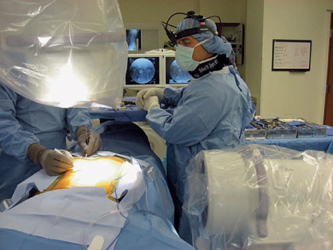
28 Spinal Fusion: Combined Anteroposterior Approach Richard D. Guyer and Thomas F. Roush Despite recent biologic advancements such as improved osteoconductive and particularly osteoinductive materials and proteins, achieving a successful lumbar spinal fusion remains a difficult task. In many respects, the dorsal lumbar spine represents the worst of all anatomic regions for fusion due to the large dorsal tension forces on the lumbar spine, difficulty in immobilization, limited bony surface area available for biologic fusion, and poor avascular healing bed following surgical exposure via periosteal stripping. In contrast, the anterior interbody region is a favorable area for fusion, as witnessed by the roughly 80% share of spinal compressive load through the anterior and middle columns1 in accordance with Wolff’s law, 90% share of the spinal osseous surface area, and relatively strong and vascular bony endplates on which to obtain fixation strength. Additional proposed benefits are improved restoration of sagittal alignment and neuroforaminal height.2 The surgical implementation of anterior lumbar interbody fusion (ALIF), however, destabilizes the spine in extension and axial rotation by the variable sectioning of anterior longitudinal ligament and outer anulus,3 as well as possibly producing facet joint distraction. The sectioning of various stabilizing structures as is necessary during surgical procedures diminishes spinal stability in a complicated fashion not yet fully understood.4 Furthermore, the original concept of “distraction compression”5 due to the pretensioned anulus has been found to be short-lived due to the viscoelasticity of the ligamentous tissue.6 Recognizing these drawbacks, combined anterior lumbar interbody/posterolateral lumbar fusion (ALIF/PLF) constructs have become increasingly popular in the treatment of recalcitrant lumbar disc degeneration.7–13 When considered independently, several biomechanical drawbacks exist for anterior and posterior fusions. ALIF has long been recognized to have limited control of extension and axial rotation.14–18 Furthermore, these studies found that the addition of posterior fixation, either pedicle or facet screws, yielded significantly superior biomechanical stability. This improved stability was also noted during lateral bending and flexion. Posterolateral lumbar fusion (PLF) alone imparts even less stability to the lumbar spine and demonstrates deficiency in a multitude of loading conditions,19–22 all of which are improved with the addition of interbody fusion. Intuitively, when the degenerated disc is implicated as the nociceptive generator, PLF as a treatment option makes little sense as the disc is not directly addressed. Even in the face of solid PLF, cantilever interbody motion remains,23,24 thereby engendering motion about the disc. Bono et al25 noted that this cantilever motion can yield sagittal range of motion of up to 6 degrees following solid PLF and, more interestingly, up to 3 degrees following solid ALIF. These residual motions have a clinical correlation in that further stabilization in the form of postoperative bracing following PLF leads to higher union rates.26 Such findings indirectly support combined constructs such as anteroposterior (360 degrees or ALIF/PLF) lumbar fusion to yield more complete motion cessation. More direct evidence was offered by Oda et al,10 who noted the importance of an ALIF/PLF construct to increase stiffness and decrease implant strain. From a biologic standpoint, solid interbody fusion requires ample vascular surface area, which is difficult with a posterior interbody approach. Even in a controlled cadaveric setting, Manos et al27 demonstrated ~50% to 60% disc material removal and adequate endplate preparation while performing a TLIF procedure. In addition to improved end-plate preparation and fusion bed, ALIF affords improvements in sagittal balance,28 particularly when coupled with PLF.29 An additional concern of stand-alone ALIF constructs is subsidence,30,31 which appears to be more problematic with recombinant human bone morphogenetic protein (rhBMP-2; INFUSE, Medtronic Sofamor Danek, Memphis, TN) use.30,32 The combined ALIF/PLF approach possesses a sound biomechanical basis, but the concern as to the necessity of this more extensive procedure in the treatment of degenerative disc disease (DDD) remains despite improvements in technique that will be addressed in this chapter. Indications and work-up for ALIF/PLF are no different than the work-up for any other lumbar approach. We consider a patient a surgical candidate if they fail 6 months minimum of nonoperative treatment (medication, physical therapy, chiropractic manipulation, injections, etc.), and have radiographic evidence including plain x-rays, magnetic resonance imaging (MRI), computed tomography (CT), myelography-CT, and provocative testing in the form of discography (where deemed appropriate on a case-by-case basis) that corroborates the symptoms and isolates the degenerative level as the pathologic source, and that no contraindications to either procedure exist. Furthermore, the patient must be medically fit to undergo a 2- to 3-hour operation depending upon the levels involved. Nearly all patients considering surgery at our institution for back pain primarily undergo extensive psychological evaluation performed by a spine specialty psychologist, and must be deemed a surgical candidate following the evaluation. With the availability of various motion preservation strategies, we view more than two degenerative, nondeformed levels, as a relative contraindication to any lumbar fusion, though there are exceptions. Should a patient emerge with solid evidence that the pathologic disc is primarily responsible for symptoms, we proceed with providing them information on anterior and posterior surgery, and involve them in decision making concerning whether to have surgery and which approach to take. We begin all ALIF/PLF procedures with the anterior approach, which allows us to obtain improved restoration of disc height and lumbar lordosis. Following preoperative antibiotic infusion and application of mechanical deep vein thrombosis prophylaxis, patients are placed supine on a standard operating table configured to allow improved unencumbered fluoroscopic access under the table. It is imperative that the table be radiolucent, flexing and extending through the involved disc space. ALIF/PLF is particularly useful with advanced collapse of the degenerated disc to obtain better visualization and access to the entirety of the endplates. Endotracheal anesthesia is then commenced. The remainder of the surgery is based on the mini-anterior retroperitoneal approach as described by Brau.33 Each anterior surgical approach is performed by a general or vascular surgeon with expertise in such approaches. Transverse incisions are used whenever possible for cosmetic reasons. Hand-held retractors are used for efficiency and the perceived circulation improvement afforded by the transient relaxation of retraction pressure on the great vessels. A single image intensifier is used to mark the appropriate level and center of the disc space via an anterior view, then the intensifier is converted to a lateral view for the remainder of the case to better assess posterior end-plate preparation and implant sizing. The disc space is prepared as carefully as one would for a disc arthroplasty. That is, care is taken not to violate the endplates to prevent subsidence and clean the endplates down to bleeding subchondral bone with a variety of Cobb elevators, pituitary rongeurs, straight, angled, and ring curettes. After verifying by lateral C-arm images that the disc tissue has been evacuated to the posterior longitudinal ligament, a variety of implants can be placed including allograft bone, cylindrical tapered cages of metal or polyetheretherketone (PEEK), and PEEK grafts with or without incorporated screws and plates. Care should be taken not to overdistract the disc space greater than the next normal adjacent one. Implants can be used with autograft bone, demineralized bone matrix (DBM), or rhBMP-2. Our preference is allograft bone with rhBMP-2. Following anterior wound closure and generous wound dressing to minimize removal during the transition to posterior procedure, a radiolucent Jackson table with either a Wilson or a four-post frame placed adjacent to the patient’s operative table. The patient is transferred carefully, while intubated, to the prone position with ample padding of bony prominences and careful positioning of the upper extremities. The standard operating table is then removed from the room, and the Jackson table is then centered under the operative lighting. Prior to preparation and draping, two image intensifiers are positioned on either side of the table. The intensifier placed on the side of the surgical scrub technologist or nurse and operative instruments and implants is denoted the permanent lateral view, so as to minimize the bulkiness which is imparted when the intensifier is placed in an anteroposterior configuration. The image intensifier for the lateral view is then rotated whereby the bar connecting the x-ray source is rotated such that is moves in the cephalad direction until it reaches its limitation conferred by the underneath structure of the Jackson table. A second image intensifier then is introduced from the opposite side of the table and is designated as the anteroposterior image. Once a truly orthogonal view is obtained, which is centered on the image intensifier screen, sterile preparation and draping commence so that the image intensifiers are first covered in a sterile fashion, followed by sterile draping of the surgical site. We find that using biplanar fluoroscopy improves both the duration and accuracy of the posterior procedure. Figure 28.1 shows the biplanar imaging configuration. Like all new techniques, however, experience must be rapidly gained by all involved; experienced fluoroscopists are vital to the success of such a technique. Regardless of whether performing concomitant posterior fusion for DDD with or without spondylolysis or spondylolisthesis, we prefer to use the bilateral paramedian Wiltse incisions due to improved biomechanical stability of bilateral over unilateral posterolateral fusion,34 despite evidence35–38 that unilateral PLF leads to similar clinical outcome. Figure 28.2 shows the skin markings for the posterior approach. Care is taken to expose the relevant transverse processes/facet/pars complexes to prepare an adequate fusion bed. Following posterior instrumentation with pedicle screws taking care to avoid damaging the zygapophyseal articulation, a high-speed burr is used to decorticate the transverse processes, lateral aspect of the superior articular process, and pars interarticularis region. Following decortication, bone graft is placed over the surface and the pedicle screws and rods are placed. Bone grafting materials most commonly used include both osteoconductive and osteoinductive materials. A common combination is tricalcium phosphate for its osteoconductive properties and a DBM as a graft extender and osteoinductive source. Rarely, autograft from the posterior iliac crest is used. If a decompression is necessary, this can be performed from a separate midline approach and the local bone is admixed with DBM. Alternatively, if decompression is not needed, we favor the percutaneous pedicle screw technique using electrophysiologic pedicle screw monitoring that is demonstrated in the video. In these cases, we depend primarily on the anterior fusion, though the facet joints can be easily decorticated by the use of pituitaries and the guidance of the C-arm prior to rod placement.
Biomechanical and Biologic Rationale
Indications and Work-up
Surgical Procedure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








