Chapter 51 Surgery for Temporal Lobe Epilepsy
• The most common medically intractable epilepsy appropriate for epilepsy surgery has a temporal lobe origin.
• Mesial temporal lobe epilepsy (MTLE) represents a large percentage of all localization-related epilepsy and has a strong association with an early injury before the age of 4 years. The seizures arise in the hippocampal and parahippocampal areas and in the amygdala. MTLE also presents common diagnostic features including unilateral interictal and ictal electroencephalography (EEG) and magnetic resonance imaging (MRI) features showing sclerosis of the mesial structures, resistance to medical therapy, and responsiveness to selective resection. The pathogenesis of this syndrome represents a special substrate: the so-called mesial temporal sclerosis (MTS).
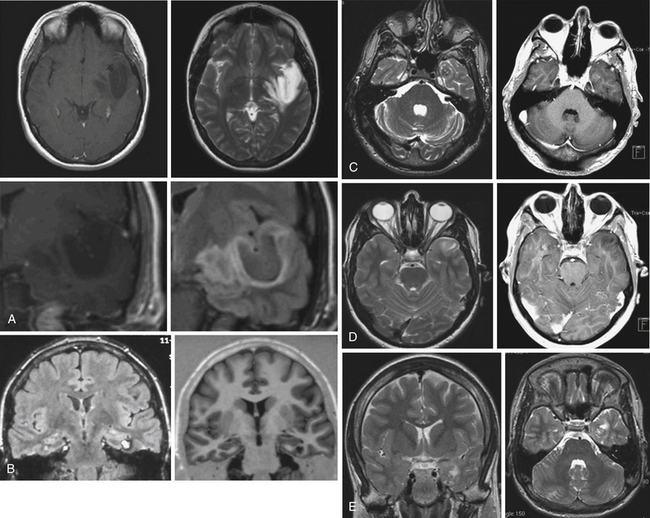
• Lesional temporal lobe epilepsy may be seen in the presence of many different histopathological entities such as tumors (astrocytoma, dysembryoplastic neuroepithelial tumor, ganglioglioma) (see Fig. 51.1), vascular malformations, and developmental lesions (cortical dysplasia, neuronal heterotopias, and others).
• Cognitive impairment in cases of pharmacoresistant epilepsy of early onset is not uncommon, and is related to different overlapping factors: the electrophysiological abnormalities of continuous seizures on a developing brain, the constant need for anticonvulsant drugs, and the presence of multiple medication regimens.
General Features
When epilepsy is uncontrolled by traditional anticonvulsant therapy, surgical resection of the epileptogenic region may be performed. The most common uncontrolled epilepsy eligible for epilepsy surgery has temporal lobe origin. In fact, the temporal lobe neocortex and the amygdalohippocampal complex are highly prone to seizure-induced brain injury. The efficacy of temporal lobe resection for the treatment of epilepsy in children was reported over 40 years ago by Davidson and Falconer.1
Natural History
The natural history of medically intractable epilepsy has demonstrated the prognosis to be poor,2 especially as children with temporal lobe epilepsy of early onset can be affected in all areas of cognitive functions and intelligence as well as behavior and psychosocial skills. The cognitive impairment of pharmacoresistant epilepsy of early onset is postulated to be related not only to the continuous seizures and their electrophysiological abnormalities on a developing brain but also to different overlapping factors. These factors include the constant need for anticonvulsant drugs, the presence of multiple medication regimens, and the use of near-toxic doses in case of status epilepticus, as demonstrated in animals models.3,4 An onset of uncontrolled seizures under the age of 3 carries the worst prognosis, with a low IQ and motor delays with tonic and myoclonic features and spasms.5 Other adverse prognostic factors are the presence of daily complex partial seizures, at least five episodes of grand mal, at least one episode of status epilepticus, and a long duration of seizures prior to control.6
Classification of Temporal Lobe Epilepsy
The most recent classification of the International League Against Epilepsy reports mesial temporal lobe epilepsy (MTLE) with hippocampal sclerosis as a specific electrophysiological syndrome.7 MTLE represents a large percentage of all localization-related epilepsy and has a strong association with an early injury before the age of 4 years, particularly febrile seizure, which may be present in 40% of cases. The seizures arise in the hippocampal and parahippocampal areas and in the amygdala. MTLE also presents common diagnostic features including unilateral interictal and ictal electroencephalography (EEG) and magnetic resonance imaging (MRI) features showing sclerosis of the mesial structures, resistance to medical therapy, and responsiveness to selective resection.
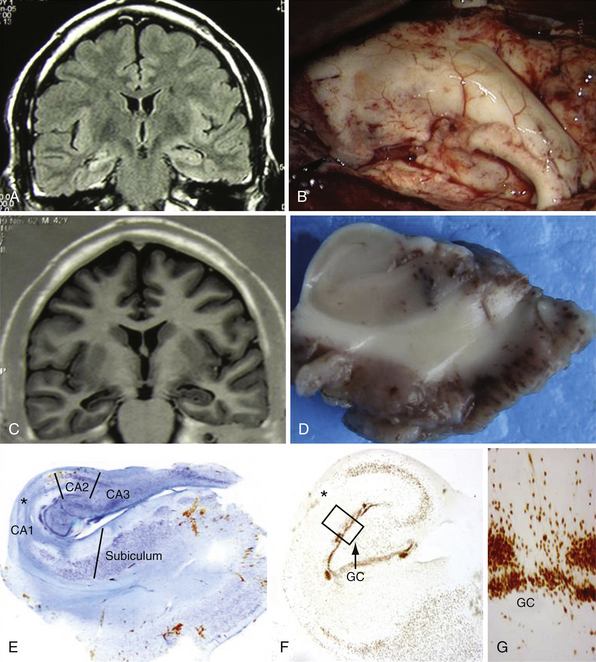
The pathogenesis of this syndrome represents a special substrate: the so-called mesial temporal sclerosis (MTS). The cause of MTS is unclear. An association with a history of febrile seizures has been proposed8 and it is suggested to be associated with complex features of febrile seizures.9 However, this connection has been questioned.10 Instead of being the consequence of recurrent temporal lobe seizures the MTS is frequently associated with a history of unusual febrile seizures in childhood with a later development of complex partial seizures. From a histopathological point of view the mesial structures show a loss of neurons with gliosis in the hippocampus associated with a modification of the normal gray matter architecture. Especially there is an involvement of the Sommer’s sector, the end folium, and the CA3 regions.11 MTS tends to be more common in adults than children but occurs frequently in association with cortical dysplasia in these patients, accounting for the early onset of epilepsy in this group.12,13 Despite the multitude of clinical and basic science studies on MTS the cause and maintenance mechanisms of epileptogenicity are not fully understood.
Lesional temporal lobe epilepsy may be seen in the presence of many different histopathological entities such as tumors (astrocytoma, dysembryoplastic neuroepithelial tumor, ganglioglioma) (Fig. 51.1), vascular malformations, and developmental lesions (cortical dysplasia, neuronal heterotopias and others). Even in patients with long-lasting histories of seizures when the hippocampus has been resected, the neuronal loss is small (up to 25%) and there is no architectural reorganization (see Fig. 51.1).14,15
Seizure Semiology
Temporal lobe seizures may originate in hippocampal and immediately adjacent structures or arise from extrahippocampal neocortical regions. The clinical differentiation of hippocampal and extrahippocampal temporal lobe epilepsy may help guide the extent of resection.16 The presence of an aura with experiential phenomena, such as a feeling of depersonalization or familiarity, or visual or auditory illusions is associated with a neocortical temporal ictal origin. In 70% of cases the epigastric aura occurs in the setting of mesial temporal lobe epilepsy; however, this rate significantly increases (98% of cases) if the abdominal aura evolves into an automotor seizure.17 Fearful and olfactory auras are commonly associated with involvement of the amygdala, whereas gustatory auras may occur in the hippocampal region or arise from extrahippocampal neocortical regions.18 The “déjà vu” onset needs a concomitant activation of hippocampus and temporal neocortex and it is not specific for the activation of hippocampus or neocortex.
Usually the ictal behavior shows different patterns including behavioral arrest and motionless staring that are described as typical symptoms of mesial temporal origin. Oral automatisms (chewing, lip smacking, tongue protruding, and lip pursing) and manual automatisms (manual exploratory behavior, grabbing, and rubbing) are both associated with temporal lobe epilepsy (TLE).19 The early onset of automatisms is frequently associated with a primary involvement of the mesial temporal lobe; moreover, the association of ipsilateral hand automatism with simultaneous contralateral dystonic posturing allows a correct lateralization. Contralateral head rotation just before seizures and secondarily generalized and unilateral tonic and dystonic posturing can be of value in localization, but they are not always accurate.20 A variety of autonomic symptoms, including elevated blood pressure, tachycardia, skin pallor, pilomotor erection, and mydriasis, have been reported in the course of MTLE seizures.21 Ictal speech and postictal dysphasia has been demonstrated to be consistent with seizure onset in the dominant hemisphere; in addition, active testing of postictal reading ability indicated a seizure focus in the language-dominant hemisphere. It has been reported that early postictal nose wiping is a reliable lateralizing sign pointing to the ipsilateral hemisphere.22
Temporal lobe seizures are more likely to occur during wakefulness, whereas frontal lobe seizures have a greater chance of occurring during sleep, implying that sleep has distinct effects on seizure threshold in different brain regions.23 Furthermore, sleep can influence the extent of seizure spread, such that seizures in temporal lobe epilepsy are more likely to secondarily generalize during sleep than during wakefulness.24,25
Evaluation for Surgery
Presence of mesial temporal lobe sclerosis resistant to pharmacotherapy is the most common indication for surgery in adults, accounting for 75% in adult surgical series.26,27 The major indication for TLE surgery in children is a lateral neocortical alteration, frequently cortical dysplasia alone, or eventually in combination with MTS and lesion-related TLE, especially gangliogliomas and dysembryoplastic neuroepithelial tumors (DNET).
The correlation between electroclinical data and hippocampal sclerosis in MTLE has been known for over half a century.8,28–30 To examine the evidence base for surgery for uncontrolled seizures, the Quality Standards Subcommittee of the AAN (American Academy of Neurology) performed a systematic review of the efficacy and safety of anterior temporal lobe resections.31 Based on one randomized trial and observational studies, they found that anterior temporal lobe resection reduced the occurrence of disabling seizures and improved patients’ quality of life. Furthermore, they speculated that the “greater potential for achieving freedom from disabling seizures offered by surgical treatment, as opposed to continuing pharmacotherapy, may reduce the risks of long-term mortality.” Moreover, pharmacoresistant epilepsy has been associated with decreased survival,32,33 and in the appropriate candidate, temporal lobectomy has actuarial benefit on life expectancy.34
Noninvasive Evaluation
The semiology, interictal and ictal scalp video-EEG, and imaging (anatomical, metabolic, and functional) as well as neurocognitive evaluation, represent the armamentarium of evaluation for surgical candidacy in the patient with pharmacoresistant epilepsy. Most evaluations would include interictal scalp recordings and inpatient video-EEG to capture typical seizures. However, the best outcomes are found in patients with concordant MRI and interictal scalp EEG concordant for unilateral MTLE.35 In fact, lack of concordance with interictal EEG and other studies is a worse predictor of surgical outcome than ictal disconcordance. However, in all but the most straightforward of cases, several typical clinical spells are usually of value to be recorded by inpatient EEG.
Imaging of MTLE by MRI has made the diagnosis of hippocampal sclerosis much more straightforward, in addition to a variety of evolving imaging tools.36 Fine-cut coronal sequences perpendicular to the axis of the hippocampus are particularly useful. Coronal fluid-attenuated inversion recovery (FLAIR) sequences suppress the cerebrospinal fluid signal and enhance the increased tissue free-water signal characteristic of mesial temporal sclerosis. Volumetric hippocampal MRI studies through serial thin-cut coronal images permit a comparison of the volumes of both hippocampi, unmasking eventual differences in volume between the normal and the affected mesial structure (Fig. 51.2A and B).37 Recent advances in high-field MRI have been particularly helpful in identifying subtle abnormalities in hippocampal or neighboring mesial temporal structures.38,39
Magnetic resonance spectroscopy (MRS) can also detect abnormalities in various metabolites using the proton signal.40,41 Nuclear medicine studies are also employed, primarily interictal positron emission tomography (PET) and ictal/interictal single-photon emission computed tomography (SPECT). Fluorodeoxyglucose (FDG) PET looks for interical hypometabolism. It can be helpful in identifying surgical candidates in the setting of normal MRI scans, and also may detect those with bilateral disease with poorer outlook for seizure freedom.42 However, the area of hypometabolism demonstrated by FDG PET often shows greater extent than foci demonstrated by EEG or MRI. Although PET is a sensitive diagnostic method, it provides only approximate localization of the epileptic zone and may not be adequate for precise localization, though it may be useful for differentiating between TLE of mesial or lateral origin.43
SPECT studies require injection very soon after seizure onset for maximal accuracy. The use of SPECT image registration to subtract an ictal from interictal injection, when coregistered with the anatomical MRI, can result in useful ictal localization information, even with a normal MRI scan.44
Invasive Evaluation
When noninvasive methods do not indicate the seizure focus, invasive diagnostic recordings can be used. When bilateral MTLE is of concern, bitemporal electrodes can be used. The two strategies most often employed are depth electrodes that penetrate the brain structures, or subdural electrodes that are passed subtemporally and medially toward mesial structures. Though these methods are typically used on a less favorable group of patients, good outcomes can be achieved in this subset and are generally well tolerated.45 Invasive monitoring indications and interpretation can be more challenging in the pediatric population because of the frequent temporal and extratemporal neocortex involvement.46
Neuropsychology
A thorough neuropsychological evaluation is part of the presurgical assessment for medically intractable seizures.47 Neuropsychological testing can help lateralize the seizure focus48,49 because dominant temporal lobe epilepsy is typically associated with verbal memory deficits as a predominant factor. Excessive speech difficulties, global memory problems, or extensive difficulties in other domains should raise concerns as to the localization of medial temporal lobe epilepsy.
Neuropsychological testing is also critical to assess for the emotional, behavioral, and psychiatric disorders that are very common in this population.50 Depression in particular can be quite significant, and many preoperative factors and expectations can impact surgical decision making, perceived benefit from surgery, and quality of life outcomes.51
Testing is also important to establish language dominance and define memory risks preoperatively. The cerebral amytal test has long been used to determine dominance and assess the effect on memory of anesthetizing one hemisphere through intracarotid injection.52 The test is invasive and there is variability in its use. Because right-handed patients with right-sided lesions have a very small chance of being dominant,53 some of these patients, for example, may not require extensive study of dominance through intracarotid amytal testing. Functional MRI can make assessment of dominance and memory, but the exact tests and their reliability are still a subject of debate.54 The precise language test to be used in functional MRI is unknown, but some batteries have been quite successful in establishing dominance.55,56 Memory in particular is difficult to assess by either method.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree