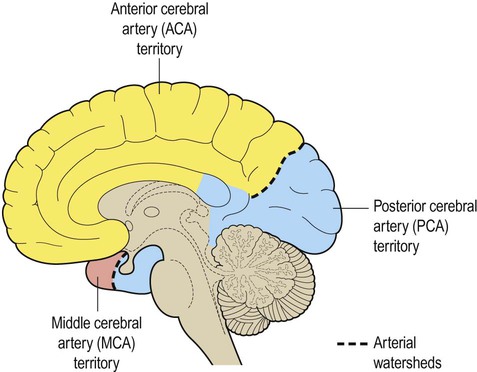
Stroke is caused by inadequate tissue perfusion or ischaemia (Greek: isch-, restriction; haema, blood). The brain is particularly sensitive to ischaemia because neurons have a high metabolic rate and can only survive a few minutes without oxygen and glucose. Tissue death as a result of inadequate blood flow (e.g. due to an occluded blood vessel) is called infarction (Latin: infarctus, stuffed). The area of dead tissue is referred to as ‘an infarct’. There are two main types of stroke (Figs 10.1 and 10.2): In both types of stroke the brain tissue normally supplied by the vessel is suddenly deprived of blood and its function is lost. This causes focal neurological deficits that develop very rapidly (e.g. sudden weakness or loss of speech) and abrupt onset is the clinical hallmark of stroke. In haemorrhagic stroke, there may also be mechanical damage, such as tearing and compression of brain tissue, caused by blood escaping under arterial pressure. Cerebral blood vessels may be occluded by an embolus (Greek: embolos, wedge or plug). This is a small piece of coagulated blood (or occasionally some other material such as fat) that travels in the circulation and lodges in the vascular tree of the brain. Emboli often originate from the heart (cardioembolic stroke) in association with valve disease or an abnormal heart rhythm (Clinical Box 10.1). Disease in the neck vessels or aortic arch may also give rise to emboli. Both types of pathology cause arteriosclerosis or ‘hardening’ of the arteries (Greek: sklerōs, hard). Sclerotic vessels are unable to dilate in response to reduced flow, leading to lacunar infarcts (see Ch. 12, Figs 12.18 and 12.19). These are small areas of ischaemic tissue damage (often in the basal ganglia or internal capsule) measuring less than 1 cm in diameter (Latin: lacūna, hole or gap). Small vessel disease is also an important cause of vascular cognitive impairment and dementia (Ch. 12). Spontaneous intracerebral haemorrhage is more common in people with high blood pressure and frequently occurs in the basal ganglia, cerebellum or pons (Fig. 10.2A). However, the incidence of hypertensive intracerebral haemorrhage is declining in the UK and USA and an increasingly important cause is cerebral amyloid angiopathy, particularly in the elderly (see Ch. 12, Clinical Box 12.5). This is caused by deposition of amyloid beta peptide in the walls of cortical and meningeal arteries, leading to atypical (or lobar) haemorrhages that are close to the cortical surface (Fig. 10.2B). Up to a third of spontaneous intracranial haemorrhages are caused by rupture of an aneurysm (a dilatation in the wall of an artery). Since the cerebral blood vessels travel and branch within the subarachnoid space, this results in subarachnoid haemorrhage (Clinical Box 10.2). Aneurysms that rupture in the substance of the brain may cause devastating brain damage or sudden death. The brain is supplied by two pairs of arteries that arise from branches of the aortic arch (Fig. 10.4): The arteries at the base of the brain are linked by communicating vessels to form the circle of Willis. This gives rise to anterior, middle and posterior cerebral arteries on each side, which supply most of the cerebral hemisphere. The cerebral blood supply can be divided into anterior and posterior circulations (Fig. 10.5A). The internal carotid artery divides at the base of the brain, giving rise to the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). The MCA is the larger of the two and receives 80% of the internal carotid blood flow. For this reason, cardiogenic emboli are much more likely to enter the MCA than the ACA. The middle cerebral artery continues laterally between the frontal and temporal lobes and emerges from the lateral sulcus to supply most of the hemispheric convexity (Figs 10.6A and 10.7). The anterior cerebral artery passes forward and medially to meet its partner between the cerebral hemispheres. It winds around the corpus callosum and supplies the medial aspect of the hemisphere as far back as the parieto-occipital sulcus (Figs 10.6 and 10.8). Posterior to this point the posterior cerebral artery takes over to supply the occipital lobe. Perforating branches of the anterior circulation supply the optic radiations, so that anterior circulation strokes may cause a contralateral visual field defect. The posterior circulation supplies the remainder of the cerebral hemisphere and the posterior fossa contents (brain stem and cerebellum). The two vertebral arteries unite in front of the brain stem, at the junction between pons and medulla, to become the basilar artery. For this reason, the posterior circulation is also known as the vertebrobasilar circulation. The basilar artery ascends along the basal pons to reach the midbrain where it splits into the two posterior cerebral arteries (PCA). These vessels wind around the midbrain and pass posteriorly, above the tentorium cerebelli, to supply the occipital lobe and the inferior surface of the temporal lobe (Figs 10.6B, 10.7 and 10.8). The circle of Willis is a polygonal arrangement of blood vessels surrounding the optic chiasm and pituitary stalk. It connects the anterior and posterior circulations via the single anterior communicating artery and the paired posterior communicating arteries (Figs 10.5B and 10.9). The circle of Willis shows considerable anatomical variation and is incomplete in 50% of people. It is an example of a collateral circulation, an arrangement of interconnected vascular channels permitting blood flow via an alternative route in the event of an obstruction. Interconnections between vessels exist elsewhere (e.g. between the distal territories of the three main cerebral blood vessels) but are not always effective, particularly in the elderly. Some structures do not have a collateral blood supply (e.g. the internal capsule and basal ganglia) and are therefore more vulnerable to stroke.
Stroke
Types of stroke
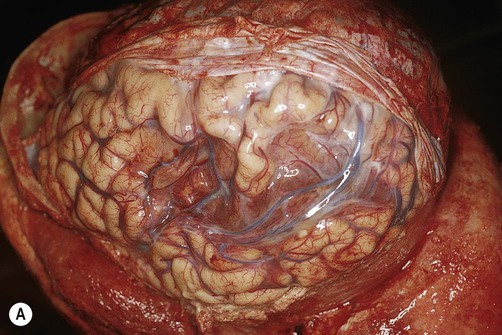
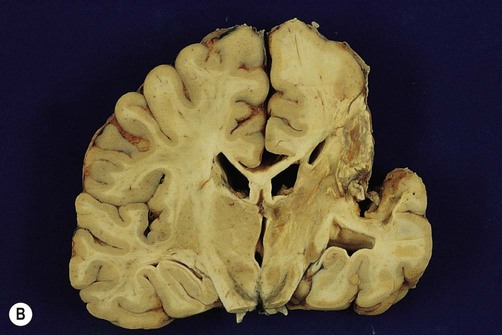
(A) Photograph taken during a post-mortem examination in a patient with an old (‘healed’) left hemisphere ischaemic infarct in the territory of the middle cerebral artery. Extensive resorption of brain tissue (leaving only a fluid-filled cyst cavity) indicates a prolonged period of survival after the stroke; (B) Coronal section of a fixed (preserved) brain slice in a different patient showing an old ischaemic stroke, also in the territory of the middle cerebral artery. From Ellison and Love: Neuropathology 2e (Mosby 2003) with permission.
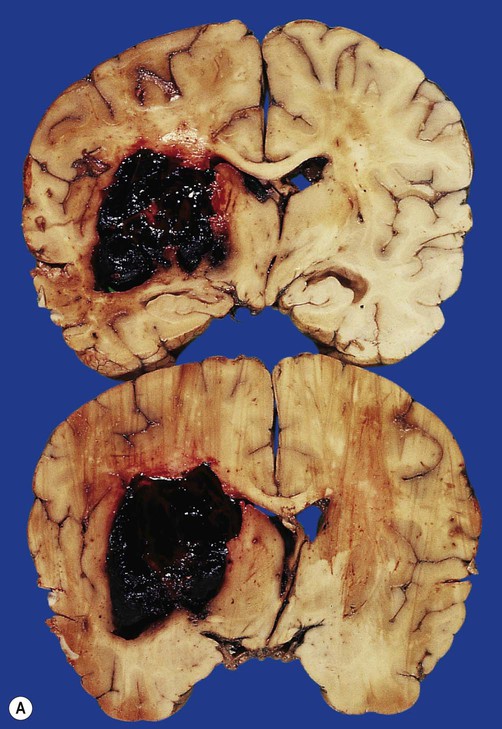
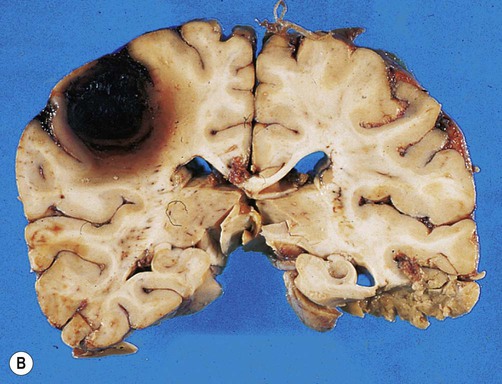
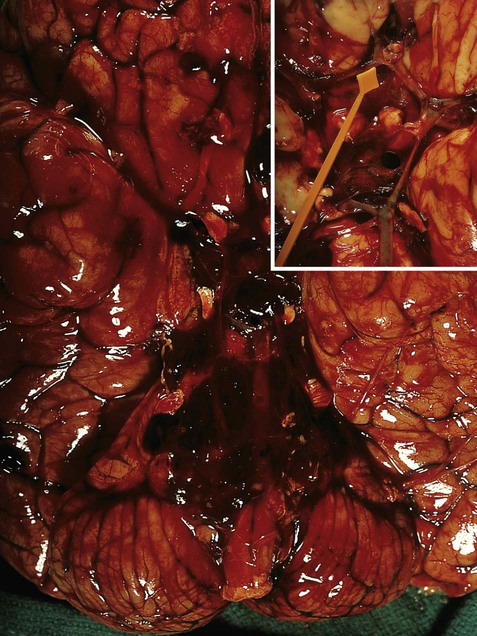
(A) Coronal sections showing a typical hypertension-associated haemorrhagic stroke replacing the basal ganglia; (B) Coronal section showing an atypical (‘lobar’) haemorrhage due to cerebral amyloid angiopathy affecting the cortical and meningeal arteries. Note the superficial (cortical) position of the atypical haemorrhage. From Ellison and Love: Neuropathology 2e (Mosby 2003) with permission.
 Ischaemic stroke (85% of cases) is caused by reduced blood flow to a particular part of the brain, usually following occlusion of a cerebral artery.
Ischaemic stroke (85% of cases) is caused by reduced blood flow to a particular part of the brain, usually following occlusion of a cerebral artery.
 Haemorrhagic stroke (15% of cases) is due to a ruptured blood vessel. This is commonly associated with high blood pressure and diseases that weaken the arterial wall.
Haemorrhagic stroke (15% of cases) is due to a ruptured blood vessel. This is commonly associated with high blood pressure and diseases that weaken the arterial wall.
Ischaemic stroke
Large vessel and cardioembolic stroke
Small vessel disease
Haemorrhagic stroke
Blood supply to the brain
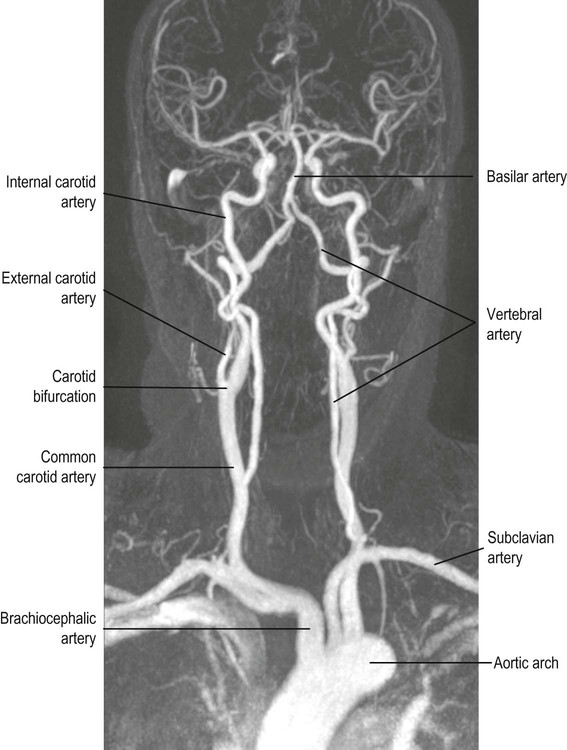
 The internal carotid arteries ascend in the anterior part of the neck, arising from the bifurcation of the common carotid arteries.
The internal carotid arteries ascend in the anterior part of the neck, arising from the bifurcation of the common carotid arteries.
 The vertebral arteries arise from the subclavian arteries and ascend in the lateral cervical region, passing through foramina in the transverse processes of the upper six cervical vertebrae.
The vertebral arteries arise from the subclavian arteries and ascend in the lateral cervical region, passing through foramina in the transverse processes of the upper six cervical vertebrae.
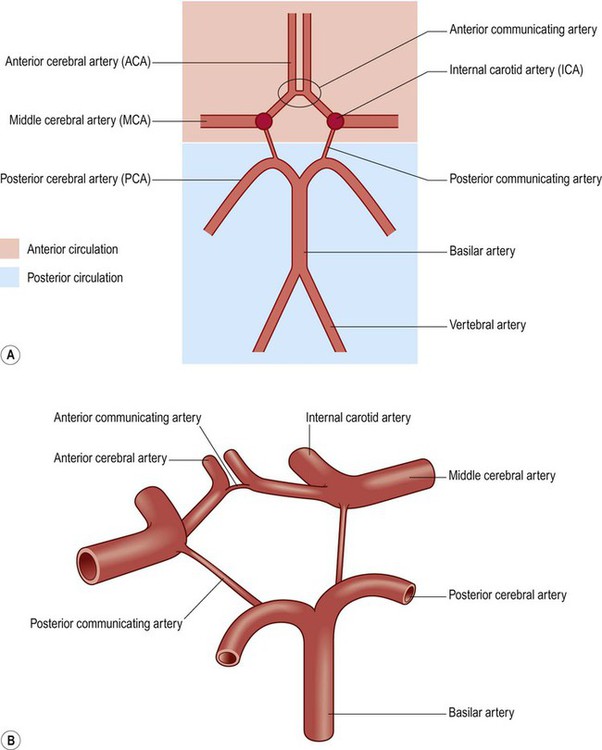
(A) Schematic, two-dimensional, representation of the circle of Willis, showing the anterior (internal carotid) and posterior (vertebrobasilar) circulations; (B) Illustration of the three-dimensional arrangement of the circle of Willis.
Anterior circulation
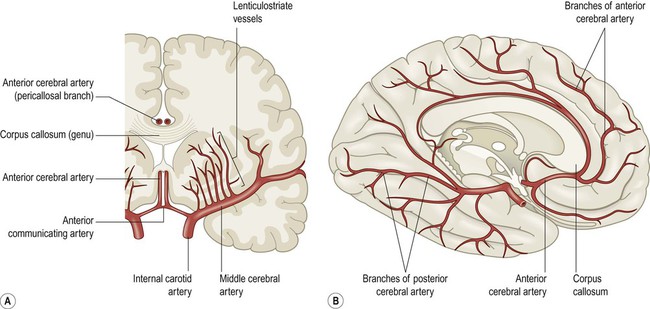
(A) Coronal view of the cerebral hemisphere showing the anterior and middle cerebral arteries. The lenticulostriate (deep perforating) vessels can be seen arising at right-angles from the middle cerebral artery; (B) Midsagittal view of the cerebral hemisphere showing the anterior and posterior cerebral arteries.
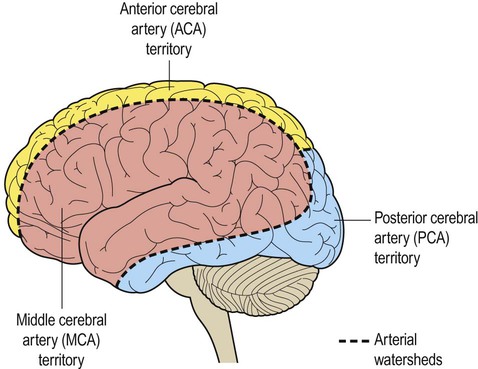
The dashed line indicates the arterial watershed zone between the main vascular territories.
Posterior circulation
Circle of Willis
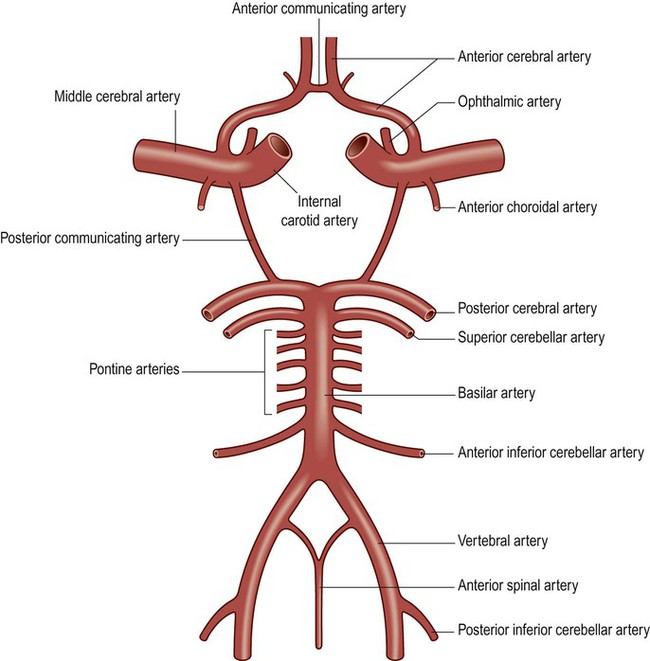
The ophthalmic artery is the first branch of the intracranial portion of the internal carotid artery and supplies the orbital contents. The anterior choroidal artery is the final branch of the internal carotid artery (or an early branch of the MCA); despite being a small vessel, it supplies portions of the central visual pathways, medial temporal lobe (e.g. hippocampus, amygdala) and part of the basal ganglia and internal capsule. The anterior spinal artery supplies the basal portion of the medulla and the anterior two thirds of the spinal cord.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Stroke