Time after injury
Cell type
Route of administration
Reference
24 h
Umbilical cord blood
Intra-arterial
[10]
24 h
Umbilical cord blood
Intraperitoneal
[11]
24 h
Umbilical cord blood
[12]
24 h
Dental pulp stem cells
Intracerebral
[13]
24 h
Umbilical cord blood
Jugular vein
[14]
Mesenchymal stem cells
Striatum
[15]
3 h
Umbilical cord blood
Intraperitoneal
[16]
Umbilical cord blood
Intracerebral
[17]
7 days
Multipotent adult progenitor cells
Hippocampus
[18]
7 days
Multipotent adult progenitor cells
Intravenous
[19]
3 days
Fetal neural stem cells
Cerebral ventricle
[20]
24 h
Mesenchymal stem cells
Intraperitoneal
[21]
The remarkable observation among the above studies (described in more detail below) is that the route of treatment does not seem to affect the generally favorable outcome. Most of the reports do not show significant incorporation of the transplanted cells into the brain substance. Therefore, the beneficial action of the cells seems to occur by neurotrophic or paracrine influences.
In the report of intra-arterial transplantation [10], the transplant did not restore stereological volume in the damaged tissue even though long-term adverse cognitive effects were reduced. This would suggest that there were positive influences of the transplant on remaining intrinsic cells, but not cell replacement.
Huang et al. [11] reported that even the route of intra-peritoneal administration was effective. Once again, the message from this study is the importance of trophic influences.
Wang et al. [12] showed that the cell loss in the CA1 sector of the hippocampus was reduced. The mechanism of cell preservation would also be invoked by these results.
Dental pulp cells only rarely differentiated into neural cells, but neurological function was improved with the inhibition of proinflammatory cytokines, increased expression of anti-inflammatory substances, and reduced apoptosis [13].
De Paula et al. [14] reported the dose dependency of intravenous transplantation. This result suggests that dosing experiments would be an important component of clinical studies.
The cells reported by Part et al. [15] were derived from human placenta. With the striatal transplantation, they theorized that the improved motor behavior was related to dopaminergic differentiation. Similarly, Pimental-Coelho et al. [16] observed a neuroprotective effect was observed in the striatum.
In the work of Xia et al. [17] above, the damage had been inflicted prenatally demonstrating a similar effect to that in the more standard postnatal model.
The two reports by Yasuhara et al. [18, 19] demonstrated positive results using the potentially commercial preparation of MAPCs. The critical outcome from these two papers is that the result was favorable with both direct brain injection and intravenous administration.
Qu et al. [20] used human fetal neural stem cells by direct cerebral ventricle brain injection. They found extensive cell survival and migration, which generally was not noted by other investigators.
Meier et al. [21] reported the intraperitoneal injection of human umbilical cord blood-derived mononuclear cells postnatal day 8 following carotid ligation and hypoxia on day 7. A few of the cells were incorporated into injured brain.
We should point out, however, that at least one report has been negative. DePaula et al. [22] have reported that severe neonatal rodent HI was not altered by intravenous human umbilical cord blood.
Mechanism of Action of Stem Cells in Acute Studies
The mechanism of benefit with stem cell treatment in acute neonatal HI is uncertain, but there are several lines of thinking which are prominent, and all of the proposed mechanisms may play a role in an additive fashion.
The first possible beneficial action is the actual replacement of intrinsic cells by the transplanted stem cells. While a small percentage of stem cells engraft, and some may even take the form of neurons, their numbers do not appear to be adequate to support much enhancement of outcome [19, 23, 24].
The other prominent ideas include blood vessel regeneration, an effect on the spleen to reduce its release of inflammatory cells which might have a deleterious effect in brain, and finally, and most likely, improved survival of intrinsic brain cells promoted by various noncellular factors.
Blood vessel regeneration potentially occurs via adhesion of CXCR4-positive cells onto vascular endothelium [25], recruitment of endothelial progenitor cells [26], and formation of periendothelial vascular cells [27]. Also, crude bone marrow cells have been shown to form endothelial cells [28].
Vendrame et al. [29, 30] proposed that umbilical cord blood may reduce the brain’s inflammatory response by acting on the spleen to reduce its output of inflammatory cells. These splenic inflammatory cells apparently increase the blood–brain barrier permeability, which produces an adverse effect. Walker et al. [31] showed that intravenous injection of MAPCs impeded the splenic response to injury by lessening the spleen’s release of inflammatory cells in association with improved outcome. The work of Leonardo et al. [32] confirmed these findings.
There are numerous mechanisms by which increased intrinsic cell survival might occur. Wang et al. [12] reported that neural stem cell proliferation in the affected tissue may be promoted by the Sonic hedgehog signaling pathway. Rosenkranz et al. [33] noted that umbilical cord cell transplantation decreased the expected rise in pro-inflammatory cytokines (interleukin-1 alpha, interleukin-1beta, and tumor necrosis factor alpha) occurring after HI injury. A marker for activated microglia and macrophages was reduced. Inflammation would thereby be reduced in brain [33]. Bae et al. [34] agreed that paracrine influences were the primary influence on intrinsic cell survival. Human umbilical cord blood cells reduced upregulation of CX43 and thereby brought about attenuation of reactive gliosis [35]. Overall, inflammation was reduced. Rosenkranz et al. [36] found various increased proteins which promoted angiogenesis. Apoptosis was lessened. Vascular endothelial growth factor (VEGF) increased perhaps increasing the proliferation of blood vessels. The same group also reported that human umbilical cord blood cells increased SDF-1 expression acting in concert with the SDF-1/CXCR4 axis [2], which appears to be a major factor for cell homing. In a primate model, Li et al. [37] reported that cell transplantation caused upregulation of IL-10 expression. Neuronal apoptosis was decreased and neuronal proliferation increased. Dayer et al. [38] found that neural progenitor cells increase expression of fibroblast growth factor-2, thereby improving HI outcome. These cells are thought to generate a pool of immature neurons available for repair (Fig. 16.1).
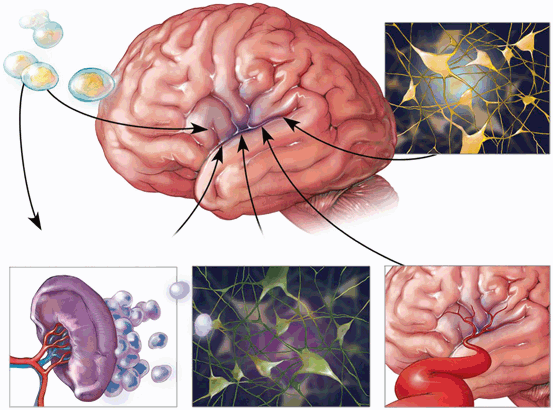

Fig. 16.1
The possible mechanisms of stem cell benefit are shown above. The drawing in the upper right hand corner depicts replacement of damaged cells. In the left lower drawing, the reduction in splenic release of inflammatory cells is shown. In the right lower corner, blood vessel regeneration is shown. In the lower center, the salvage of intrinsic neurons is depicted. (Courtesy of pediatric research)
Summary of Acute Clinical Studies
There are no reports available as yet of controlled, double-blinded trials with stem cells in acute neonatal HI injury.
Luan et al. [39] reported a 75-day-old male who suffered severe HI injury at birth. The infant received transplantation of fetal neural stem cells into the cerebral ventricle. The infant showed improvement. There were no controls noted. In view of the relatively late transplant, it is unclear whether this child should be considered an example of acute or chronic HI injury.
Three trials in progress are listed in ClinicalTrials.gov. These are (1) Cord Blood for Neonatal Hypoxic-Ischemic Encephalopathy at Duke University, (2) Autologous Umbilical Cord Blood Transfusion for Preterm Neonates at Ain Shams University in Cairo, and (3) Autologous Stem Cells in Newborns with Oxygen Deprivation at Hospital Universitario in Monterrey, Mexico. These trials appear to be confined to single institutions.
In any single medical center, there are only relatively small numbers of term infants with acute HI encephalopathy (HIE). Consequently, considerable time will be required to acquire the necessary numbers of subjects from one hospital system. In view of the fact there is ample preclinical laboratory evidence for benefit and a robust, multileveled rationale for the use of adult stem cells in the treatment of acute HIE, it is striking that multicentered, controlled trials have not been initiated. The cost of such a trial will be significant.
Cotton et al. [40] reported the treatment of 23 infants suffering from acute HI injury with intravenous autologous umbilical cord blood. This study demonstrated that, with considerable institutional preparation and coordination, it is feasible to conduct a trial with fresh, autologous umbilical cord blood in acute HI injury in newborns. The effort used to bring about this preliminary trial was considerable.
One of the problems inhibiting such a trial is the lack of a uniform, widely available cell preparation. Although autologous umbilical cord blood could be used for this purpose, there is some variability in the quality of preparation among the samples obtained, which would undoubtedly influence the outcome of the study. A widely available commercial preparation would be advantageous.
Characteristics of Chronic Clinical Cerebral Palsy
As we have noted, CP is a heterogeneous group of motor disorders of childhood. The condition is nonprogressive and may occur as a result of many specific disorders of brain. HI injury in the neonate comprises only a minority of the disorders among children with CP. While CP may manifest as hypotonia, ataxia, hemiparesis, dystonia or spasticity, the form resulting from neonatal HI brain injury is either spastic CP or spastic–dystonic CP. Therefore, in any clinical trial of chronic HI injury CP, the clinical inclusion criteria would need to be confined to spastic or spastic–dystonic CP.
In order to choose the subjects for such a trial the inclusion and exclusion criteria will need to be carefully constructed. The study should be confined to term or near term infants. The criteria composed by the American Congress of Obstetricians and Gynecologists [41] may prove useful. The four critical criteria are: (1) evidence of metabolic acidosis in arterial umbilical cord blood, (2) neonatal encephalopathy in infants born at 34 or more weeks of gestation, (3) CP of the spastic or dyskinetic type, and (4) exclusion of other causes. Other criteria that could be used as confirmatory would include a specific HI event, significant fetal bradycardia with abnormal decelerations, persistently low Apgar, evidence of adverse effect in other organs, and a confirmatory imaging study. Magnetic resonance imaging (MRI) is the best tool for this determination [42]. In the first 24 h, diffusion-weighted images may demonstrate increased signal intensity in central brain regions such as basal ganglia. This finding is seen in so-called severe asphyxia. T1 and T2 weighted images are more conveniently done at around 7 days of age when they diagnostically useful. Less severe or “partial” asphyxia results in a watershed pattern of injury with relative sparing of the basal ganglia.
Animal Models of Chronic HIE
Among the various animal models for CP perhaps the greatest difficulty is producing an injury which is sustained over a significant period of time. Rodents tend to die or recover quickly from brain injuries and consequently their motor manifestations of the injury fade rapidly. A sustained motor disability is essential to the use of the model in the experimental treatment of an injury designed to mimic long-standing brain injury.
Most of the models are variations of the Rice–Vanucci method [43] which impede delivery of oxygen to the animal brain either before or around the time of delivery. In the Rice–Vannucci method, the investigator ligates a unilateral carotid artery and then delivers a period of hypoxia. Variations on this theme have been conducted in rodents, piglets, and fetal sheep [44]. None of these variations are totally satisfactory.
Prenatal HI injury has been produced in the rabbit [45], but the injury is not sufficiently long-lasting to mimic CP in children. The induction of HI injury in prenatal rabbits by uterine ischemia results in motor disability in the animals, but once again the disability is not long lasting, perhaps less than 2 weeks. The motor disability may be assessed with a swim test [46]. The model is also amenable to study with MRI [47].
Maternal administration of lipopolysaccharide to pregnant rats late in gestation results in significant motor impairment of the offspring, but these findings tend to fade by 5 weeks [48]. A similar model has been produced in rabbits [49, 50]. This type of injury, however, does not necessarily mimic term infant HI injury and therefore would not be appropriate for a preclinical study of stem cell therapy for chronic HI injury. The model is more reminiscent of the periventricular leukomalacia of premature infants rather than term infant HI injury.
All these models suffer from the lack of similarity in structure to the human brain. The rodent models are particularly dissimilar in that the white matter is considerably less than in the mammal and differing in structure. The models overall are comparatively short-lived in terms of the motor abnormalities.
Clinical Trials in Chronic HIE
Stem cell therapy is widely proposed as a treatment for CP. Most of these therapies are offered for a fee in countries outside the USA and on a compassionate basis in at least one site in the USA. The treatment, in general, appears to be safe but the efficacy of the treatment is not clear. The problem is compounded considerably by the fact that CP is comprised by many different disorders only one of which (neonatal HI injury) is the subject of this chapter.
We are aware of several reports showing benefit. None of the reports address specifically the issue of the treatment of chronic neonatal HI injury.
Min et al. [51] reported benefit in a controlled study with allogeneic umbilical cord blood. To date, this is best study of the possible benefit of stem cell therapy in CP. In this study, erythropoietin was added to the cells. The three groups of patients were (1) the cells plus erythropoietin, (2) erythropoietin alone, and (3) the placebo group. They measured the gross motor performance measure, Bayley scales of infant development, F-fluorodeoxyglucose positron emission tomography, and diffusion tensor imaging. The first group had had significantly improved scores on the motor function measure and the Bayley scales. The diffusion tensor imaging and positron emission studies seemed to relate to the benefit seen with the cells plus erythropoietin. The subjects had a variety of diseases as a cause of their CP: periventricular leukomalacia, “diffuse encephalopathy”, focal ischemia or hemorrhage, multicystic encephalomalacia, or cerebral malformations. It was not clear how many of the children sustained HI injury as a cause of their chronic disability. Because the children were not segregated as to the cause of their CP, we cannot draw conclusions about the possible benefit of the cells in chronic HI injury.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






