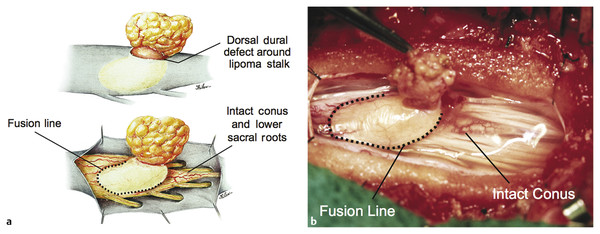
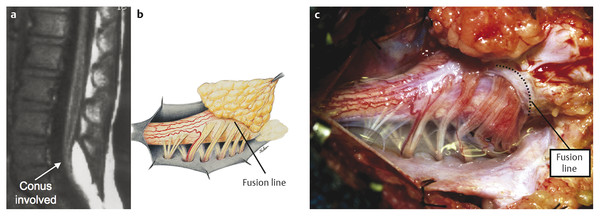
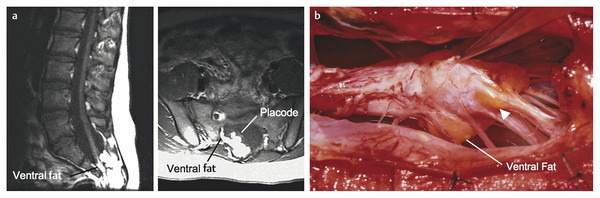
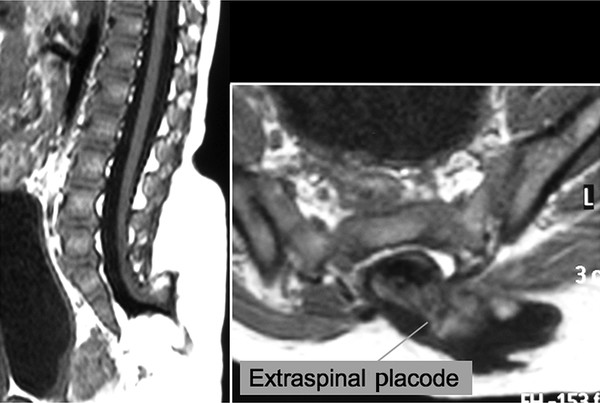
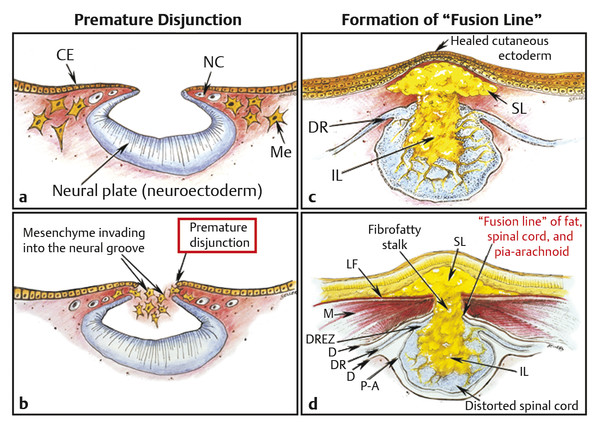
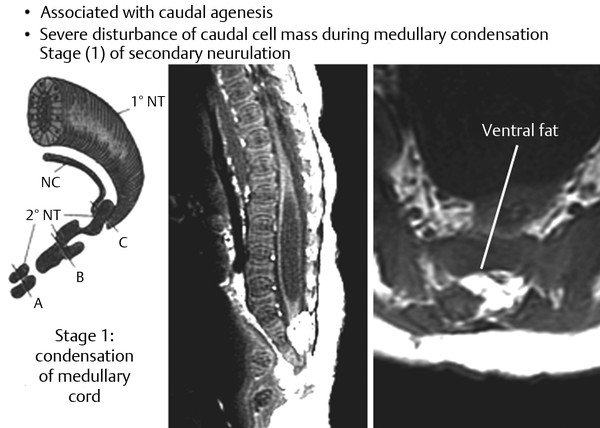
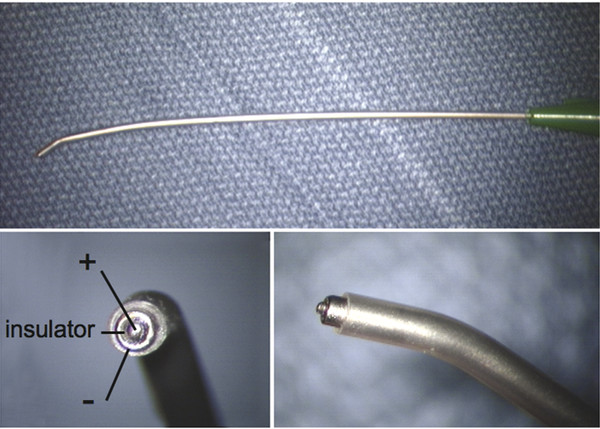
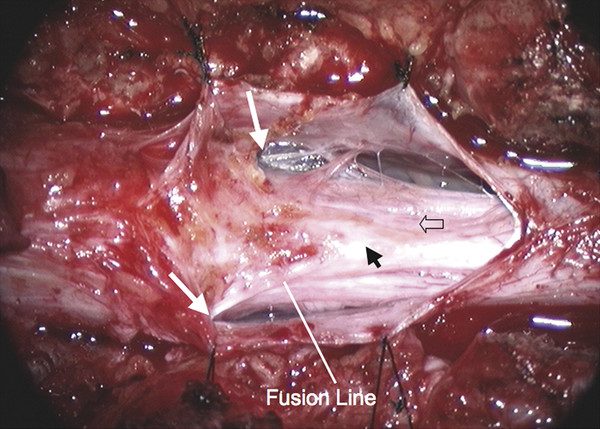
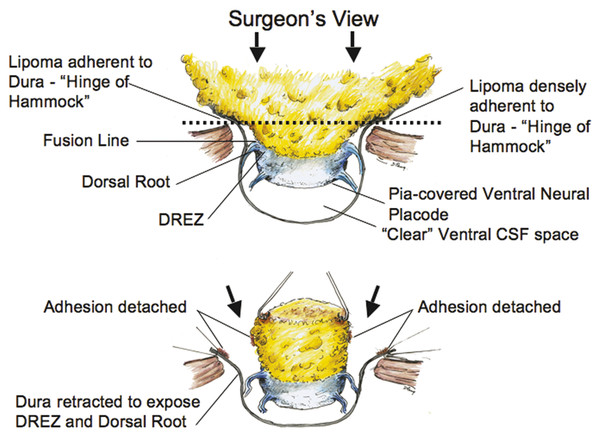
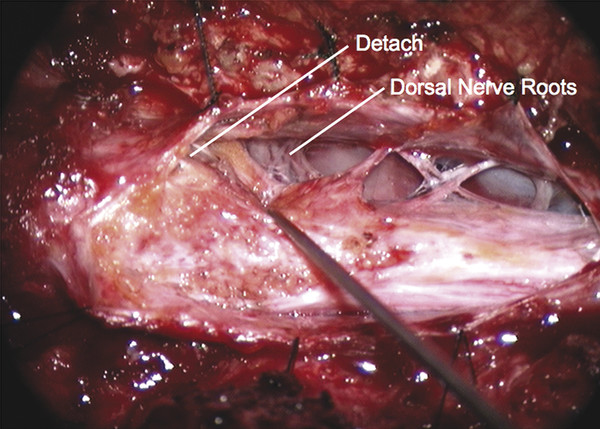
Surgical Management of Complex Spinal Cord Lipomas The partial resection of complex spinal cord lipomas is associated with a high rate of symptomatic recurrence due to retethering.1 Since 1991, the author has performed more than 330 total or near-total resections of complex lipomas with radical reconstruction of the neural placodes, designed to minimize the preconditions of retethering. Twenty years of follow-up have proven the long-term benefits of this technique.1,2 I now strongly advocate for total resection of spinal cord lipomas and radical reconstruction of the neural placode over partial resection because aggressive surgery, contrary to traditional teaching, is safe and gives far better long-term progression-free survival.1 The rationale for total lipoma resection is based on three hypotheses: The high rate of symptomatic recurrence after partial resection is due to retethering. Retethering is promoted by three factors: a tight content–container relationship between spinal cord and dural sac; a large, “sticky,” raw surface of residual fat; and incomplete detachment of the terminal neural placode from residual lipoma. Total resection can eliminate the factors conducive to retethering and thus reduces the probability of symptomatic recurrence. The object of surgery is therefore to create conditions that will minimize retethering. The first condition relates to the fact that the normal spinal cord exhibits intradural motions to gravity and postural changes on ultrasonography and dynamic imaging.3,4 Reducing the content–container ratio and amplifying the degree of freedom of the cord within the dural sac must lessen re-sticking by limiting sustained contact between the cord and the dura, this sustained contact being intuitively a necessary condition preceding the formation of fibrous adhesions. To do this, the cord bulk must be drastically reduced. For large, rambling, “virgin” lipomas, this means resection of all or most of the fat down to the thin, supple neural placode. For redo lipomas, the hard, grasping cicatrix must also be removed. The aim is to render the thinnest, most pliant neural placode possible that can be atraumatically neurulated (sutured from pia to pia; see below) without distortion or strangulation to form a slender, round tube. (The term neural placode in lipoma is borrowed from the familair essential element of an open neural tube defect [ONTD] to emphasise its equivalent “neural” nature once purified of fat. The synonymous use of the term in lipoma and ONTD is logical if one ponders the embryogenesis of the two entities (see below); the “placode” in each case represents the original embryonic neural plate blighted in its final completing process, one invaded by paraxial mesenchyme, the other thwarted in its midline dorsal fusion.)The raw, sticky lipoma bed is simultaneously concealed within the tube, and the sac is enlarged by a capacious dural graft. Finally, total resection also enhances the chances of terminal untethering. In the literature, the nomenclature of spinal cord lipoma is imprecise and inconsistent. Here, we define the types of lipomas as follows: The lipoma–cord interface is entirely on the dorsal surface of the lumbar spinal cord, always sparing the distal conus (▶ Fig. 26.1). The junctional demarcation between the lipoma, cord, and pia, the fusion line (see below), can always be traced neatly along a roughly oval track, separating fat from the dorsal root entry zone (DREZ) and dorsal nerve roots laterally (▶ Fig. 26.2). The lipoma therefore never contains nerve roots. The lipomatous stalk runs through an equally discrete dorsal dural defect to blend with extradural fat. The uninvolved conus often ends in a thickened filum terminale. Fig. 26.1 Dorsal lipoma on magnetic resonance images. Sagittal image: Intact conus is caudal to the lipoma stalk. Axial images: Upper image shows site of lipoma attachment to cord; lower image shows free conus just caudal to the level of the lipoma attachment. Fig. 26.2 Dorsal lipoma. (a) Intraoperative drawings show neat dorsal dural defect through which lipoma stalk goes. Lower drawing shows circumferential fusion line and intact conus. (b) Intraoperative picture shows neat oval fusion line around lipoma–cord interface in a horizontal plane. Note intact conus and caudal sacral roots. The rostral portion of this type is identical to that of a dorsal lipoma, with a discrete fusion line and easily identifiable DREZ and dorsal roots. Unlike the dorsal type, however, which always spares the conus, the transitional lipoma then plunges caudally to involve the conus as the plane of the fusion line cuts ventrally and obliquely toward the tip of the conus, likened to making a slanting, beveled cut on a stick (▶ Fig. 26.3a). The lipoma–cord interface thus created may be undulating and tilted so that the neural placode is rotated to one side or even spun into a parasagittal edge-on orientation, but the neural tissue is always ventral to this interface (i.e., on the side of the nerve roots exit), and the DREZ and the nerve roots are predictably localizable lateral and ventral to the fusion line and therefore do not course through the fat (▶ Fig. 26.3b). There may or may not be a discrete filum. The dorsal dural defect extends to the caudal end of the thecal sac and may be much larger on the biased side. Fig. 26.3 Transitional lipoma. (a) Sagittal magnetic resonance image shows that lipoma begins dorsally but involves the entire conus. The ventral side of the neural placode is free of fat. (b) The plane of the fusion line begins dorsally, then cuts obliquely toward the tip of the conus. The array of DREZ and dorsal roots is also forced to slant dorsoventrally. (c) Intraoperative picture showing a massive lipoma but a very distinct dorsoventral fusion line separating fat from the DREZ and dorsal roots, which always lie lateral and ventral to the fusion line. The ventral side of the placode is always free of fat in a regular transitional lipoma Unlike the dorsal and transitional types, terminal lipomas insert into the caudal extremity of the conus without blending with the spinal cord or its root entry zones. All the sacral roots clearly leave the conus rostral to the lipoma, and in most cases the conus itself looks normal. The dural sac and the dorsal myofascial coverings are intact. The lipoma either replaces the filum entirely or is separated from the conus tip by a short, thickened filum. The surgery for terminal lipoma is relatively straight forward and therefore beyond the scope of this chapter. This novel type is so named because it does not “follow the rules” of either the dorsal, transitional, or terminal lipoma. It begins dorsally in an orderly fashion, like a dorsal or transitional lipoma, but its caudal portion is ventral to the neural placode and does engulf neural tissue and nerve roots (▶ Fig. 26.4a). The fusion line may be distinct rostrally but quickly becomes blurred distally, and the location of the DREZ and nerve roots is less predictable. The moniker “chaotic” depicts the sometimes confusing blend of the ventral fat and neural placode, and the often impossible task of separating fat from neural tissue at surgery (▶ Fig. 26.4b). Chaotic lipomas are uncommon but are characteristically seen with sacral agenesis.2 Fig. 26.4 Chaotic lipoma. (a) Sagittal magnetic resonance image shows ventral as well as dorsal fat in relation to the neural placode. Note sacral agenesis, with only two visible sacral segments. Axial image shows ventral fat and an extremely irregular lipoma–fat interface. (b) Intraoperative picture showing fat ventral to the placode and on one of the sacral roots (arrowhead). Note absence of a discrete fusion line. The literature5,6 describes one other lipoma type, the lipomyelomeningocele, in which part of the distal conus extends into the extraspinal compartment, dragging with it a small collar of dural sac (▶ Fig. 26.5). The basic structure is that of either a transitional or a dorsal lipoma. Accordingly, we choose to classify this type as either a transitional or dorsal lipoma, with a descriptive qualifier of “extraspinal extension.” Fig. 26.5 Transitional lipoma with extraspinal extension (“lipomyelomeningocele”). The lipoma, cerebrospinal fluid sac, and part of the neural placode extend out of the spinal canal through a dorsal defect. An understanding of the embryogenesis of lipomas is helpful in appreciating the surgical nuances. In the embryo, a progressive disparity exists between the spinal cord and vertebral column as a result of the faster growth rate of the latter.7–10 The caudal end of the cord ascends gradually from opposite the coccyx in the 30-mm human embryo to the L1–L2 level at birth.9–12 Proper ascent of the cord requires a well-formed neural tube and a smooth pia–arachnoid covering. If during early development a dorsal defect exists in the dura (duraschisis) and neural tube (myeloschisis), mesodermal elements from the surrounding mesenchyme will enter the dural sac and form an attachment with the sliding neural tube in the form of a fibrofatty stalk, resulting in its entrapment. This theory features a fundamental defect in neural tube closure during primary neurulation (secondary neurulation does not involve dorsal neural fold closure), and thus it applies only to dorsal and transitional lipomas (see below). It is compatible with the observation that these two types of lipomas are always associated with neural arch defects. Normal separation of the cutaneous ectoderm and neuroectoderm (disjunction) occurs after dorsal neural folds fusion, so that the surrounding mesenchyme is permanently segregated from the dorsal (ependymal) surface of the neural plate. The embryologic error leading to the mesodermal invasion of the neural tube probably lies in premature disjunction between the cutaneous and neural ectoderm13,14, i.e., the separation of one from the other occurs before the converging neural folds fuse with each other. This allows the paraxial mesenchyme to roll over the still gaping neural folds and enter the central canal. Once contact between the mesenchyme and ependymal neuroectoderm is made, further closure of the neural tube is permanently prevented, and a segmental dorsal myeloschisis is created (▶ Fig. 26.6a,b). Alternatively, the fault may lie in a delay in neural folds fusion secondary to an insufficiency of the paraxial mesoderm in impelling their dorsal convergence,15–21 so that ectodermal disjunction again precedes neural folds fusion. Lastly, faulty fusion of the neural folds due to a metabolic disturbance of the cell membrane–bound glycosaminoglycans, which are vital to cell–cell recognition and adhesion,22–25 can likewise reverse the temporal relationship between disjunction and neural folds fusion. Fig. 26.6 Embryogenesis of a dorsal lipoma, a purely primary neurulation defect. (a,b) Premature disjunction before complete closure of the neural plates allows the migration of mesenchymal cells into the neural groove to be in contact with the ependymal surface. (c,d) Formation of a fusion line between the lipoma, cord, and pia–arachnoid. The DREZ and dorsal roots are always lateral to the fusion line and thus not entangled in fat. CE, cutaneous ectoderm; NC, neural crest; Me, mesenchyme; SL, subcutaneous lipoma; DR = dorsal root; IL, intramedullary lipoma; DREZ, dorsal root entry zone; D, dura; P-A, pia–arachnoid; M, muscle; LF, lumbodorsal fascia. Experimental studies show that the pluripotential mesenchyme forms derivatives according to the inductive properties of the adjacent neuroectoderm (▶ Fig. 26.6c).26,27 The ependymal side of the neural tube induces mesenchyme to form fat, muscles, collagen, and occasionally bone and cartilage, whereas the outer surface of the neural tube induces the formation of meninges.28 However, no dura can now form over the dorsal opened portion of the neural tube, and the dural defect neatly surrounds the evolving lipomatous stalk, which tethers the neural tube to the subcutaneous adiposity. In like manner, deficiencies in the overlying myofascial layers (from myotomal mesoderm) and neural arches (from scleromesoderm) also neatly surround the lipomatous stalk (▶ Fig. 26.6d). Within the neural tube, the intramedullary fat and muscles fuse with the developing alar and basal plates. Because the dorsal root ganglions develop from neural crest cells at the outer aspect of the neural fold lateral to the site of failed fusion, the dorsal nerve roots grow outward ventrolateral to, but never traverse, the lipomatous stalk. The DREZ must correspondingly lie very near, but always lateral to, the exact junctional boundary between the lipoma and spinal cord. This boundary, called the fusion line, is of tremendous surgical significance2,29,30 (▶ Fig. 26.6d). Meanwhile, the cutaneous ectoderm, long detached from the neuroectoderm, heals over in the dorsal midline to form healthy skin over the subcutaneous lipoma. The genesis of dorsal lipoma perfectly exemplifies mistimed disjunction during primary neurulation. Its fibrofatty stalk always involves cord segments above the conus, which forms mainly from secondary neurulation. Furthermore, the failure of primary neural tube closure appears to be segmental, and normal closure takes place—business as usual—immediately following the abnormal event. This “square pulse” nature is illustrated by the fact that the sharp fusion line between fat, spinal cord, and pia–arachnoid can be neatly traced circumferentially around the lipomatous stalk29–31 (▶ Fig. 26.2). Dorsal lipomas therefore result from a segmental closure abnormality involving only primary neurulation. They accounted for fewer than 15% of spinal cord lipomas in our series.1,2 In transitional lipoma, the myeloschisis involves much more than an isolated segment of the primary neural tube. Even though its rostral part resembles the dorsal lipoma, the involvement of the whole of the caudal spinal cord means that not only primary but also secondary neurulation has been profoundly disturbed by the mesodermal invasion. This is supported by the observations that in many transitional lipomas, the filum is incorporated into the distal fat, and within the lipomas are often spaces resembling the vacuoles found within the secondary neural tube during its cavitary phase. Also, whereas the rostral part of the transitional lipoma is always dorsal and aptly reflects premature disjunction of primary neurulation, the distal part involves the core of the conus, a situation compatible with misguided mesenchymal inclusion during the much less orderly events of secondary neurulation. Intramedullary mesenchyme may migrate within the neural tube after invasion and travel caudally across the boundary from the primary to the secondary neural canal since the two neural canals are in continuity.32 In fact, the hypothesis that the rostral part of the transitional lipoma arises from aberrant primary neurulation (involving only the dorsal cord) and the caudal part arises from abnormal condensation of the secondary neural cord (affecting the deeper central core of the conus) furnishes at least one explanation for the dorsoventral obliqueness of the lipoma–cord interface. Despite the larger field of involvement of the neural tube, the lipoma–cord interface remains relatively distinct in a transitional lipoma. Chaotic lipomas do not quite fit into either the dorsal or the transitional schema. They often do not have a distinct dorsal part with the symmetry of a dorsal lipoma, and the lipoma–cord interface is irregular and ill defined, with fat running through the neural placode to the ventral side in large and unruly measures. Even in the context of the less orderly transitional lipoma, the interplay between lipoma and cord in this type of lesion seems to be in constant chaos. This degree of anatomical unpredictability in chaotic lipoma and its strong association with caudal agenesis (82% in our series2) suggest that the embryogenetic error occurs during the early stage of secondary neurulation as part of the general failure of the caudal cell mass (▶ Fig. 26.7).33,34 Secondary neurulation comprises three distinct stages: (1) condensation of neural material from the caudal cell mass to form the solid medullary cord; (2) intrachordal cavitation of the medullary cord32,34,35 and its integration with the primary neural tube; and (3) partial degeneration of the cavitary medullary cord through massive apoptosis to result in the thin filum terminale.9,32 It is possible that formation of the chaotic lipoma involves the entanglement of lipogenic mesenchymal stem cells with cells from the caudal cell mass during aberrant condensation of the medullary cord, forming an inseparable mixture of neural tissue and fat, with nerve roots projecting out haphazardly.2 Fig. 26.7 Embryogenesis of chaotic lipomas. Left: Basic error probably occurs with the inclusion of abnormal lipogenic mesenchymal cells into the caudal cord during the condensation stage (stage 1) of secondary neurulation, with formation of the medullary neural cord, thereby generating fat tissue throughout the substance of the mature neural placode. Middle and right: Dorsal and ventral fat with associated sacral agenesis. 1° NT, primary neural tube; 2° NT, secondary neural tube; NC, notochord. Terminal lipomas result from abnormal secondary rather than primary neurulation, as evidenced by the unexcepted rule that the lumbar and upper sacral cord segments, products of primary neurulation, are never affected in a terminal lipoma. Furthermore, dorsal myeloschisis and duraschisis, both hallmarks of failed (primary) neural fold fusion, are never seen. Lastly, the terminal lipoma either replaces or forms part of a thickened filum, which temporally places the pathogenetic process at secondary neurulation. The fact that the distal conus in terminal lipomas always remains fat-free argues against an abnormal condensation phase during early secondary neurulation. On the other hand, the terminal lipoma often contains (disorganized) spinal cord elements and ependymal tubules,36,37 which suggests rather an incomplete or ineffective degeneration phase at late secondary neurulation, possibly due to failed apoptosis.38 Intraoperative monitoring has become a sine qua non in lipoma surgery.2,39,40 Elaborate electromyographic preparations are made to capture triggered motor responses from the relevant lumbosacral nerve roots. Standard electromyographic needles are inserted into the rectus femoris (L4), anterior tibialis (L4–L5), gastrocnemius (S1), and abductor hallucis (S2). Smaller-gauge (No. 27) electromyographic needles are inserted obliquely into the external anal sphincter through the anal verge on each side, which are insulated from each other by a plug of dry muslin gauze so that sphincter contractions from one side can be unequivocally distinguished from the other side.39 All stimulations and recordings in our cases are done with the Cadwell Cascade Intraoperative Monitoring System (Cadwell Laboratories, Kennewick, WA) and the Cascade software version 2.0. Stimulating needle electrodes are placed subcutaneously along the shaft of the penis in male patients and between the periclitoral skin and the labia minora in female patients. Stimulation of the sensory domain of the pudendal nerve via these electrodes generates an “electric” bulbocavernosus reflex, which is a form of H-reflex for the conus useful in monitoring the integrity of the central connections of the conus apart from motor root mapping.39,41 Standard stimulating electrodes are placed near the posterior tibial nerve behind the medial maleolus and near the common peroneal nerve at the fibular neck to enable somatosensory evoked potential monitoring for the spinal cord segments above S2.39 All motor root and direct spinal cord stimulations are done with a concentric coaxial bipolar microprobe electrode that has an end plate diameter of 2 mm (▶ Fig. 26.8). The concentric configuration and small size of the anode–cathode complex allow extremely focused current delivery to a very small target volume, thus making the electrode ideal for the fine discrimination of small and crowded electroresponsive units.2 Stimulating currents from 0.5 to 3.0 milliamperes are used depending on target impedance. The stimulation frequency is usually set at 10 per second. This permits spontaneous random firing due to nerve irritation from surgical manipulation to be distinguished from rhythmic evoked contractions. Fig. 26.8 Concentric coaxial bipolar microprobe stimulator, in which the concentric cathode and anode are separated by a coaxial insulator. Tip diameter is approximately 1.75 mm. The skin and soft tissue incision should go straight through the subcutaneous lipoma if one is present. Removing this will leave behind a large subdermal space into which cerebrospinal fluid (CSF) can collect under tension and consequently hinder wound healing. Frequently, a discrete fatty stalk connects the subcutaneous with the intraspinal lipoma through a defect in the lumbodorsal fascia. This stalk is continuous with the spinal cord and cannot be tugged on during fascial dissection. The upper extent of the bony exposure should include one level above the rostral end of the lipoma. This reveals, for proper orientation, the “last” normal set of nerve roots and DREZ before the lipoma resection is started. Wide laminectomy is essential to afford full access to the lateral edges of the dural sac (see below). Visualization of the normal dura rostral to any lipoma gives a depth perspective as to how far out the neural tissue and CSF sac may have extruded beyond the plane of the dura. The heavy bulk of extradural fat can then be safely lopped off to give room for intradural dissection and lighten the tug on the conus. The dura is always opened in the midline about 1 cm rostral to the lipoma regardless of whether it is dorsal or transitional. For a dorsal lipoma, the midline incision is then carried circumferentially around the discrete lipoma stalk and down the middle again to expose the conus (▶ Fig. 26.2a). For a transitional lipoma, the midline cut is carried right up to the lipoma, where the dura quickly thins out to nothing in the middle, although decent dura can usually be picked up just beyond the lateral edge of the lipoma stalk. Unlike with the dorsal type, the bifurcated dural incisions with a transitional lesion can seldom be made to rejoin neatly in the midline caudal to the lipoma stalk, usually not until the whole of the lipoma–placode complex had been exposed, many steps later (see below). At this stage, the freed-up portion of the dural edge on each side must be tautly and widely retracted with sutures. This is crucial maneuver because full lateral exposure of the intradural span, made possible by generous bone removal, reveals the “crotch” where the far lateral fringe of the lipoma attaches to the inner surface of the dura (▶ Fig. 26.9). Fig. 26.9 Exposure of the rostral lipoma, showing the normal cord to the right and the swath of yellow fat indicating the beginning of the lipoma (small black arrow). The large arrows locate the far lateral adhesion points (the “crotches”) between the flanges of the lipoma and the inner dura. The rostral starting point of the fusion line is also indicated (small open arrow). Next, the fusion line is identified, where the pia, spinal cord, and lipoma join in a continuous furrowed border that travels in a rostral to caudal direction, outlining the entire attachment of the lipoma stalk to the cord. In a dorsal lipoma, the fusion line forms a neat, complete oval or circle from side to side, usually upon a leveled horizontal plane, often bilaterally symmetrical, and always sparing the conus below (▶ Fig. 26.2a,b). In a transitional lipoma, the rostral fusion line starts distinctly enough but then edges ventrally toward the tip of the conus and tends to wander laterally and asymmetrically, often becomes sheltered by the overhanging fat, and never meets its counterpart from the other side at the caudal end (▶ Fig. 26.3a,b). True to the events of embryogenesis, the DREZ and dorsal roots are always lateral to the fusion line, and at the rostral end of the fatty stalk of both lipoma types, this orderly arrangement can be depended upon on both sides, thus presenting a convenient place at which to start the dissection. In most transitional lipomas, however, the more caudal nerve roots are quickly covered from view by the overflowing fat, which tends to fuse with the dura at a far lateral point (▶ Fig. 26.10 upper). Hence the use of the term crotch dissection, which depicts the key step of grasping the overhanging fat and pulling it medially under tension against the tagged dural edge, then sharply separating the fat–dura attachment with the dissecting scissors (▶ Fig. 26.10 lower and ▶ Fig. 26.11). It is absolutely essential to lean the round curve of the scissors firmly against the inner lining of the dura while this attachment is cut to avoid blindly injuring the nerve roots, which project from the cord slightly medial to the “crotch” and lie just deep to the fat. The hidden roots should spring into view wherever the detached fat is pulled back, and they can be gently coaxed away from the dura by blunt dissection toward the exit foramina (▶ Fig. 26.12). At the same time, the free CSF space ventral to the dorsal roots and the fat-free, pia-covered ventral surface of the neural placode, hitherto hidden by the overhang, now “pop” into view (▶ Fig. 26.13). Fig. 26.10 Drawing depicting the relationships between the lipoma, neural placode, nerve roots, and dural sac in an axial slice. Upper: The lipoma–cord assembly is suspended at the dural edge at far lateral adhesion points, like a hammock against side hinges. The dotted transverse line that joins the two side hinges divides the assembly into a dorsal disorderly fibrofatty half that completely blocks the surgeon’s view and a much more orderly ventral half containing the important anatomical landmarks of the fusion line, DREZ, dorsal roots, fat-free ventral placode, and pristine ventral cerebrospinal space. Lower: After the far lateral adhesion points (the hinges) have been detached by careful “crotch dissection” and the fatty mass has been folded in, the ventral anatomical landmarks can be visualized. Fig. 26.11 Step 2 of surgical technique, “crotch dissection,” in which the far lateral adhesion points between the fringes of the lipoma and the inner dura are sharply detached to unhinge the hammock of the lipoma–cord assembly. Note how the proximal sets of dorsal roots are revealed with just the first efforts to unhinge the hammock.
26.1 Anatomy and Classification
26.1.1 Dorsal Lipoma
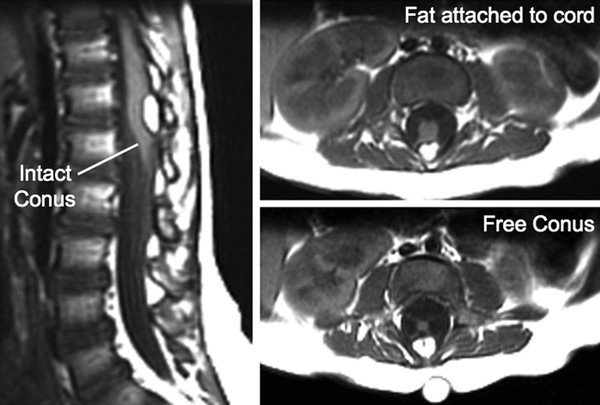
26.1.2 Transitional Lipoma
26.1.3 Terminal Lipoma
26.1.4 Chaotic Lipoma
26.2 Surgically Relevant Embryology
26.2.1 Embryogenesis of Dorsal and Transitional Lipomas
26.2.2 Embryogenesis of Chaotic Lipomas
26.2.3 Embryogenesis of Terminal Lipomas
26.3 Intraoperative Electrophysiologic Monitoring
26.4 Surgical Technique for Total or Near-Total Resection
26.4.1 Step 1. Exposure
26.4.2 Step 2. Detachment of Lipoma from Dura
Surgical Management of Complex Spinal Cord Lipomas
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree








