumbosacral region may be masses of developmental origin and not true neoplasms.
| Spinal Level | No. of patients | Percentage (%) |
| Cervical | 80 | 19 |
| Cervicothoracic | 23 | 6 |
| Thoracic | 109 | 26 |
| Thoracolumbar | 48 | 12 |
| Lumbar | 87 | 21 |
| Lumbosacral | 29 | 7 |
| Sacral | 27 | 7 |
| Holocord | 10 | 2 |
| Source: Adapted from Raffel C, McComb JG. Spinal cord tumors. In: Weinstein SL, ed. The Pediatric Spine: Principles and Practice. New York, NY: Raven Press; 1994:917–930.68 | ||
45.2 Pathology and Pathobiology
45.2.1 Intradural Extramedullary Neoplasms
In adults, tumors in the intradural extramedullary compartment are the most common intraspinal tumors. In children, they account for approximately 25% of intraspinal tumors (▶ Fig. 45.1 and ▶ Fig. 45.2). Dermoid and epidermoid tumors comprise about one-third of these lesions, originating in sinus tracts that form during faulty separation of the neuroectoderm from the overlying cutaneous ectoderm at the time of disjunction. Meningiomas are common tumors in adults but account for fewer than 5% of tumors in children. Although psammomatous meningiomas are the most common type, hypercellular lesions with high mitotic rates occur more often in children than in adults, representing aggressive, sarcomatous lesions.18,19 Nerve sheath tumors, such as neurofibromas and schwannomas, account for about 10% of spinal tumors in children and about 30% of intradural extramedullary tumors. Meningiomas and tumors of the nerve sheath are often associated with neurofibromatosis types 1 and 2 (NF-1 and NF-2)20. Myxopapillary ependymomas can also be intradural extramedullary tumors that develop at the filum, enveloping roots in the cauda and/or involving the conus (▶ Fig. 45.3).
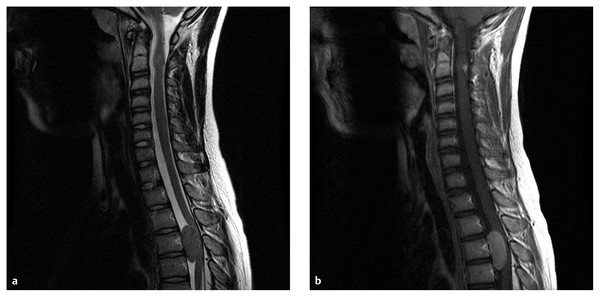
Fig. 45.1 An 11-year-old boy with neurofibromatosis type 2 presented with left leg weakness. (a) T2-weighted- and (b) contrast-enhanced sagittal magnetic resonance images of the cervical and upper thoracic spine exhibit a 2.5 × 3.5-cm homogeneously enhancing lesion ventral to the spinal cord centered at T4. Incidentally, there is also a contrast-enhancing intramedullary lesion at C2. The patient underwent a thoracic laminectomy with resection of this meningioma.
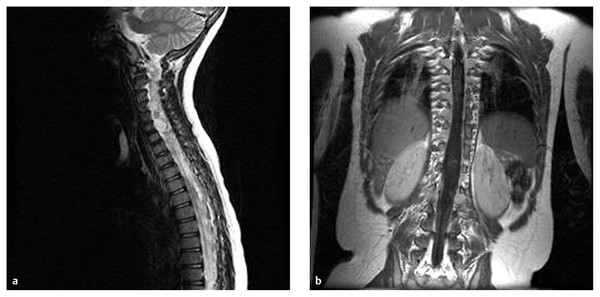
Fig. 45.2 (a) Sagittal and (b) coronal gadolinium-enhanced magnetic resonance images of the cervical and thoracic spine of a 6-year-old girl with neurofibromatosis demonstrate multiple enhancing tumors involving most nerve roots of the cervical and thoracic spinal cord.
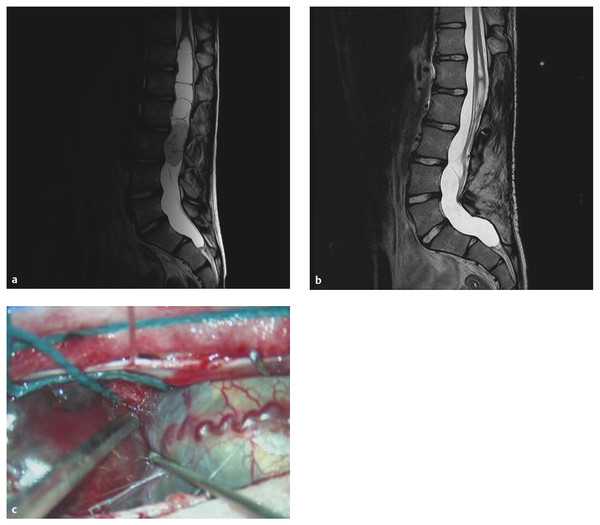
Fig. 45.3 A 14-year-old boy presented with progressive back pain. (a) T2-weighted magnetic resonance image shows a solid mass at the tip of the conus/proximal cauda equina. (b) Image after decompression and gross total resection of a myxopapillary ependymoma. (c) Intraoperative photograph.
45.2.2 Diffuse Subarachnoid Tumors
The rarest spinal lesions in children are diffuse subarachnoid or leptomeningeal tumors. They usually arise from the dissemination of a posterior fossa tumor along cerebrospinal fluid (CSF) pathways. CSF dissemination most commonly occurs in primitive neuroectodermal tumors (PNETs), germ cell tumors, ependymomas, and malignant gliomas.21 These tumors rarely present with spinal symptoms; however, 20% of PNETs in the posterior fossa show dissemination upon presentation.22 Subarachnoid dissemination of an intracranial tumor invariably leads to a poor prognosis.
Histologic grade influences the risk for leptomeningeal metastases in children with primary brain tumors. Civitello et al23 reported a 2% incidence of leptomeningeal metastases in low-grade gliomas compared with a 33% risk reported in children with supratentorial high-grade gliomas.24 Leukemia is one of the most common systemic malignancies to involve the CNS. Leukemic meningitis may occur either at the time of initial diagnosis (3%) or at the time of leukemic relapse (15%).25,26 Surgical approach is indicated only for tissue diagnosis.
45.2.3 Extradural Neoplasms
Thirty-five percent of intraspinal neoplasms in children are extradural. ▶ Table 45.3 summarizes the extradural pathologies reported in four series.15,27–29 Children younger than 3 years of age with an extradural spinal tumor were more likely to have a neuroblastoma/ganglioneuroma (▶ Fig. 45.4). Sarcoma was more common in patients older than 3 years.14 Cord compression develops in 3 to 5% of children with a systemic cancer.2
| Tumor type | No. of patients | Percentage (%) |
| Neuroblastoma | 64 | 26 |
| Ewing sarcoma | 52 | 21.1 |
| Rhabdomyosarcoma | 31 | 12.6 |
| Osteogenic sarcoma | 29 | 11.8 |
| Lymphomaa | 19 | 7.7 |
| Undifferentiated sarcoma | 12 | 4.9 |
| Germ cell tumorb | 12 | 4.9 |
| Leukemia | 7 | 2.8 |
| Wilms tumor | 4 | 1.6 |
| Other | 16 | 6.5 |
| Source: Adapted from references 15,22,27,28. aHodgkin and non-Hodgkin. bEmbryonal cell carcinoma, endodermal sinus tumor, and teratoma. | ||
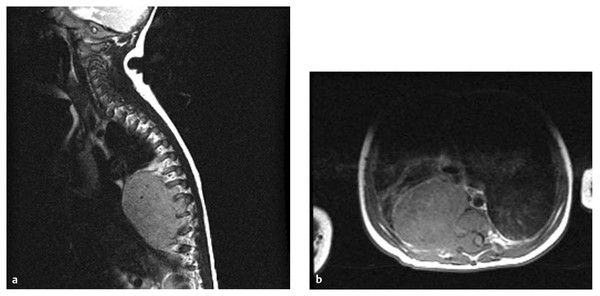
Fig. 45.4 A 5-month-old boy presented with chronic cough. (a) Magnetic resonance imaging demonstrates a large neuroblastoma with extraspinal and intraspinal components. (b) On T1-weighted axial image, the intraspinal component is extradural and compresses the spinal cord laterally to the left.
45.3 Signs and Symptoms
The clinical manifestations of extramedullary tumors vary with location and the involvement of neural elements and bony structures.30 The onset of symptoms may be rapid, with acute paraplegia and spinal shock, and symptoms presenting for months or years before presentation. The symptoms may also develop following spinal trauma, as a result of increased edema.
In patients with extramedullary spinal tumors, weakness and pain are the most common presenting symptoms (▶ Table 45.4 and ▶ Table 45.5). The weakness is usually in the upper motor neuron distribution, with increased tone, hyperactive deep tendon reflexes, and extensor plantar reflexes (Babinski sign). If the conus medullaris or cauda equina is involved, asymmetric, flaccid weakness may occur. In infants and toddlers, the weakness may be subtle; failure to meet gross motor milestones, refusal to walk, irritability, and frequent urinary tract infections may result from spinal cord compression. At presentation, the incidence of weakness was found to range from 53 to 82% depending on the degree of cord compression.29,31,32
| Symptom | No. of patients | Percentage (%) |
| Weakness | 292 | 54 |
| Back pain | 150 | 30 |
| Extremity pain | 60 | 12 |
| Incontinence | 118 | 23 |
| Muscle spasm | 36 | 7 |
| Sensory changes | 22 | 4 |
| Curvature | 26 | 5 |
| Mass | 8 | 2 |
| Meningitis | 4 | 1 |
| Hydrocephalus | 4 | 1 |
| Subarachnoid hemorrhage | 2 | 1 |
| Source: Adapted from Raffel C, McComb JG. Spinal cord tumors. In: Weinstein SL, ed. The Pediatric Spine: Principles and Practice. New York, NY: Raven Press; 1994:917–930.68 | ||
| Sign | No. of Patients | % |
| Weakness | 351 | 70 |
| Reflex changes | 226 | 45 |
| Sensory changes | 183 | 36 |
| Curvature | 141 | 28 |
| Atrophy | 88 | 17 |
| Tenderness | 62 | 12 |
| Mass | 56 | 11 |
| Muscle spasm | 55 | 11 |
| Torticollis | 33 | 6 |
| Hydrocephalus | 6 | 1 |
| Source: Adapted from Raffel C, McComb JG. Spinal cord tumors. In: Weinstein SL, ed. The Pediatric Spine: Principles and Practice. New York, NY: Raven Press; 1994:917–930.68 | ||
Pain occurs most commonly in the back and is reported in 28 to 80% of patients.31–33 Distention of the dural tube may cause vague back pain with palpable tenderness. The afferent nociceptive neurons are activated by both the mechanical distortion and the release of inflammatory mediators.4,33 Children may have radicular pain radiating into an arm, leg, or the chest wall. Radicular pain can be paroxysmal and spontaneous or provoked by motor or sensory stimuli. Pain with neck flexion or straight leg raise may indicate dural traction, and recumbency may exacerbate pain by altering canal and foramen size.33 Back or radicular pain in a child with known malignancy requires immediate evaluation. In children without a known malignancy, a careful evaluation is required as to avoid diagnostic delays.33 Sensory disturbances are also common in patients with spinal tumors.29,31,32 In comparison with pain or temperature, fine touch is a more sensitive indicator of sensory level, although this is not critical with the current noninvasive imaging techniques.7
Bladder or bowel dysfunction is also common and occurs in one-half of patients with severe cord compression.29 Changes in bladder function are difficult to detect in young children, especially before toilet training.6 Loss of bladder control in a toilet-trained child should raise the suspicion of a spinal lesion. Dysfunction of the bowel or bladder is found only through a careful neurologic examination, and formal urodynamic studies may be merited.
Scoliosis of the spine occurs in about one-fourth of children with spinal tumors and may be the initial presenting sign. In any child with a progressive abnormality of spinal curvature, the diagnosis of intraspinal neoplasia should be considered.4,6,7,12,34 Kyphosis is usually a late finding associated with extensive bony metastatic disease.
Patients with spinal tumors, especially malignant tumors, may have hydrocephalus and associated increased intracranial pressure (ICP).35,36 Decreased CSF absorption secondary to elevated CSF protein content may lead to hydrocephalus.33 Patients with spinal cord tumors may have symptoms related to sequelae of their disease: infectious or radiation-related myelopathy, spinal cord stroke, intradural or extradural hematoma secondary to thrombocytopenia or coagulopathy, or an epidural abscess.
The most common nontraumatic acquired cause of paraparesis in children is malignant epidural spinal cord compression by epidural tumor deposits. Cord compression may be the presenting sign of malignancy in 28 to 76% of patients.15,27,31,32 These epidural deposits differ from those found in adults in their histologic features, the location and direction of compression, the degree of bone involved, and the general medical condition of the patient.27,31,37,38
During early childhood, sympathetic tumors such as neuroblastoma and ganglioneuroma predominate. A series reported from St. Jude Children’s Research Hospital concluded that 18% of children with Ewing sarcoma, 8% with neuroblastoma, and 7% with osteogenic sarcoma had compression of the spinal cord at some time during their disease.28 In one series, 26% and 21% of cases of cord compression were secondary to neuroblastoma and Ewing sarcoma, respectively.33 Malignant epidural spinal cord compression is the most frequent neurologic complication of Ewing sarcoma; sarcomas have a higher incidence of spinal cord compression and usually present at a slightly later age.27,39
45.4 Diagnostic Studies
45.4.1 Plain Radiography
Plain radiographs of the spine are a reasonable first step in the evaluation of a patient suspected to have an intraspinal tumor. Plain radiographs show abnormalities in one-half of patients with spinal cord tumors.5,16 Intradural extramedullary tumors can thin or cause sclerosis of the pedicles. A tumor that extends through and enlarges a neural foramen is best visualized on oblique films. An enlarged foramen indicates a tumor of the nerve sheath or a paraspinous tumor. Malignant tumors and epidural metastases erode bone and may cause vertebral body collapse. The loss of a pedicle through bony destruction causes the “winking owl” sign.40 Plain radiographs are also important in the evaluation of and surgical planning for patients who present with spinal deformity. They are rarely of value in the evaluation of spinal cord compression. In one series, only 30% of children with metastatic spinal disease presenting with spinal cord compression had abnormal plain radiographs.27 Abnormalities are more often detected in adults with cord compression, in whom most epidural invasion is from vertebral sites versus paraspinous sites in children.
45.4.2 Magnetic Resonance Imaging
Magnetic resonance (MR) imaging is the study of choice to identify spinal cord neoplasms. It provides anatomical detail while obviating the need for lumbar puncture, thus eliminating two risks: (1) bleeding from thrombocytopenia or coagulopathy and (2) acceleration of the rate of neurologic deficit from the loss of CSF. MR imaging is at least as sensitive as myelography combined with computed tomography (CT), providing superior anatomical details in and around the spinal canal.
MR imaging should be performed both with and without intravenous contrast enhancement (gadolinium diethylene–triamine–pentaacetic acid [Gd-DTPA]) and in multiple planes of view. In studies done without contrast, both T1- and T2-weighted images should be obtained. Usually, the surgeon is able to identify abnormal areas in the spinal canal without contrast; however, contrast is useful for defining the extent of tumor.
In a study of patients with documented intradural extramedullary disease, the administration of Gd-DTPA aided in the identification of 2- to 3-mm intradural extramedullary nodules and the demonstration of leptomeningeal spread.4 Larger intradural extramedullary lesions, such as nerve sheath tumors and meningiomas, are readily imaged within the subarachnoid space with standard imaging techniques, although the use of Gd-DTPA makes these lesions much more conspicuous and confirms the compartment and extent of the lesion (▶ Fig. 45.1). Lesions containing fat or cholesterol, such as dermoids, epidermoids, and lipomas, are visualized without the use of contrast.
Intradural extramedullary lesions often displace the cord contralaterally, widening the ipsilateral CSF space and creating what is known as the “meniscus” sign.41 In patients with extradural lesions, T1-weighted images provide adequate tissue contrast. The metastatic deposits are hypointense against the high signal of normal marrow and epidural fat. Metastatic lesions usually have an increased signal on T2-weighted images, but signal characteristics may vary because of hemorrhage, inflammatory debris, and necrosis. Intravenous Gd-DTPA may be helpful, although enhancement may make vertebral and epidural metastatic deposits less conspicuous; the signal is similar to that of normal epidural fat and vertebral marrow on T1 weighted imaging.4
Neuroblastomas are usually isointense or hypointense to the spinal cord, and there may be areas of necrosis (▶ Fig. 45.4). On T2-weighted images, neuroblastoma demonstrates some hyperintensity, and there may be areas of hypointensity secondary to calcification, although rarely. After the administration of Gd-DTPA, there is homogeneous or heterogeneous enhancement.42 Neurofibromas and schwannomas can be difficult to differentiate; both are isointense on T1-weighted images and hyperintense on T2-weighted images. Schwannomas may have mixed signal intensity on T2 secondary to different cellular populations, Antoni A and B cells.41 They may hemorrhage and undergo fatty or cystic degeneration, but rarely do they undergo malignant degeneration. Most neurofibromas are in both the intradural extramedullary and extradural compartments, although a small percentage may be totally extradural. They can be single, multiple, or diffuse (▶ Fig. 45.2). Neurofibromas can demonstrate a central hypointensity, or “target,” on T2, thought to be a dense collection of collagenous stroma. They may also undergo malignant change.41 Although usually larger and eroding into bone, neurofibrosarcomas may be difficult to differentiate. Areas of necrosis may become evident with contrast. Once an intraspinal lesion is identified, complete neural axis imaging is warranted to examine for intraspinal dissemination or a primary cranial lesion.2
45.4.3 Myelography
Myelography with water-soluble contrast has previously been used extensively in the evaluation of intraspinal lesions in children.5,6,12,15,43 However, deterioration after a lumbar puncture has been reported in patients with intraspinal neoplasms,38 and the risk for hematoma exists with coagulopathy in pediatric patients with cancer.29 Myelography should be reserved for patients who require an emergent study when MR imaging is not immediately available.
In intradural extramedullary tumors, the cord is displaced, with a filling defect in the contrast column. Epidural lesions displace both the cord and the subarachnoid space. A complete block of contrast material may prevent a determination of the upper extent of the tumor.
45.4.4 Computed Tomography
CT with intrathecal water-soluble contrast is an important adjunct to myelography.12,44 Contrast medium may pass around a high-grade block seen on a myelogram, defining the upper extent of the lesion without the need for a second injection of contrast material. CT with intrathecal contrast is more sensitive than myelography alone in detecting drop metastases and lesions in the epidural space.45 High-resolution CT with intravenous contrast may demonstrate whether a tumor, such as a paraspinal neuroblastoma, is in the canal. As well, contrast enhancement can identify the involvement of great vessels with large paraspinal masses.
CT is the optimal study for assessing the degree of bone involvement. The neuroforaminal widening, bone erosion, and vertebral body scalloping caused by neurofibromas and schwannomas are evident on CT. This information is critical for surgical planning.
45.4.5 Ultrasonography
Ultrasonography is of little value in detecting intraspinal lesions because bone is impenetrable to sound waves. However, a primary lesion can be detected in children up to 3 months of age, when the posterior spinal elements remain largely cartilaginous. Ultrasonography is a valuable intraoperative tool.46,47 The thecal sac can be scanned before dural opening to delineate the extent of the tumor. In a comparison of preoperative MR imaging with intraoperative ultrasound, it was found that tumors of the cauda equina can migrate rostrally during positioning.48
45.5 Operative Technique
The goals of the operative management of extramedullary tumors are to (1) obtain tissue for histologic diagnosis, (2) preserve neurologic function, (3) treat pain, and (4) maintain or restore spinal stability and load-bearing capacity.33 The histological diagnosis requires a percutaneous CT-guided or an open biopsy. Open procedures are performed when the initial presentation is with spinal cord compression or when the lesion is too small or dangerous to be sampled percutaneously. The location of the tumor, suspected or prior diagnoses, and the presence of metastatic disease from another known source also direct the choice of tissue sampling.
Corticosteroid use in the preoperative management of patients with extramedullary masses compressing the spinal cord is important for preserving neurologic function. However, no studies have compared type of steroid, route of administration, or duration of therapy. High-dose dexamethasone therapy is most commonly used until the spinal cord is decompressed and neurologic symptoms are improving. Preoperative and postoperative care includes deep venous thrombosis prophylaxis and neurogenic bowel and bladder training regimens.
In adults, spinal cord compression from malignancy is most often caused by the epidural extension of a metastatic lesion into the vertebral body. Thus, the mass compressing the cord is anterior to the spinal cord. This finding led Sundaresan and colleagues49,50 to recommend an anterior approach to these lesions, with resection of the vertebral body. Epidural compression in children with malignant tumors is usually caused by direct extension of the tumor through the vertebral foramen without significant involvement of the bone.5,10,38,51,52 The mass compressing the cord is lateral to the vertebral body and can easily be reached through the posterior approach. Accessing the tumor with a laminectomy, laminotomy, or transpedicular approach is usually preferred. The goal is an optimal decompression with maximal safe resection. A gross total resection of a malignant tumor is rarely accomplished. However, subtotal removal can be done with minimal morbidity and no mortality.29 Postoperative spinal stability may be an issue, depending on the extent of tumor involvement, resection, and radiation therapy. Most malignant tumors are not vascular and can be removed with conventional microsurgical technique. The ultrasonic aspirator (Cavitron Ultrasonic Aspirator, Tyco Healthcare, Mansfield, MA) can help remove larger lesions. If the tumor invades the dura, the involved area may be resected and reconstructed with a dural graft. Somatosensory and motor evoked potentials may be useful in cases in which the tumor involves nerve roots.2
45.5.1 Intradural Extramedullary Tumors
The treatment of intradural extramedullary tumors is primarily surgical. These tumors are usually nerve sheath tumors or benign meningiomas, which are separated from the surrounding tissue by a distinct margin (▶ Fig. 45.1). This margin allows total excision in almost all patients. Some nerve sheath tumors extend into the extradural space through the neural foramen, and into the paravertebral soft tissue. A second extraspinal resection may be required to remove the remaining tumor. Careful histologic examination is critical in patients with neurofibromatosis because these tumors may contain malignant elements. Asymptomatic intradural neurofibromas in patients with neurofibromatosis can be clinically followed up with serial MR imaging studies. Surgical removal is recommended for patients with cord compression, intractable pain, or radiographic enlargement.
Dermoid tumors of the spinal canal are most often associated with dermal sinus. The treatment of these tumors is surgical, and the operation is best undertaken before any infection has occurred. Scarring induced by infection makes resection more difficult. If meningitis is present when the sinus is discovered, surgery should be delayed until the infection has been treated. The wall of these tumors is made up of epidermis and dermis. They enlarge slowly, filling with desquamated epithelium, sweat, and sebaceous material. The wall of the tumor may be densely adherent to the conus medullaris or the cauda equina roots, and the tumor may tunnel into the cord. Attempts to remove the tumor wall may result in a significant postoperative deficit; thus, debulking should be the approach. The portion not in contact with neural tissue can then be removed, and the remaining adherent portion can be coagulated or treated with a carbon dioxide laser. Spilling of tumor contents into the subarachnoid space should be avoided because sterile meningitis may occur.2 Serial MR imaging is required to monitor for recurrence.
45.5.2 Extradural Tumors
In children, most epidural tumors are extensions of malignant paraspinous neoplasms that infiltrate the spinal canal through neural foramina. The treatment options for malignant tumors in the spinal canal are surgical decompression, radiation therapy, and chemotherapy. Asymptomatic patients with a small intracanal tumor burden or with chemo- or radiosensitive histology should be treated nonsurgically. Considerable controversy exists regarding the best management of intraspinal tumors producing neurologic deficits. Tumor type, extent of spinal cord compression, and deterioration during radiation or chemotherapy all play a role in the management decisions for patients with these tumors. The location, extent of tumor, and previous radiation also play a role in the surgical approach.
45.6 Treatment Alternatives
The multidisciplinary management of children with primary or metastatic spinal neoplasms has become the mainstay of treatment with surgical decompression/maximal resection, chemotherapy, and/or radiation. Consultations with radiation and medical oncology are necessary to determine a treatment plan, during which chemotherapy protocols and prior treatment are considered and treatment goals are clarified. Pediatric anesthesia, critical care, and rehabilitation are adjuncts available at pediatric cancer centers providing a holistic treatment approach. Treatment plans are affected by the type of tumor, extent of spinal cord compression, and whether a diagnosis has been established. These factors are examined in the next sections with respect to the two most common intraspinal malignant tumors—neuroblastoma and sarcoma. The degree to which surgery, chemotherapy, and radiation play a role in treatment with respect to overall survival and best neurologic outcome has been studied in several clinical trials.
45.6.1 Outcomes
Neuroblastoma
Treatment options for patients with neuroblastoma and spinal involvement include chemotherapy alone, chemotherapy followed by surgical resection of residual radiographic disease, and surgical resection followed by chemotherapy and radiation therapy. In an early series, nine patients with neuroblastoma and evidence of spinal cord compression underwent chemotherapy alone and had successful elimination of tumor bulk, and all patients were able to walk at the completion of treatment.53 In another study treating patients who had dumbbell neuroblastomas initially with chemotherapy, Plantaz et al showed a reduction in the size of the intraspinal mass in 58% and improvement of neurologic deficits in 92%.54 Surgical decompression was avoided in 60% of patients. In a series of 112 patients with epidural compression of the spinal cord, Klein et al28 emphasized that the type of tumor is important in the efficacy of nonsurgical therapy. Spinal cord compression from neuroblastoma developed in 32 of their patients; 12 underwent surgical decompression, 16 received only medical treatment, and 3 were not treated because the compression appeared at the end-stage of the disease. No significant difference was seen in the functional outcomes of patients treated surgically or medically. The combined results of the patients with so-called small cell tumors (neuroblastoma, germ cell tumors, and lymphomas) were similar to those for patients with neuroblastoma. Patients without severe spinal cord compression can be treated successfully without surgical decompression. These results have led to the conclusion that surgery is not indicated for asymptomatic children with intraspinal neuroblastoma and other small cell tumors. Massad and associates38 evaluated 80 patients with neuroblastoma admitted to the Beirut Medical Center over a 21-year period. Twelve of these patients had intraspinal involvement sometime during the course of their disease. Intraspinal disease was more common in those patients with mediastinal versus retroperitoneal disease. Only 5% of the 80 patients presented with spinal cord compression, and none had disseminated disease. Patients with intraspinal involvement and less disseminated disease had better survival at 5 years (50% vs. 21%). The factors that affected survival in this study were age, histologic differentiation of the tumor, duration of neurologic symptoms, and mode of therapy.
Prognosis and surgical outcome may also depend on the International Neuroblastoma Staging System (INSS) stage. In a study from Memorial Sloan-Kettering Cancer Center in New York City,55 46 patients with epidural or neural foraminal neuroblastoma were stratified retrospectively into high- and low-risk groups according to INSS stage (high risk, 4; n = 31; low risk, < 4). Patients with normal neurologic examinations regardless of INSS stage had a low risk for neurologic deterioration following surgery or chemotherapy. Patients with high-grade spinal cord compression and neurologic deficits can respond to chemotherapy, but 25% will worsen and may require an operation for progressive neurologic deficits. Chemotherapy may be avoided in low-risk patients who respond to surgery alone. A significant number of patients who underwent operations (30%) developed spinal deformities postoperatively. Most of the operations (34%) were posterior approaches in the lumbar or thoracic segments without instrumentation. Median survival was not significantly different between high-risk patients who did or did not have high-grade spinal cord compression and between patients whose initial treatment was chemotherapy or surgery. This was consistent with the findings in the low-grade groups. There was a trend toward longer survival in the patients without neurologic deficits (5.2 vs. 2.7 years), but it was not statistically significant (p = 0.07) and may have been due to lower statistical power.
Sarcoma
The experience of Klein et al28 also suggests that some tumors are better treated with surgical resection. In their series of 29 patients with Ewing sarcoma, 20 underwent surgical decompression and 9 were treated medically. The patients in each group were comparable with respect to their neurologic status. Seven of the surgical patients were able to walk independently when they first came for treatment, and all maintained this functional level after treatment. Of the 13 patients who underwent surgery but were not able to walk, all but 1 improved, and 11 were able to walk independently after treatment. Of the 9 patients treated medically, only 3 were able to walk after undergoing treatment. Likewise, of the 14 patients in this series who had rhabdomyosarcoma, all 5 who underwent surgical decompression were able to walk independently afterward. Of the 9 patients treated medically, 1 remained the same and 1 got worse. This series suggests that surgical decompression is the initial treatment of choice for children with sarcomas and compression of the spinal cord.
The degree of compression noted on initial imaging studies may affect outcomes.56 The 33 patients in one series had complete or nearly complete blockage on myelography, or the tumor occupied more than 50% of the spinal canal on MR imaging. Twenty-six patients underwent surgical decompression followed by radiation and chemotherapy. At the time of follow-up, 18 of these patients showed neurologic improvement, 7 were unchanged, and 1 was worse. The other 7 patients underwent treatment with radiation and chemotherapy alone. Among these patients, 1 improved and 4 deteriorated while receiving chemotherapy. Surgical outcomes were superior to medical therapy outcomes in an assessment of bowel and bladder function after treatment. This was confirmed in a subgroup of patients from St. Jude Children’s Research Hospital who had complete motor and sensory level deficits, recognized regardless of tumor type.28 In patients who present with cord compression as the first sign of malignancy, surgery is required for diagnosis regardless of the degree of cord compression or tumor type. Surgical therapy is also required in those patients whose condition deteriorates during medical therapy.
Surgical decompression is indicated for patients with severe compression of the spinal cord regardless of tumor type, symptomatic patients with sarcomas, patients with symptomatic compression who have no diagnosis, and patients whose neurologic function deteriorates during nonsurgical therapy. Others can be treated primarily with chemotherapy and radiation therapy.
45.6.2 Complications
Immediate complications of surgical decompression of the spinal cord are rare. These are most commonly postoperative hematomas and wound complications (infection, dehiscence, and CSF fistulas). Reduction of morbidity and mortality in these cases requires careful preoperative planning. Proper positioning of the patient minimizes pressure sores and reduces ventilatory and venous pressures during extensive operations. Anemia from acute blood loss is a common complication, and the preparation of blood products for perioperative transfusion is essential. Maintenance of normothermia lessens the risk for coagulopathy and metabolic acidosis. In the surgical approaches to patients with extradural and intradural extramedullary spinal masses, consideration of en bloc resection is sometimes required for maximal oncologic treatment. This often involves the collaboration of pediatric thoracic and abdominal surgeons to allow a larger surgical field for more complete removal and the placement of stabilizing instrumentation.
Short-term and delayed spinal deformity may occur as a consequence of posterior decompression with laminectomy, with rates varying from 16 to 100% of cases in children, versus 10% of cases in adults.57 In an early series of patients with intraspinal tumors treated with laminectomy, cervical kyphosis, anterior subluxation, and disabling scoliosis were observed.58,59 Causes of instability may be vertebral erosion, paraspinous denervation or fibrosis, asymmetric irradiation of the spine, or postsurgical destabilization.60 This may also be related to a wedging change in the cartilaginous portion of the vertebral body and to the viscoelasticity of ligaments in children.61 In one series of 31 patients treated for intraspinal tumors, 7 developed a spinal deformity after laminectomy.12 The incidence of deformity after multilevel laminectomy is related to the patient’s age and the spinal level of the laminectomy (▶ Table 45.6). Reports from the Children’s Memorial Hospital, Chicago, Illinois,62 suggest that laminotomy is less likely to cause a deformity than laminectomy.12,62 After laminectomy, 39% of patients have shown abnormal curvature, whereas 27% have done so after laminotomy. Using a high-speed pneumatic drill, Abbott et al encountered only one technical complication in 180 laminotomies.63 However, other authors have not been convinced of the superiority of the laminotomy.55,64 Laminoplasty has also been associated with a high incidence of postoperative deformity; McGirt and colleagues reported similar risks for deformity with laminoplasty and laminectomy after 20 months postoperatively. Laminoplasty has, however, been associated with a reduction in incisional spinal fluid leaks and reduced hospital stay, but further studies in pediatric populations are required to determine if this association is significant.57
| Characteristic | Percentage of patients with subsequent deformity (%) |
| Age | |
| Younger than 15 | 46 |
| Older than 15 | 6 |
| Site of laminectomy | |
| Cervical | 100 |
| Thoracic | 36 |
| Lumbar | 0 |
| Source: Yasuoka S, Peterson HA, MacCarty CS. Incidence of spinal column deformity after multilevel laminectomy in children and adults. J Neurosurg 1982;57(4):441–445. 69 | |
One series of 255 children with intramedullary spinal tumors compared the occurrence of postlaminectomy spinal deformity in children who underwent decompression and fusion at the time of surgery with the occurrence of deformity in those who underwent decompression alone. Moderate and severe deformity, defined as scoliosis of more than 25 degrees and/or sagittal imbalance of more than 20 degrees requiring bracing or surgery, developed in 21 of 37 resections (57%) without fusion and in 4 of 15 resections (27%) with fusion. Laminectomy involving laminae at more than three levels demonstrated a significant association with postoperative deformity. Although this series includes only intramedullary tumors, it raises the question of considering postoperative outcomes when a surgical plan is made. For younger patients who have undergone cervical decompression with a multilevel laminectomy, immobilization with a cervical collar is recommended for 3 to 6 months. The first-line treatment for many children with spinal tumors will be a posterior decompression with resection; these patients must be followed closely to monitor for postoperative deformity.65
Spinal deformity is also a complication of radiation therapy. Mayfield and colleagues60 reported that significant spinal deformities developed in 32 of 57 patients (56%) with neuroblastoma treated with radiation therapy and chemotherapy. A rate as high as 71% has been reported.66 A higher rate of deformity is associated with younger age at the time of radiation, doses greater than 20 Gy, and asymmetric radiation fields.60,66,67 Radiation-induced myelopathy occurs more frequently at the midthoracic region, the watershed area of the spinal cord. The degree of injury varies with total radiation dose, fraction size, and length and duration of treatment. A threshold for radiotherapy of 45 to 50 Gy has been suggested, delivered in fractions of 1.8 to 2 Gy. With image guidance systems, radiosurgical methods can now be applied to extracranial targets. Mainly used in adult patients with spinal metastases, the concern for posttreatment deformity and radiation-induced myelopathy persists. Further studies are needed to examine the impact on the pediatric population.30
The combination of laminectomy and radiation can pose severe risk for the development of spinal deformity.60,66,67 In children who have an epidural tumor without evident cord compression or neurologic deficit, both surgery and radiation therapy should be avoided. If possible, a child with an epidural tumor should undergo chemotherapy alone. For those patients who require surgery, consideration should be given to bone grafting and/or implants with instrumentation if the degree of tumor or bone removal may result in spinal instability.
Pearls
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








