Spine Tumors
The classification of spinal tumors is based on their anatomical origin and extension, specifically with relation to the spinal cord (intra- or extramedullary) and dura (extra- or intradural). This chapter focuses exclusively on pediatric extradural tumors involving the spinal column. These tumors may arise from osseous or paravertebral structures and may be primary or metastatic. Spinal column tumors are rare in children and young adults, accounting for fewer than 1% of all spine and spinal cord tumors combined. The clinical symptoms, usually pain, allow early diagnostic consideration and evaluation. This chapter focuses on the more commonly encountered benign and malignant conditions, with an emphasis on diagnostic and treatment paradigms.
47.1 Epidemiology
Considered as a group, intramedullary, intradural extramedullary, and extradural spinal tumors account for 5 to 10% of all pediatric tumors of the central nervous system. Extradural primary or metastatic tumors account for approximately 50% of cases, followed by intradural intramedullary tumors (40%) and intradural extramedullary tumors (10%).1–3 By comparison, pediatric brain tumors are six to seven times more common than pediatric spinal tumors. Extramedullary tumors are equally distributed throughout the mobile spine; sacral involvement is less common, except in the case of giant cell tumors and chordomas.
The primary tumors emphasized in this chapter include osseous tumors (osteoid osteoma, osteoblastoma, osteochondroma and osteogenic sarcoma, aneurysmal bone cyst [ABC], and eosinophilic granuloma). Malignant tumors (neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, and chordoma) and metastatic tumors arel also included here.
47.2 Clinical Symptoms
Spinal tumors in children may present with symptoms due to osseous or neural involvement, which causes pain, neurologic deficit, and/or spinal deformity. Most commonly, pediatric patients present with axial or radicular pain. The onset of symptoms may be acute in the case of vertebral collapse or epidural spinal cord compression. Slow-growing tumors may result in an insidious progression of symptoms. Persistent back pain, particularly when nocturnal, in a child should prompt diagnostic evaluation.4–6
The symptoms of spinal tumors in children are age- and location-dependent. Tumors causing compression of the spinal cord may produce upper motor neuron signs: weakness, hypertonia, hyperreflexia, clonus, Babinski sign, and sensory deficits. Lower motor neuron findings, including weakness, hypotonia, hyporeflexia, and sphincter dysfunction, may predominate in children with tumors involving the lumbar and sacral segments. The presentation of spinal tumors in infants and toddlers may be subtle, with irritability and regression of motor milestones.5
Spinal deformity, including kyphosis, scoliosis, and lordosis, occurs in up to 25% of children with spinal tumors and rarely is the sole presenting sign of a spinal tumor.5,7,8 The severity of spinal deformity generally relates to the extent of osseous destruction and neurologic deficit, although occasionally, a small tumor may cause a significant deformity.4,5,9 Spinal deformity may also develop after the treatment of spinal tumors; multilevel laminectomy and radiation therapy significantly increase the risk for deformity.10,11
47.3 Diagnostic Imaging
Modern imaging permits a detailed anatomical visualization of the spinal and paravertebral structures, often allowing an accurate and concise differential diagnosis. Plain radiographs, often the initial study, can identify lytic or blastic lesions with a high rate of sensitivity.8 Additionally, spinal alignment can be accessed and the degree of scoliosis, if present, quantified.8,9 Computed tomography (CT) provides further detail and may reveal abnormalities not demonstrated on plain radiographs. Three-dimensional reconstruction of CT scans may also be useful for surgical planning. Magnetic resonance (MR) imaging adds anatomical detail of soft tissue and neural structures, demonstrating edema or compression of the spinal cord or nerve roots. Technetium bone scan may be helpful in selected cases, particularly when symptoms persist and the results of alternative imaging modalities are negative or equivocal.12,13 Spinal angiography is rarely indicated, although endovascular interventional techniques may be an important adjunct in the treatment of highly vascular lesions, such as ABCs and giant cell tumors.14–17
47.4 Treatment
The goals of treatment of spinal tumors are histologic diagnosis, complete tumor removal, and improvement or preservation of neurologic function, in addition to the correction and preservation of vertebral alignment. The treatment of choice for patients with symptomatic spinal cord compression is surgical decompression,6 although specific tumors, such as neuroblastomas, may be appropriately treated with primary radiation or chemotherapy.18–21 Corticosteroids may be given in anticipation of definitive treatment, although no specific agent or regimen has demonstrated superiority.5
47.4.1 Biopsy
Biopsy may be an appropriate initial procedure; CT-guided or fluoroscopically guided and open procedures have been described. CT-guided biopsy may be technically difficult with small, firm tumors and those in contact with neural or vascular structures. Additional concerns include seeding of tumor cells along the biopsy tract, although the risk for this often-cited complication may in fact be overestimated.22 Techniques including transpedicular approaches with closure of the biopsy tract with methylmethacrylate or hydroxyapatite may reduce the risk for tumor seeding.23 Given the small amount of material obtained by needle or trocar, there is a possibility of nondiagnostic tissue or sampling error. Although needle biopsy of spinal lesions has become widely used, the potential for nondiagnostic tissue or misdiagnosis is relatively high (18%) in the case of benign primary bone tumors.24
Open biopsy may be performed by a transpedicular approach, laminectomy, or costotransversectomy. Laminectomy may be appropriate for lesions in the epidural space. However, for lesions of the vertebral body without epidural extension, laminectomy may result in the spillage of tumor cells into the epidural space.25 The incision used for biopsy should be planned such that it can be included within resection margins should en bloc or wide excision be required.
Biopsy may be unnecessary in cases of suspected metastasis from a known primary tumor, or those in which the imaging characteristics are highly suggestive of a specific tumor type for which an initial attempt at complete resection is appropriate, such as ABC or osteoid osteoma.4,7,15
Oncologic Staging
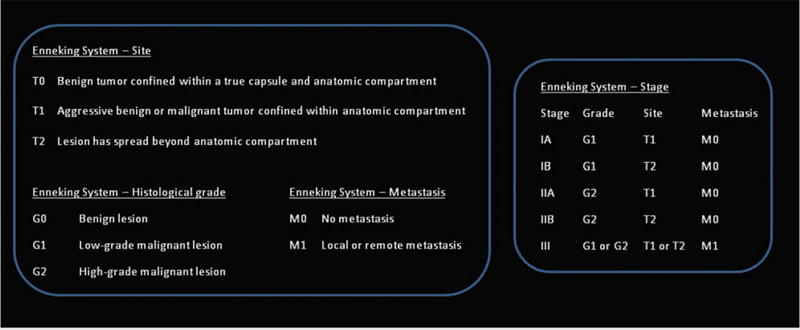
Boriani et al23 emphasized the importance of oncologic staging in spinal bone tumors to evaluate accurately the relationships of histology, management, and outcome. They applied and expanded the principles of the Enneking system (▶ Fig. 47.1) for classifying the stages of musculoskeletal tumors to the spine.23,25 The Enneking staging system divides benign tumors into three stages (S1, S2, and S3) and local malignant tumors into four stages (IA, IB, IIA, and IIB). Two additional stages include metastatic high-grade intra- and extracompartmental tumors (IIIA and IIIB).23,25 This grading system incorporates clinical characteristics, radiographic features on CT, MR imaging and isotope scanning, and histologic diagnosis.
The first stage of benign tumors is S1 (latent, inactive), during which tumors do not grow, or do so very slowly. These tumors have well-defined margins and are contained within a true capsule and do not extend beyond the anatomical compartment of origin. Surgery is indicated in cases of neural compression or instability.26–28 S2 tumors are benign or active and grow slowly; they are bordered by a thin capsule and a layer of reactive tissue. The results of technetium Tc 99 pertechnetate bone scans are often positive, depending on the presence of osteoblastic activity, which is associated with deposition of tracer. These lesions may be treated with en bloc resection or intralesional excision, both of which have reported low recurrence rates.15,29–32 The recurrence rate is further lowered by the addition of cryotherapy, embolization, or radiation therapy in appropriate cases.23 S3 tumors are aggressive, rapidly growing benign tumors with or without a thin or incomplete capsule and have the potential to invade adjacent structures, creating a wide, reactive, and highly vascular pseudocapsule. Appropriate treatment for S3 lesions is en bloc resection or intralesional excision followed by adjuvant therapy. Despite aggressive measures, recurrence is common.8,23,33,34
Low-grade malignant tumors, stages IA and IB, have no true capsule, are surrounded by a wide reactive pseudocapsule, and should be treated by en bloc resection and adjuvant radiotherapy. Stages IA and IB are differentiated by containment of the tumor within the vertebral body (type IA) versus invasion of the paravertebral compartments (type IB). High-grade malignant tumors, stages IIA and IIB, grow rapidly; there is no pseudocapsule, leading to the formation of local tumor nodules, and the continuous seeding of neoplastic cells results in distant seeding. These tumors often result in pathologic fractures. The favored surgical approach includes wide en bloc resection, if possible, followed by radiation and chemotherapy according to tumor type.2,33,35–40 Stages IIIA and IIIB are differentiated from stage II tumors by the presence of distant metastases.
Surgical Staging
The first proposed staging system for spinal bone tumors was described by Weinstein and McLain8 and was subsequently revised by a team at the Rizzoli Institute in Bologna.23,25 The resulting classification, the Weinstein, Boriani, Biagini surgical staging system (▶ Fig. 47.2), uses location of the tumor within the vertebral column and bases the surgical approach on specific anatomical factors. Each vertebra is divided into 12 transverse radiating zones and 5 concentric layers, beginning in the paravertebral extraosseous region and extending to the dura. Tumor location is further categorized within longitudinal zones based on the number of levels involved. The oncologic and surgical staging of each tumor affords a plan for resection and adjuvant therapy based on the specific characteristics of each case.
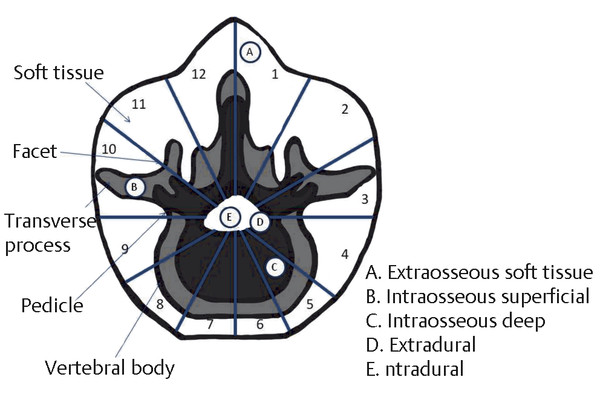
Fig. 47.2 The Weinstein, Boriani, Biagini surgical staging system. (After Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine [Phila Pa 1976] 1997;22(9):1036–1044.23)
47.4.2 Indications for Surgical Treatment
Indications for surgical treatment include the preservation of neurologic function and the restoration or maintenance of mechanical stability.6–,7,8 For many benign pathological processes (e.g., ABC or osteoid osteoma), resection may be curative.4,15,27,41,42 Malignancies involving the pediatric spine (e.g., neuroblastoma and rhabdomyosarcoma)10,19,43 usually require surgical intervention, most often as a key element of a multidisciplinary approach. Surgical management may include biopsy, tumor resection, and spinal stabilization.
47.4.3 Surgical Treatment
For cases in which it is deemed appropriate, en bloc resection remains the operative goal. Three basic approaches for en bloc excision, based on anatomical considerations, have been described: vertebrectomy, sagittal resection, and posterior arch resection.7,8,23,25
Vertebrectomy is indicated if the tumor is confined to the vertebral body and at least one pedicle is spared (zones 4–8 or 5–9). Vertebrectomy begins with a posterior approach and excision of the posterior elements at the involved level, with stabilization, followed by an anterior approach for vertebrectomy and anterior reconstruction. Depending on the involved spinal level, anterior resection may be performed by an anterior cervical approach, a transpleural thoracotomy, or a thoracoabdominal or retroperitoneal abdominal approach.23
Sagittal resection is indicated when the tumor involves the vertebral body eccentrically, including the pedicle or transverse process (zones 3–8 or 8–10). Sagittal resection includes a posterior approach for excision of the posterior elements and pedicle, followed by an anterior approach for partial vertebrectomy and the completion of pedicle and transverse process removal.23
Resection of the posterior arch alone is indicated when the tumor is located exclusively in the posterior elements (zones 10–3). Surgery includes a wide laminectomy and exposure of the dura above and below the involved levels, with extension laterally to the pedicles.23
Instrumentation and stabilization, when required in children, should take into consideration the location and extent of initial tumor involvement, the spinal levels, and anterior/posterior element deficiency. Future growth capacity and the potential for asymmetric growth and progressive deformity are additional considerations in the pediatric population. Removal of more than two laminae and facetectomy both significantly increase the incidence of postoperative deformity.11 As a general principle, the fewest number of levels possible should being included in the construct. Asymmetric fusion should be avoided, and the use of nonbiological interpositional materials (methylmethacrylate) and growth factors, such as recombinant bone morphogenic protein (rBMP), should be cautiously considered.7,23
47.4.4 Managing Spinal Deformity and Instability after Treatment
The risk for instability and progressive deformity after treatment of spinal column tumors is significant. Reported rates of instability after pediatric spinal tumor resection range from 24 to 100%.5,8,9,11,45,46 This may result from bone destruction by the tumor, resection of the tumor or extensive bone removal, neurologic impairment caused by the tumor or its treatment, or postoperative irradiation.47 Although the extent of bone removal correlates with deformity, the extent or safety of tumor resection should not be compromised.
The surgical approach itself may result in immediate instability, particularly if multiple spinal levels are involved or if facetectomy is required. Patients with tumors involving more than one spinal column are at significant risk for immediate instability. In cases of immediate spinal instability, instrumentation at the time of initial surgery is indicated. Cases not requiring immediate postoperative stabilization may nonetheless develop instability and progressive deformity. The risk for delayed instability is higher in younger patients and in those undergoing multilevel laminectomy in the cervical or thoracic region.47 Radiation therapy significantly elevates the risk for delayed deformity, with a reported incidence from 10 to 100%.47 The degree of deformity has been shown to relate to the initial radiation dose and the duration of follow-up.45
Numerous techniques have been suggested to mitigate the risk for postoperative deformity: limiting the number of laminectomies performed, avoiding facetectomy, immediate rather than delayed spinal instrumentation, preservation of the intraspinous ligament, laminoplasty, posterior lateral fusion, and prophylactic orthotic treatment.11,47–47 Laminoplasty has been advocated as a technique for minimizing postlaminectomy kyphotic deformity; however, some authors suggest that the rate of deformity is not decreased.46 Replacement of lamina (not infiltrated by tumor) after resection restores normal anatomy and may facilitate reoperation and/or subsequent fusion if required.
Surgical indications for treatment of postsurgical or postirradiation spinal deformity include the degree and rate of progression, pain, and neurologic impairment. Otsuka et al47 advocate posterior instrumentation and fusion for cases of moderately severe but flexible kyphosis. Cases of severe and/or rigid kyphosis are treated with a combined anterior release or decompression and fusion augmented by posterior instrumentation and fusion. They reported no pseudoarthrosis and an average kyphosis regression of 5% during follow-up. They reported that bracing was ineffective in preventing the progression of kyphosis in all cases.47
47.5 Primary Bone Tumors
47.5.1 Osteoid Osteoma and Osteoblastoma
Osteoid osteoma and osteoblastoma are benign tumors and account for approximately 3% of all primary bone tumors.50,51 Osteoid osteoma accounts for 1.4% of primary spinal tumors and most commonly occurs in the second decade of life, with a male preponderance.28 Ten percent of osteoid osteomas occur in the spine, with a large majority found in the posterior vertebral elements. Osteoblastoma is less common and has a peak incidence in the third decade of life.50,51 These tumors have a similar histology, with bone formation by osteoblasts producing osteoid and woven bone. They are differentiated primarily by size, intraoperative appearance, and behavior. Osteoblastomas are larger than 1 to 1.5 cm and more vascular, and they are more likely to involve the vertebral body.52 Although exceedingly rare, cases of malignant transformation have been reported.53 Osteoid osteomas typically have a sclerotic pattern, appearing hyperdense and expansile, without bone destruction. On MR imaging, high signal intensity on T2-weighted images of the surrounding muscle and bone is seen. Radiographically, osteoblastomas more commonly have a ground glass appearance on CT. They usually occur in cancellous bone of the lamina or pedicles of the cervical or lumbar spine.
Osteoid osteoma (▶ Fig. 47.3) commonly presents with localized pain, although radicular pain may be present because of nerve root irritation.50 Other clinical findings may include point tenderness, scoliosis,9 and neurologic deficit.51 The pain is often severe and intermittent and is worsened by activity. Often, nocturnal pain relieved by nonsteroidal anti-inflammatory medications or aspirin is noted.50 Osteoblastoma (▶ Fig. 47.4), shares many of the clinical features of osteoid osteoma; however, neurologic deficit is more common because of the larger size of this lesion.28 The onset of symptoms may precede detectable abnormality on plain radiographs or CT. MR imaging and radioisotope bone scans may detect lesions not initially identified on plain radiographs or CT.54,55
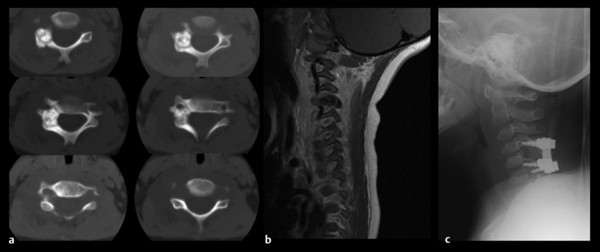
Fig. 47.3 This 5-year-old boy presented with severe dysesthetic pain of the right upper extremity. On examination, he had torticollis and weakness of the right deltoid and bicep. (a) Axial computed tomography shows a sclerotic lesion of the lateral mass and pedicle with a typical “ground glass” appearance, consistent with osteoid osteoma. (b) On sagittal T1-weighted magnetic resonance imaging, the lesion is hypointense. (c) The lesion was resected, and segmental posterior fusion with lateral mass screws was completed.
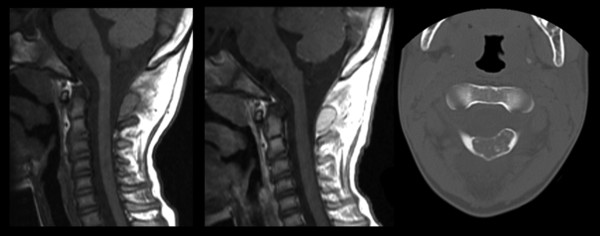
Fig. 47.4 This 17-year-old boy presented with persistent neck pain and torticollis. Osteoblastoma.
(Courtesy of Dr. David Harter and Dr. Sarah Milla.)
Observation alone may be appropriate management for small, intermittently or minimally symptomatic lesions consistent with osteoid osteoma. Spontaneous remission and involution may occur. In patients with persistent pain or neurologic deficit, gross total excision is the surgical goal. Osteoid osteoma appears firm and sclerotic with an occasional fibrotic or granulomatous component, whereas osteoblastoma more typically a friable, hemorrhagic mass, well circumscribed from the normal surrounding bone.
Results of surgery are usually excellent for symptomatic relief and tumor control. However, the risk for local recurrence remains, with most series reporting a recurrence rate of approximately 10%.27,28,51 Intraoperative localization may be challenging, given the small size and sclerotic appearance of these lesions. Techniques, including preoperative CT-guided dye injection or guidewire placement and intraoperative radioisotope bone scanning, have been described.56,57 Although the estimated risk for radiation-induced malignancy is low (< 1%),58 reoperation rather than radiotherapy is recommended for recurrent or residual osteoid osteomas.27,50,51,53,55 CT-guided radiofrequency ablation and other techniques have also been described for the “deactivation” of osteoid osteoma with good results.59,60
47.5.2 Osteochondroma and Osteosarcoma
Osteochondromas (▶ Fig. 47.5) account for 30 to 40% of benign osseous tumors and 4% of solitary spine tumors. They may occur as solitary, sporadic lesions or multifocally in the setting of an autosomal-dominant syndrome—hereditary osteochondromatosis. Approximately 1 to 7% of all osteochondromas occur in the spine. Solitary spinal lesions on average occur toward the end of the third decade of life, whereas multiple lesions occur earlier, toward the beginning of the third decade of life.26 There is a significant male preponderance (2.5:1).26,61 Osteochondromas commonly affect the cervical spine, with approximately 50% of cases reported there.26,61,62 Osteochondromas can involve multiple contiguous vertebral levels and encroach upon the spinal canal and neural foramina, resulting in spinal deformity and neurologic impairment.26,61
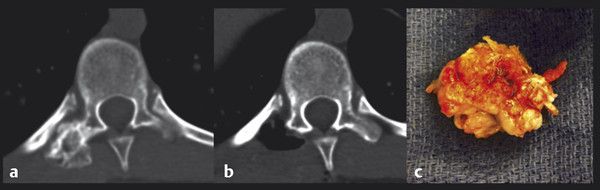
Fig. 47.5 This 15-year-old boy presented with back pain. (a) Axial noncontrast computed tomography demonstrates a lesion originating from the T10 transverse process. (b) The lesion was removed en bloc. (c) Osteochondroma.
Given the cartilaginous quality of these tumors, plain radiographs are often normal. CT and MR imaging are often required for the diagnosis. As for other benign spinal tumors, complete excision is usually curative, with excellent reported neurologic and functional outcomes.
Malignant transformation occurs in approximately 10% of cases, resulting in osteosarcoma. Although they are very uncommon in children, a small number of spinal osteosarcomas have been reported.63,64 The prognosis for spinal osteosarcoma remains poor, even with contemporary, multimodal treatment at experienced centers.65,66 The presence of metastatic disease and the inability to achieve en bloc resection are significant negative prognostic factors.65,66
47.5.3 Aneurysmal Bone Cyst
ABCs occur most commonly in children and adolescents (median age, 10.2 years).67 These expansile, vascular lesions have no known cause, although they have been reported to occur in conjunction with other pathologic conditions, such as giant cell tumor, fibrous dysplasia, and osteoblastoma.41,42,68 Histologically, the lesions often have a hemorrhagic component, with hemosiderin-containing macrophages; other features are multinucleated giant cells, fibrous tissue, and expansion of the cortical margins. Localized pain, worse with activity, is common. Neurologic symptoms occur as the lesion encroaches upon the neural foramina or spinal canal. Pathologic fracture or spinal instability may occur as the normal vertebral elements are replaced.
Radiographically, these lesions appear distinct—expansile, eccentrically located masses with fluid–fluid levels in the central region of trabeculae surrounded by a thin “eggshell” rim of cortical bone. They are often primarily located in the posterior elements, although they may also extend into the pedicle and vertebral body (▶ Fig. 47.6). There may be a significant soft tissue component, with variable enhancement on MR imaging or CT. Radionucleotide bone scan may show areas of peripheral uptake and a central area of hypoactivity within the cystic component. Arteriography often shows a highly vascular lesion with extensive collateral formation. Preoperative embolization, on a case-by-case basis, is advocated by many centers because blood loss may be significant.15,30,31 Embolization should be considered for large ABCs, those that involve multiple vertebral levels, and those with significant ventral extension. Intraoperative fatality from uncontrolled hemorrhage has been described.31 Although biopsy has been performed safely,24,69 the unique radiographic appearance of these lesions often obviates the need for histologic diagnosis before definitive surgical treatment.
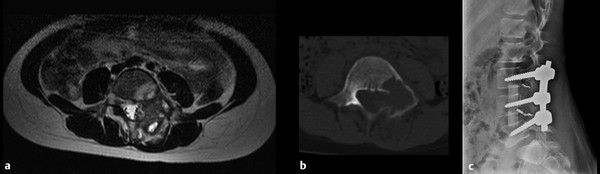
Fig. 47.6 This 8-year-old girl presented with persistent back pain that extended down the left lower extremity. She had paraspinal tenderness and weakness of the left dorsiflexors. (a) T2-weighted magnetic resonance imaging demonstrates a complex mass involving the lamina, pedicle, and vertebral body of L4, consistent with aneurysmal bone cyst. (b) Computed tomographic scan shows a thin rim of cortical bone. Preoperative angiography and embolization were performed. (c) The lesion was removed by a posterior approach, and primary reconstruction was performed with allograft and posterolateral fusion (note coils from embolization). (Courtesy of Dr. Joseph Dryer, New York University.)