14 Spinal Metastases
Introduction
Spinal metastases may affect different components of the spine, and the approach to treatment depends on correct localization of the affected compartment. Metastases to the spine most frequently involve the vertebral elements and the epidural space.1–4 In a small proportion of patients, symptoms and signs may arise from metastases that spread to the intradural spinal compartment, either extramedullary or intramedullary (Table 14-1).5–7 The approach to treatment varies according to the affected site, and ideally requires the shared decision of a multidisciplinary team. The specific treatment may include various combinations of steroids, irradiation, surgery, chemotherapy, hormonotherapy, and bisphosphonates, as appropriate for the underlying neoplasm.
| Vertebral metastases Vertebral metastases with epidural extension Epidural metastases without vertebral lesion Intradural metastases |
Vertebral Metastases
Metastases most commonly affect the lungs, liver, and skeletal system. Bone metastases are a significant cause of morbidity, due to pain, pathologic fractures, hypercalcemia, and spinal cord compression.3,4,8,9 The vertebral column is the most common site for skeletal metastases,10 and in autopsy series, 60% of patients dying of cancer were found to have spinal and epidural metastases.11 Destructive vertebral lesions are a common source of morbidity, with pain the presenting complaint in the majority of cases.
All patients with vertebral metastases are at potential risk of developing spinal instability and neurological impairment. Therefore, it is useful to classify vertebral metastases according to biomechanical stability and the presence or absence of dural sac displacement or neural element compression (Table 14-1). This classification allows for early recognition of potential complications. Treatment can be adjusted to meet the potential risks and preserve or restore normal function.
Assessment of the extent of vertebral metastases is based on imaging findings that range from spine radiography and bone scintigraphy to positron emission tomography (PET) using F-18 2-fluoro-2-deoxy-D-glucose (FDG),12 computerized tomography (CT), and magnetic resonance imaging (MRI). MRI is sensitive to both focal vertebral lesions and bone marrow involvement, in both hematologic malignancies and in solid tumors.13
CLINICAL AND IMAGING CRITERIA FOR SPINAL STABILITY IN NEOPLASTIC DISEASE
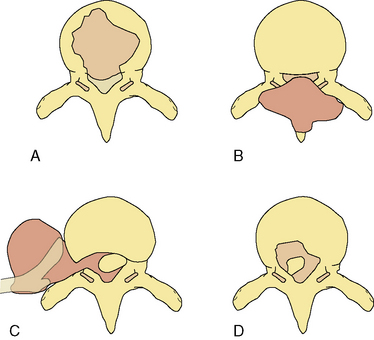
Determining spinal stability is of paramount importance in choosing the appropriate form of management for patients with spinal metastases, since a major goal of treatment is restoration or maintenance of spinal stability. In trauma, the accepted biomechanical model for thoracolumbar stability after fractures is the three-column concept of the spine (Figure 14-1).14
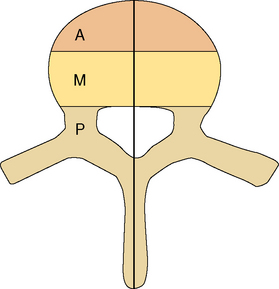
Figure 14-1 Classification system for the evaluation of spinal stability. The three-column system of Denis14 was devised for assessment of spinal column stability in trauma. The system divides the spine into the anterior (A), middle (M), and posterior (P) columns. The spine is considered unstable if two of the three columns are disrupted. The six-column system of Kostuik and Errico15 was devised for evaluating stability in spine tumors. Here the three columns, as defined by Denis, are subdivided into left (L) and right (R) halves. The spine is unstable if three to four of the columns are destroyed.
In neoplastic destruction of the spine these concepts may not always be applicable, because trauma and tumors are quite different conditions in terms of the disruption of bone, disc, and ligament; the quality of surrounding bone stock; and the ability of the spine to heal. A set of criteria, which requires validation, has been developed for spinal tumors.15 The spine is divided into six columns: the three columns defined above14 subdivided into left and right halves (Figure 14-1). The spine is considered to be unstable if three to four of the columns are destroyed, and markedly unstable if five to six of the columns are involved. Angulation of 20 degrees or more adds to the consideration of instability.
Instability does not usually develop when involvement is limited to the vertebral spongy bone core or to the anterior column. When the posterior half (middle column) of the vertebral body is also involved (cortical bone included), pathologic compression fracture can occur, producing kyphosis and extrusion of bone, tumor, or disc into the spinal canal, with resulting neural compromise. Tumor involvement of the middle and posterior column may produce forward shearing deformity. In addition, segmental instability is probably present when the clinical syndrome is characterized by pain that is aggravated by movement (in the absence of significant neural encroachment), and associated with progressive collapse of vertebral bodies or localized kyphosis on imaging studies. The MRI appearance of acute vertebral fractures was studied in a series of 100 patients evaluated for suspected spinal cord compression (SCC).16 Pathological fracture of the vertebral body was present in 51% of compressive levels. Of these, 68% had loss of height greater than 50%; vertebral bodies in the lower thoracic region were more likely to have pathological fractures.
A systematic approach to determining clinical instability of the spine should include anatomic, biomechanical, symptomatic, and therapeutic considerations.17,18 Apart from imaging studies that define anatomic details used in the three-column spinal model (Figure 14-1),16 the concept of clinical instability should be taken into account. Clinical instability is defined as “loss of the ability of the spine under physiologic loads to maintain relationships between vertebrae in such a way that there is either damage or subsequent irritation to the spinal cord or nerve roots, and in addition there is development of incapacitating deformity or pain due to structural changes.”18
TREATMENT OF VERTEBRAL METASTASES
Biomechanically stable vertebral metastases
Radiation therapy
Radiotherapy is effective in reducing metastatic bone pain and, in some instances, causing tumor shrinkage or growth inhibition.8,19–21 There is, as yet, no consensus regarding the most appropriate way of delivering radiotherapy for metastatic bone pain. The practice differs significantly between countries and, indeed, between treatment centers within the same country.
One of the controversies is whether single fraction radiotherapy is as effective as multifraction radiotherapy. Single fraction radiotherapy is more convenient for the patient, and it is also less costly compared with multifraction radiotherapy. However, there are some important concerns relating to single fraction therapy. The equivalent biological dose of single fraction treatment is usually smaller than the total multifraction treatment dose. As a result, the pain response to a single dose may be inferior to the multifraction radiotherapy response. Even if the initial pain response is similar, it may not be durable enough to ensure that the patient remains asymptomatic. In addition, with a potentially reduced tumoricidal effect, single fraction radiotherapy may not be as effective in preventing complications such as pathological fractures and SCC. Systematic reviews of randomized studies examining the effectiveness of single fraction radiotherapy versus multiple fraction radiotherapy pooled the results and used meta-analysis to estimate the effect of treatment on pain response, retreatment rate, and complications.8,19 There was no difference between the two radiotherapy schedules for both overall and complete pain response rates; however, patients treated by single fraction radiotherapy had a higher retreatment rate and a higher rate of pathological fractures compared with patients treated by multifraction schedule. The SCC rates were similar for both arms of the treatment schedules, although there was a trend of increasing SCC rates for patients treated by single fraction radiotherapy. Based on the above analysis of the two treatment schedules, we recommend multifraction radiotherapy for most vertebral metastases except in those patients whose life expectancy is considered to be extremely short, in which case a single fraction schedule may be appropriate for immediate pain control. The treatment schedule should be tailored for each patient based on the type of palliative therapy required and the complications that may yet develop.
Bisphosphonates
These agents work by several different mechanisms to reduce both bone resorption and bone formation.22,23 Bisphosphonates can be divided into two groups: those resembling pyrophosphate (for example, clodronate and etidronate), and the aminobisphosphonates (for example, pamidronate and zoledronic acid). The latter group inhibits enzymes of the mevalonate pathway, disrupting the signaling function of key regulatory proteins. The net effect in both groups is inhibition of osteoclast function, which leads to a decrease in bone resorption. Systematic review showed that bisphosphonates significantly decreased skeletal morbidity, and reduced the probability of vertebral fracture.3,4,9 However, the incidence of SCC was not reduced. This may be related to the fact that studies examining skeletal events were insufficiently powered to show a difference between bisphosphonates and controls for SCC, which is a relatively rare skeletal event. Based on the systematic review, recommendations are that treatment with bisphosphonate medications should start when bone metastases are diagnosed and continue until no longer clinically relevant.3
Vertebral metastases with biomechanical potential or overt instability
Vertebral metastases that lead to biomechanical spine instability, or potential instability, produce a range of symptoms and signs that should direct therapeutic consideration. Patients with localized kyphosis, collapsed vertebra, fracture-dislocation, retropulsion of a bone fragment, or segmental instability may require various surgical procedures tailored to meet decompression, stabilization, or pain relief requirements, depending on symptomatology (Figure 14-2). However, not every case defined by the criteria for unstable spine requires surgical intervention. If the tumor is relatively radiosensitive, radiotherapy may result in satisfactory axial settling and pain relief over a period of weeks or months (Figure 14-2). These patients should be carefully followed because they are still potentially unstable. Surgical procedures are reserved for symptomatic cases, because preventive surgery is probably unjustified in the management of metastatic disease of the spine.
Recommendations for patient selection and optimal methods of decompression and stabilization are constantly evolving. The criteria for spinal instability that should lead to consideration of spinal stabilization are summarized in Table 14-2.
TABLE 14-2 Categories of Spinal Instability in Metastatic Disease that Require Consideration for Spinal Fixation*
* Definition of potential instability relies on imaging studies and is valid only if the vertebral cortical shell is involved.
VERTEBRAL COMPRESSION FRACTURE IN CANCER PATIENTS
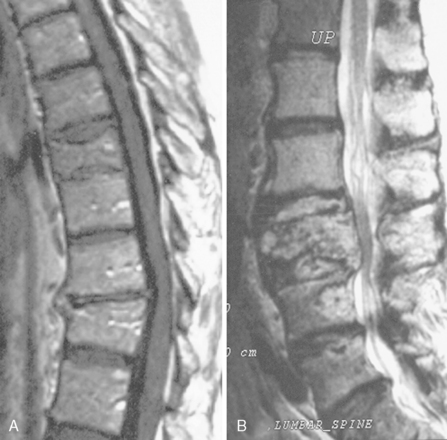
Acute vertebral compression fractures are common and may occur because of osteoporosis, neoplastic infiltration of the vertebral body, or trauma (Figure 14-3). Patients may present with osteoporotic fracture secondary to various precipitating factors such as age, steroid therapy, immobility, and prolonged use of low molecular weight heparin. Neoplastic vertebral compression is a common occurrence in both hematological malignancies and solid tumors.13,24–26
The differential diagnosis may be difficult. Imaging findings are suggestive, but often inconclusive. It has been reported, for example, that osteoporotic fractures may accumulate FDG to varying degrees, and false-positive findings may occur when FDG-PET imaging is performed to assess whether metastases are present.12,27 Accurate differentiation of simple osteoporotic fracture from pathological fracture often requires the integration of imaging findings (PET/CT combined technique and MRI) with clinical assessment. MRI findings may be useful (Figure 14-3). A convex posterior border of the vertebral body, abnormal signal intensity of the pedicle or posterior elements, epidural mass, focal paraspinal mass, and other spinal metastases are suggestive of metastatic compression fractures. In contrast, findings suggestive of osteoporotic compression fractures are: a low signal-intensity band on T1-weighted and T2-weighted images, spared normal bone marrow signal intensity of the vertebral body, retropulsion of a posterior bone fragment, multiple compression fractures, and fluid collection (the fluid sign) that results from bone marrow edema.24,25
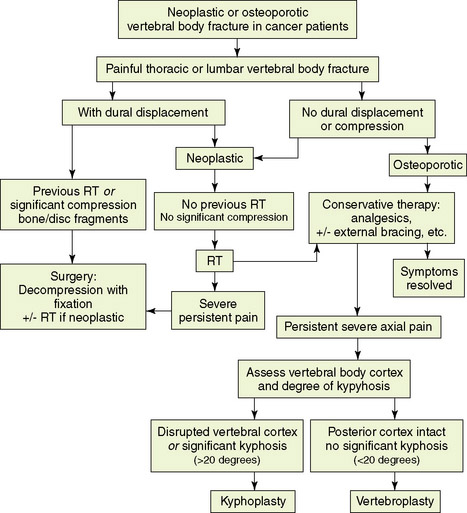
Treatment options for vertebral compression fracture (Figure 14-2)
Vertebral compression fracture is associated with severe pain and is frequently the cause of immobility. A multidisciplinary approach to patient selection and management is essential (Figure 14-2).Once vertebral body fracture is diagnosed, it is important to evaluate whether dural displacement or significant neural compression is present. In cases with significant compression of neural elements secondary to impingement of bone, disc fragments, or tumor mass, early consideration of surgical decompression combined with stabilization should take place. In neoplastic vertebral fractures where dural displacement is present but compression is not significant, radiotherapy followed by conservative therapy can form the initial approach. Conservative treatment of thoracic or lumbar vertebral fracture includes analgesic medications with or without external bracing and encouragement to maintain weight-bearing activities. Radiotherapy is also added to metastatic vertebral fractures that are not associated with dural displacement. None of these latter modalities is uniformly effective in relieving pain or improving ambulatory status.
For patients who fail to improve with conservative management and have no evidence of cord or thecal sac compression, a minimally-invasive procedure that involves percutaneous injection of polymethylmethacrylate (PMMA), a surgical bone cement, under imaging guidance can be offered.28,29 Precise indications for these techniques are still evolving.
The goal of vertebroplasty is to provide pain relief and bone strengthening in painful vertebral body compression fracture. Selected patients with focal, intense, and intractable midline spinal pain at the level of the fracture, or within two vertebral levels below it, and who failed conservative management, are candidates for this minimally-invasive procedure. Vertebroplasty does not re-expand a collapsed vertebra, but it provides a certain degree of reinforcement and stabilization of the fracture, which alleviates pain. The procedure is relatively contraindicated for patients with evidence of dural displacement or compression,30 and suitable for patients with no radicular signs. Contraindications to the procedure include bleeding disorder and unstable fracture due to posterior element involvement. Procedural complications are relatively rare; most are related to PMMA leakage through cortical defects.29–32 Cement leakage rates range from 9% to 88%, and, in most cases, leakage is asymptomatic. Neural element compression resulting from cement extravasation remains a rare event.
Current experience with vertebroplasty and kyphoplasty for painful vertebral fractures in cancer patients remains limited, but favorable results can be obtained with careful selection of appropriate patients.28–30,33,34 Marked or complete pain relief is reported in 70% to 84% of procedures performed in neoplastic vertebral fractures, and the rate exceeds 90% in osteoporotic fractures. No change in the level of pain is reported in about 9% of neoplastic fractures. Pain relief and increased mobility are expected within 24 hours postprocedure, but occasionally these responses occur after a few days. As a result, analgesic consumption is also significantly reduced in the majority of treated patients.29 Based on current knowledge, these procedures can be applied with a good safety profile in well-selected patients with osteoporotic fractures and in patients with multiple myeloma or metastatic cancer who have refractory spinal pain.
Vertebral Metastases with Epidural Extension
Sixty percent of vertebral metastases associated with epidural extension in adults are related to breast, lung, and prostate cancer.1,35–38 Other common neoplasms include lymphoma, renal cancer, sarcoma, and multiple myeloma. The origin of metastases cannot be identified in 7% to 12% of cases.13,37,39,40 All patients with vertebral metastases are at potential risk of developing compression of the neural roots, cauda equina, and/or the spinal cord. Epidural metastases are the most common cause of spinal cord and cauda equina dysfunction in cancer patients. The term SCC is used in this chapter to include cauda equina compression, unless otherwise noted.
FREQUENCY
The incidence of SCC is unknown. Retrospective clinical studies suggest that 2% to 20% of patients with vertebral metastases develop myelopathy secondary to SCC.10,37,41 A recent population-based study of neoplastic SCC in Ontario found that the cumulative probability of experiencing at least one episode of SCC in the last five years preceding death from cancer was 2.5% and ranged from 0.2% in cancer of the pancreas to 7.9% in myeloma.37 An autopsy study estimated that 5% of cancer patients develop spinal epidural tumor deposits,42 but a proportion may remain clinically silent. SCC may present as the initial manifestation of malignancy in about 8% to 20% of patients with symptomatic epidural deposits.43,44 Carcinoma of the lung, cancer of unknown primary site, multiple myeloma, and non-Hodgkin lymphoma are disproportionately represented (accounting for 78% of episodes) in a series of SCC occurring as initial manifestation of malignancy. These primary tumors account for only 26% of SCC in patients with a previously-established diagnosis of malignancy.
The incidence of SCC secondary to lymphoma, breast, and prostate cancer has decreased over the years.45 This declining incidence probably reflects a shift in oncological treatment policies towards the early use of radiotherapy, or more effective and earlier treatment of the primary tumor reducing the rate of metastatic complications. The routine use of advanced imaging techniques (CT, PET/CT, and MRI) also contributes to early detection and treatment of clinically silent spinal metastases.1,2,12,16,39,40 Imaging of the whole spine is feasible with these techniques. When performed, for example, in patients with clinical symptomatology suggestive of SCC, whole spine images often reveal that two or more levels of compression are present, affecting more than one region of the spine. Multiple levels of neural element compression are detected in 25% to 43% of suspected SCC.2,16,39,40,46
LOCATION OF EPIDURAL TUMOR IN RELATION TO THE SPINAL CORD AND THE YIELD OF DIAGNOSTIC IMAGING
Epidural SCC usually results from metastases to one of three sites: the vertebrae, the paravertebral tissue, or the epidural space itself. Extension of the tumor into the spinal canal may produce variable involvement of the anterior (ventral) compartment, lateral gutters, posterior compartment, or any combination of these sites (Figure 14-4).
Spinal radiography is predictive of epidural disease,47 and an epidural mass is identified at 86% of symptomatic spinal segments. However, radiographic results require 30% to 70% bony destruction before they become positive. An MRI study demonstrated that, although an absent pedicle is often the first radiographic sign of metastatic disease, the pedicle is involved by direct extension from either the vertebral body or the posterior elements, and is therefore a late occurrence in the disease process.48 The region of the vertebral column that is most often involved is the vertebral body, probably because of its extensive vascular supply; thus, most epidural tumors arise in a vertebral body and invade the epidural space anteriorly.16,49 MRI evaluation of neoplastic SCC showed that metastatic disease was present in the bodies of all 160 vertebral levels that were studied.16 Assessment of vertebral quadrants’ (one anterior, two laterals and one posterior column) involvement showed that 97% of the vertebrae had more than 50% involvement. Four columns were affected in 52% and three columns in 30%. Single column involvement was seen only in the anterior column, and accounted for 3% of affected vertebrae. Coexisting anterior and posterior column disease was observed in 75%. The extensive vertebral involvement associated with SCC suggests that any attempt to perform decompressive surgery should always be combined with measures to stabilize the spine.
Normal spinal roentgenograms, seen in 6% of SCC, do not exclude epidural metastases. Normal radiographs are found in 89% of lymphoma patients with epidural compression,45 in whom a paravertebral tumor mass invades the epidural space through the intervertebral foramina, rather than via vertebral extension (Figure 14-4). This mode of epidural invasion is seen in lymphomas, renal cell cancer, superior sulcus tumors (Pancoast syndrome), and neuroblastoma, and accounts for approximately 10% of all SCC.45 Up to 36% of patients with paraspinal tumor have epidural metastases on myelography.50 With the advent of CT scanning and MRI, which adequately demonstrate paravertebral soft tissues, these lesions are being more frequently recognized. A paraspinal mass is detected by MRI at the site of SCC in 28% of patients, and only one third of the masses were detected on plain radiography.40 Pure, exclusively epidural lesions are rare, and their incidence is not known. In a consecutive series of 100 patients with SCC evaluated by MRI, no isolated epidural lesion was noted.16
The location of the extradural metastases within the vertebral canal has important surgical implications. Accurate definition of the epidural mass as posterior, lateral, cuff, or anterior (Figure 14-4) requires CT-myelography or a noninvasive MRI study. MRI is the procedure of choice to evaluate the vertebral column, spinal cord, and soft tissue parts, and usually eliminates the need for other imaging studies.39,51–53 It is the imaging method of choice both for screening and for ultimate diagnosis of SCC whenever it is readily available.2,16,39,40,54,55 Still, spinal CT is an excellent screening method to diagnose patients at risk, and to verify presence of an epidural mass in an emergency setting.1
In a study of 342 episodes of suspected neoplastic SCC evaluated with spinal CT, additional MRI assessment was required due to diagnostic uncertainty in only 5%.1 However, MRI has a broader impact on management. It detects unexpected vertebral and epidural lesions that lead to a change in treatment in up to 50% of patients.2,16,39,54,55 Thus, when SCC is suspected, a complete spine evaluation is indicated, and MRI provides significant economic benefits compared with other imaging modalities used for evaluation.56
SYMPTOMS AND SIGNS
Onset of SCC symptoms may be acute or insidious, and symptom duration varies widely. Pain is the initial symptom in 96% of cases, preceding other symptoms by approximately 2 to 3 months.35,57 Pain can be localized close to the site of the lesion or can be radicular. Pain generally results from nerve root compression or infiltration, compression fractures, segmental instability, displacement of the dura, or dural invasion. Therefore, all patients with symptomatic spinal metastases must be considered at risk for SCC. The site of pain may not correspond to the site of epidural compression on imaging,35 and it may be nonspecific or referred to other sites, frequently leading to a delay in diagnosis.35,57 This is especially true in patients without a previous history of cancer.
At the time of diagnosis, neurological signs are common,35,45,51,57 and include various degrees of muscle weakness in 76%, bladder and bowel dysfunction in over 50%, and sensory deficit in about 50% of patients with SCC. However, weakness and sensory abnormalities are often reported late and identified even later, despite patients having reported pain for a considerable time. Nearly half of severely affected patients develop a complete deficit (no residual spinal cord function) after diagnosis and before undergoing any treatment. Of the paraparetic patients, 28% become paraplegic in less than 24 hours and before initiation of treatment.58 SCC should therefore be treated as soon as possible, and early diagnosis is crucial, as functional outcome largely depends on the neurological function before treatment.45,58–60
Apart from the typical manifestations of SCC, unusual clinical presentations may be seen. These includes atypical facial pain and numbness, Brown-Séquard syndrome, ataxia secondary to posterior column dysfunction, or herpetic rash along the affected dermatomes.45,51,61
PATHOPHYSIOLOGY
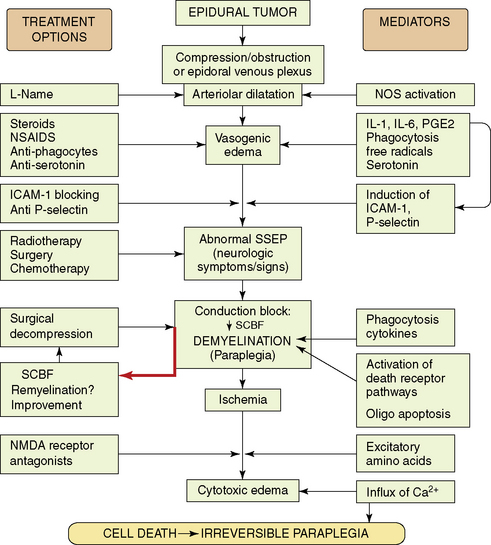
The mechanisms that determine the degree of irreversible tissue damage are poorly understood, but appear to be associated with a neurochemical cascade activated by the initial event of compressive injury. Comprehension of the secondary autodestructive processes has increased with the use of well-characterized animal models.62–76 These laboratory studies demonstrate that pharmacologic treatments modify neurochemical changes, attenuate spinal cord edema, ameliorate structural destruction, and significantly delay neurologic deterioration, even if the compressing tumor is not removed. Figure 14-5 summarizes the paradigm of secondary events in neoplastic SCC, and the pharmacological strategies used to ameliorate them in experimental animal models.
The mechanism of injury induced by the expanding extradural tumors is complex and multifactorial. The extradural tumor causes early obstruction of the epidural venous plexus, and also induces arteriolar dilatation via local spinal autoregulatory mechanisms such as activation of endothelial NO synthase.64,65,77 These changes in vascular tone and drainage enhance production of a vasogenic type of edema. With increase of edema and mechanical pressure, a decrease in spinal cord blood flow at the site of compression eventually follows. Ischemia may then play the final deleterious role, leading to cell death if compression is not promptly alleviated. In animal models, development of conduction block and neurologic signs of myelopathy were related to myelin destruction,76 which was probably caused by both mechanical compression and ischemia, and by activation of death receptor pathways associated with oligodendroglial apoptosis.78 Although demyelination can occur at sites of spinal compression,79,80 remyelination may take place after transient compression,81 possibly correlating with recovery of function after prompt decompression.
Local production of cytokines such as prostaglandins, interleukin (IL)-1 and IL-6, may promote an inflammatory response with associated physiological changes of vasodilation, plasma exudation, and edema formation.75,82 In keeping with this concept, a rapid antiedema effect is achieved by steroidal or nonsteroidal antiinflammatory drugs (e.g., indomethacin),67,69,70 or by inhibitors of phagocytic activity.75,82 It was found that IL-1 induces upregulation of adhesion molecules, such as P-selectin and ICAM-1. Blockage of this process resulted in white matter preservation and improved neurological outcome.83,84
Other studies looked at the microglia and phagocytic activity. Immunohistochemical studies showed that in tumor-bearing paraplegic rats, the normal population of resting microglia was replaced by activated amoeboid cells, probably engaged in phagocytosis.75 At onset of paraplegia, marked disruption of normal neurofilament cytoarchitecture was evident. In vivo pharmacological inhibition of phagocytosis (using chloroquine and colchicines) was associated with a reduced ratio of amoeboid microglia, marked preservation of neurofilament structure, diminished synthesis of cytokines (PGE2, IL-1, IL-6), and significant attenuation in spinal cord edema. Initiating this treatment at first sign of neurologic dysfunction significantly delayed the onset of paraplegia, and protracted the course of neurological deterioration toward paraplegia.
These results suggest that inhibition of phagocytosis may delay structural damage, and thus enhance the chance of recovery following antitumor therapy. It provides the scientific background for the clinical use of steroids, and it also explains the favorable neurological outcome in patients with SCC who are treated by radiotherapy and receive high-dose dexamethasone.85 Although dexamethasone is incapable of blocking phagocytosis, it does inhibit inflammatory responses and production of some cytokines that play a role in the phagocytic cascade.
A marked increase in serotonin utilization is present in the compressed cord segments. Inhibition of serotonin receptors results in attenuated vascular permeability and a protracted clinical course toward paraplegia, similar to the favorable effect produced by antiinflammatory agents.73–75,82 Receptor-activated serotonergic mechanisms that are distinct from the mechanism associated with the inflammatory response probably participate in the disruption of the blood-spinal cord barrier in the subacutely developing compression injury. These mechanisms can be separately manipulated pharmacologically to yield measurable effects in experimental models. Finally, in the end stage, when conduction block and ischemia set in, excitotoxins (such as glutamate) mediate the evolution of cytotoxic edema that adds its deleterious role.71,72
SURVIVAL AND SPINAL CORD COMPRESSION
The magnitude of the clinical problem of SCC is usually underestimated. The best evidence suggests that approximately 5% of patients dying of cancer have suffered from epidural metastases.37,42 This calculated incidence exceeds the annual rate of traumatic spinal cord injury. However, because of the high mortality among these patients (50% to 70% at one year), the socioeconomic impact of neoplastic SCC is less than traumatic injury. The overall median survival following the first episode of SCC is short, ranging from 3 to 4 months.36,37,86 As a result, neoplastic SCC accounts for only 10% to 14% of all spinal cord injury admissions to rehabilitation units.87 It was demonstrated that pretreatment ambulatory function is the main determinant for posttreatment gait function, and the survival of nonambulatory patients can only be improved with restoration of gait function by immediate treatment.36,38,88,89
In spite of the poor prognosis of the disease, most patients should receive active treatment36,51,90 to preserve or restore mobility and continence, and to alleviate intractable pain. SCC in itself is usually not the direct cause of death, except when it occurs in the upper portion of the cervical spine. Survival in SCC is related to the natural history of the systemic malignancy. Only about 30% of patients are expected to survive beyond 1 year, and rarely as long as 4 to 9 years.43,91–93
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree