5 Surgical Therapy of the Temporal Bone Otologic/Neurotologic Instrumentation Surgical Access to the Mastoid and Middle Ear, Grafting Materials. Considerations for Pediatric Ear Surgery. Canaloplasty of the External Ear Canal Temporal Bone Resections for Malignant Disease Surgical Management of Aural Atresia Management of Middle Ear Trauma Surgical Management of Acute Otitis Media and Chronic Otitis Media with Effusion Middle Ear Ventilation Surgery Surgery for Chronic Otitis Media Myringoplasty and Underlay Tympanoplasty Surgical Management of the Facial Nerve Management of Facial Nerve Neoplasms Management of Traumatic Facial Palsy Surgical Management of Bell Palsy Surgery for Implantable Auditory Devices Surgery for Bone-Anchored Hearing Aids Surgery for Implantable Hearing Aids Neurotologic Approaches to the Medial Temporal Bone Retrosigmoid/Suboccipital Approach Combined Approaches to the Cerebellopontine Angle Surgical Management of Chronic Vestibular Disorders Translabyrinthine and Middle Fossa Vestibular Neurectomy Retrosigmoid, Retrolabyrinthine, and Combined Vestibular Neurectomy Cochleosacculotomy and Singular Neurectomy; Posterior Semicircular Canal Occlusion Surgery for Vestibular Schwannoma Infratemporal Fossa Approaches Surgery for Lesions of the Petrous Apex In general, otologic and neurotologic surgery is quite different from most other surgical disciplines. It typically requires a very unique set-up in the operating room and a special set of instruments. Also, practically every otologic procedure is performed using the surgical microscope and selective cranial nerve monitoring. • Objective testing of the facial nerve—see p. 90 • Intraoperative monitoring—see p. 263 The unique character of otologic procedures requires a specific set-up in the operating room (Fig. 5.1A). This set-up differs from those of most other surgical disciplines. As with all other surgical disciplines, however, respect for sterile principles is fundamental. Many otologic procedures can be performed under local anesthesia, and this requires special considerations. The surgeon must be comfortable while performing microsurgery. Adequate legroom under the operating table can be achieved in most cases by turning the top of the table 180°. Also, proper posture during surgery is critical, especially for lengthy skull base procedures. A comfortable chair should be available that allows adequate back and arm support. Equipment typically used for otologic procedures includes a suction system, preferably a multi-canister suction system to avoid lengthy breaks once the canister is full and needs to be changed, a high-speed otologic drill, the surgical microscope, an irrigation system, and special otologic instruments. Irrigation and suction systems may be combined into a suction–irrigation set-up preferred by most surgeons (Fig. 5.1B, C). Intraoperative facial nerve monitoring should be used as a safety standard (see p. 263). Naturally, electrocautery equipment should be available for all procedures. A laser is currently used by many surgeons for stapes surgery but continues to evolve and might be used for various other otologic procedures in the future. Neurotologic and lateral skull base procedures might require even more equipment. Thus, a larger operating theater is desirable for those surgeries. Motor function of multiple cranial nerves might be monitored such as with surgery for lesions of the jugular foramen or brainstem implantations. For some vestibular schwannoma surgeries where hearing preservation is intended, intraoperative electrocochleography (ECochG), compound action potential (CAP), and auditory brainstem response (ABR) monitoring might be helpful (see p. 58). The CUSA (Cavitron Ultrasonic Aspirator) is typically used for skull base surgery to aid in intradural tumor dissection. Many skull base procedures require placement of an abdominal fat graft or even more sophisticated microvascular reconstruction procedures. Thus, the surgical set-up might include more than one sterile site. In those cases, a second electrocautery and suction might be helpful. Special re-tractors and head positioning systems are also used for certain procedures. Intracranial dissection often benefits from different suction irrigation systems (Brackmann suction irrigation system, Fig. 5.1D). An appropriate suction system is essential for every otologic and neurotologic procedure. A multicanister system allows for a smooth transition once a canister is full. Also, a flexible tubing system and various caliber suctions are critical (Fig. 5.1B, C). To provide adequate irrigation and suction during drilling, two set-ups are available: integrated suction–irrigation systems or regular suction and irrigation through the drill or via an assistant. Each system has pros and cons and specific utilization depends on the surgeon’s preference. In general, two types of otologic drills are available: pneumatic and electric systems. In general, pneumatic systems are more powerful and have superior torque. Electric systems, on the other hand, are typically less powerful but have improved handling over pneumatic systems. Traditionally, therefore, electric systems were used for more delicate oto-logic procedures, whereas most surgeons preferred pneumatic systems for drilling during intense neurotologic surgeries. Modern electric drills, however, feature-high power motors that are suitable for lengthy drilling procedures and are therefore the preferred choice in many centers. Fig. 5.1 A–F Various sizes of cutting and diamond burrs are available (Fig. 5.1E). The surgeon should choose wisely, as different burr characteristics can be used for various applications. Rough-cut diamond burrs are also available and provide more aggressive dissecting qualities but generally avoid lacerations of soft tissues such as the dura. Also, diamond drills may be used for hemostasis of small vessels if used correctly. Skeeter microdrills are often used for stapes surgery. They are mainly used for footplate work, especially with obliterative otosclerosis. Although these are used by many surgeons, various types of laser instruments or hand drills might be used with similar results. Otologic surgery would not be possible without the use of the operating microscope. As with most other types of equipment, devices are available from several manufacturers. The basic principles of handling the microscope are uniform, however, and the otologic surgeon must be familiar with the equipment used. Before scrubbing, the surgeon must also ensure proper set-up such as proper interpupillary distance and eyepiece setting, correct lens (typically 200 or 250 mm objective for otologic procedures), and correct angle and side of the observer arm. Adequate balancing of the microscope is crucial and should be checked by the surgeon prior to draping. Lasers were first introduced to clinical otologic practice via stapedectomy. Compared with conventional dissection techniques, tissue vaporization provided by lasers, and thus touch-free surgical manipulation, can reduce the risk of mechanical trauma to the inner ear while providing excellent hemostasis (Fig. 5.1F). Today, lasers are used mainly for stapes surgery, although new applications are evolving. Several types of lasers are available, of which the KTP-532 (potassium titanyl phosphate), argon, and CO2 lasers are most commonly used in otology. The CO2 laser is most commonly used, with a light wavelength of 10 600 nm (invisible to the human eye and thus necessitating a visible aiming beam). Traditionally, the CO2 laser required a rigid articulated arm for its delivery. Recently, a flexible tubing system has become available that allows for easy handling and precise application. Not all instruments are used for every procedure. A set of instruments should be small enough to avoid any loss of time searching for the required instrument. This is not only time-efficient but also cost-efficient and makes assisting the surgeon easier. There are different sets for different otosurgical procedures. It is therefore recommended to compile a small myringotomy set, a set for chronic ear surgery with and without mastoidectomy, one for stapes surgery, one for cochlear implantation, and a basket for skull base surgery. Specialized trays should be available for designated instruments not used for each surgery of a given type. Benecke JE, Stahl BA. Otologic instrumentation. In: Brackmann DE, Shelton C, Arriaga M, eds. Otologic Surgery. Philadelphia, PA: W.B. Saunders; 2001: 1–24 Intraoperative monitoring of cranial nerves has evolved into an essential tool for any surgical otologist/neurotologist. The facial nerve is potentially at risk during almost every otologic procedure and thus monitoring should be done routinely. Monitoring of auditory function can be used to direct intraoperative decisions during vestibular schwannoma surgery, although the feedback is markedly delayed. • Objective audiometry—see p. 58 • Evaluation of the facial nerve—see p. 87 • Surgery for vestibular schwannoma—see p. 374 Intraoperative facial nerve monitoring is widely accepted and has been established as a valuable adjunctive modality to be employed in a variety of neurotologic and skull base surgical procedures. This type of monitoring assists the surgeon in the identification and preservation of the facial nerve. It is cost-effective, and its routine use should be adopted to reduce the risk of iatrogenic facial nerve injury during otologic and skull base surgery. Various devices are currently available from different manufacturers. Although facial nerve monitoring is not a substitute for anatomical identification of the nerve, intraoperative monitoring provides an additional technique to optimize surgical safety. It might also aid in the educational process of trainees. Inherent in any effective motor cranial nerve monitoring configuration is the auditory and visual display of information obtained from both free-running electromyography and elective, controlled electrical stimulation of the monitored nerves. Neuromuscular blocking agents should be avoided except for the use of short- or intermediate-acting drugs. Clinical experience suggests that either hook-wire or subdermal needle electrodes are ideal for the monitoring of the function of cranial nerves VII, IX, XI, and XII because of their signal characteristics and reliability. For facial nerve monitoring, electrodes are typically placed in the orbicularis oculi and orbicularis oris muscles (Fig. 5.2A). Also, one or more reference electrodes are needed for recording and stimulation (Fig. 5.2B). Appropriate functioning of the system must be confirmed at the start of the case and rechecked intermittently throughout the surgical procedure. Although some nerve monitors sound a warning if an electrode is displaced, use of a clear adhesive drape over the face helps to secure and corroborate electrode position. Prior to electrical stimulation, confirm that current flow is present by first contacting the stimulus probe with soft tissue. If a stimulator probe is used, check that the device is active and, again, document current flow during contact with soft tissue. Note that as the muting circuit prevents a distracting noise from being sounded through the loudspeaker during cautery, valid monitoring does not occur during these periods. A normal response pattern is shown in Fig. 5.2C. Burst or train responses are pathologic responses that are typically observed with brief mechanical contact of the nerve or traction forces acting on the nerve, respectively (Fig. 5.2D). The encouraging experience with facial nerve monitoring has driven recent interest in monitoring of other cranial nerves during procedures that involve the lower cranial base (Fig. 5.2E). Current outcome measures of success regarding the management of a tumor of the lower skull base include not only the complete removal of the disease but also the functional preservation of those cranial nerves that are involved in the primary process. Depending on the site of origin and extent of the lesion, surgical exposure is gained through varying combinations of the infratemporal fossa approach, the transjugular approach, the translabyrinthine or transcochlear approach, the transcondylar approach, the suboccipital approach, or an extended retrosigmoid approach. An acute surgical injury to the proximal portion of a functioning vagus nerve may result in an anesthetic larynx, a breathy, weak voice, and, most problematically, aspiration. These profound changes are further aggravated when there is an additional paralysis of one or more of the other lower cranial nerves. Iatrogenic injury to these cranial nerves is reduced with appropriate monitoring of at-risk, physiologically intact nerves. Although the best way to reduce neural trauma is early localization of the nerves, microtrauma associated with dissection may result in significant facial nerve injury as a result of prolonged dissection or traction. Fortunately, these surgical maneuvers may often provoke a mechanically evoked nerve response that can alert the surgeon as to their presence. These mechanically evoked potentials appear to be a result of rapid neural deformations resulting in ion depolarization. The most efficient stimuli appear to be abrupt contact or traction of the nerve (Fig. 5.2D). Slower, more progressive traction, however, may result in little or no response. When traction responses occur, they tend to be multiple asynchronous potentials, or “trains,” in contrast to the single synchronized potential, or “burst,” seen with brief direct mechanical contact. Fig. 5.2 A–E Intraoperative ABR: Intraoperative monitoring of auditory brainstem responses (ABR, see p. 64) has been the method traditionally used intraoperatively in surgery aiming to preserve hearing in appropriate acoustic neuromas or other cerebellopontine angle (CPA) lesions. Click-evoked potentials are recorded by a needle electrode placed through the inferior part of the tympanic membrane on the cochlear promontory (electrocochleography) and by electrodes in the scalp. ABR measurements in the operating room have several disadvantages. Recording of the response may take a relatively long time. Also, competing electrical interference from the many parts of the equipment may make it difficult if not impossible to record reliable ABRs. Finally, to obtain clear recordings, all direct contact with the patient may need to be suspended during the recording period, potentially increasing duration of the surgery. A more functional method of acoustic nerve monitoring during tumor removal may be recording of the compound action potential (CAP). The amplitude of the recorded signal is larger, potentially reducing recording time significantly. Recording of CAP responses can generally be accomplished in ~20 seconds. CAP measurement also minimizes interfering noise from surrounding devices and patient artifacts that compromise intraoperative ABR recordings. Electrocochleography: Electrocochleography (ECochG) records near-field potentials and provides rapid feedback of the compound action potential of the auditory nerve, probably generated near the cochlea and cochlear microphonic potentials, which are generated by the outer hair cells (see p. 67). ABRs are far-field potentials that have a slower feedback. In practice only wave V, which is generated within the brainstem, is mon itored because the other potentials are much smaller and often undetectable. The short-latency ECochG potentials are the most useful for monitoring during surgery because they are generally not affected by anesthesia, they are almost always detectable, and they have an instant feedback. On the other hand ABRs, while useful, are undetectable in some patients even when there is useful hearing; in addition, it may take up to a minute or more to obtain satisfactory recordings because of the small amplitude of the potentials. By monitoring both ECochG and ABRs, the entire portion of the auditory system at risk during CPA surgery can be monitored. The presence of N1 indicates the integrity of the auditory nerve peripheral to the tumor: wave V is an indication of auditory nerve activity central to the tumor and the cochlear microphonic potential indicates the condition of hair cells in the cochlea, which are at risk from interrupted blood supply or from damage to other structures essential for cochlear function. The waveforms of the electrocochleogram are stable and reproducible in the majority of patients. The potential problems with the recording include displacement of the electrode in the ear, blood or fluid entering the middle ear and blocking sound transmission, the inability to recognize the cochlear microphonic waveforms in some patients because of their small amplitude, the possibility that direct trauma to the cochlear nerve may not cause immediate changes in the electro-cochleogram, and the theoretical possibility that potentials will be generated from a site distal to the lesion. When the status of N1 and wave V at the end of an operation were correlated with the hearing outcome, it was found that if N1 and wave V were lost there was no hearing. If wave V and N1 were present, most patients had a useful hearing; if N1 was present and wave V was lost or never detected, the results were not predictable. The aim is that monitoring will give an indication of early hearing compromise that is reversible and will allow the surgeon to alter the dissection. This is the case in some patients in whom a change occurred that recovered when the dissection was stopped or altered. Monitoring has not made a definite difference in the outcome when there has been abrupt loss of function without warning that does not recover, presumably due to interruption of vascular supply, when gradual loss of function occurs, and when there is no change in any waveform during the operation. Brackmann DE, Owens RM, Friedman RA, et al. Prognostic factors for hearing preservation in vestibular schwannoma surgery. Am J Otol 2000;21(3):417–424 Edwards BM, Kileny PR. Intraoperative neurophysiologic monitoring: indications and techniques for common procedures in otolaryngology-head and neck surgery. Otolaryngol Clin North Am 2005;38(4):631–642, viii Several routes can be chosen to access the tympanomastoid compartment. Almost always, a tympanomeatal flap has to be raised to work beyond the soft tissues of the external auditory canal and tympanic membrane. Each route offers distinct features and exposure to various locations within the middle ear. Naturally, advantages and disadvantages are associated with each. • Chronic suppurative otitis media—see p. 130 • Surgical management of AOM and COME—see p. 291 • Ossiculoplasty—see p. 306 • Surgery for otosclerosis—see p. 317 For procedures performed under local anesthesia, preoperative injections are fundamental. They not only provide a pain-free experience for the patient but also serve to reduce intraoperative bleeding. Therefore, injections should be used regardless of whether surgery is performed under local or general anesthesia. Typically 1% lidocaine with epinephrine (1:100 000) is used for otologic surgery. Injections should start in the periauricular skin. Specifically, the area of the postauricular skin should be infiltrated. The needle can be directed toward the posterior aspect of the external auditory canal medial to the conchal cartilage. Then, the soft tissues of the endaural incision in the gap between the tragal and conchal cartilage may be injected as well. The tragus should also be injected, especially when a conchal graft will likely be obtained for the procedure (Fig. 5.3A). Once, the periauricular soft tissues have been injected, a nasal speculum may be used to inject the four quadrants of the external auditory canal (Fig. 5.3A). Over-injecting these areas should be avoided since this might interfere with surgical exposure. The surgical site prepping should be finished to maximize the vasoconstriction effect of the injected material. Then, with the help of the operating microscope, additional injections should be placed into the canal skin. Specifically, the injection needle should be placed under the periosteum of the bony posterior canal wall to outline the vascular strip. A reasonable amount should be injected and special care must be taken not to over-inject this area. The injection should be continued inferior to the tympanomastoid suture line and into the inferior portion of the posterior EAC. With planned excision or manipulation of the anterior canal wall, the anterior portion of the EAC should be injected just lateral to the bony cartilaginous juncture. With a proper injection technique, the skin of the EAC should turn white as a result of the vasoconstriction. Once that effect has been observed, the surgeon may proceed with raising the tympanomeatal flap. Only a few skin incisions have been described for accessing the tympanomastoid compartment. Each incision features advantages and disadvantages. As a general rule, larger exposures and more extensive surgical procedures require a postauricular approach. Also, the location of a standard postauricular incision is usually not cosmetically disfiguring and many patients prefer this route of access over an endaural incision. Transcanal surgery without superficial skin incision necessitates operating through the speculum, which requires adequate experience. Also, harvesting of a tissue graft is impossible through a transcanal route and thus requires a separate incision. This approach is mostly used for smaller procedures involving the posterior aspect of the middle ear and tympanic membrane. Classically, stapes surgery and ossiculoplasties are performed using this approach. Small perforations of the posterior quadrants of the tympanic membrane can also be managed this way. Bimanual operating through the speculum (Fig. 5.3B) requires some practice and speculum holders are available for less experienced surgeons. Often, harvesting of a temporalis fascia graft or any other soft-tissue graft requires a small postauricular incision beneath the superior aspect of the auricle. Harvesting of tragal cartilage can be performed via a small vertical incision in the skin overlying the tragus. An endaural approach typically provides sufficient access for many tympanoplasty procedures. One advantage of this approach is that the fascia graft can be obtained directly from the temporalis fascia (Fig. 5.3C). No additional incision is needed. The use of two small tissue retainers allows for improved bimanual dissection. Fig. 5.3 A–F The postauricular incision is the standard incision and the workhorse for mastoidectomy and tympanomastoid procedures. In general, this incision should be used whenever mastoid access might be required and it is also used for cochlear implantation. When trans- or intracanalicular manipulation is required such as for most tympanomastoid procedures, elevation of a vascular strip flap becomes necessary. Another option of accessing the ear canal from posterior is to elevate and incise the canal skin from posterior. Care must be taken to leave enough lateral ear canal skin to avoid postoperative epithelial migration into the mastoid and subsequent cholesteatoma formation. The postauricular incision should be made ~2 cm behind the crease (Fig. 5.3D). Placing the incision directly in the postauricular crease should be avoided since this might cause painful scarring. While dissecting the subcutaneous soft tissues, the superior portion of the incision should be elevated laterally to provide easy access to the temporalis fascia. By using an adequate technique, the soft tissues in this area splay open and reveal the loose areolar tissue overlying the fascia. This may be carefully dissected off. Typically, an assistant or the scrub nurse may aide in pulling the lateral soft tissues superiorly and thus provide better access to the temporalis fascia (Fig. 5.3G). Conchal cartilage is also readily available through this approach (Fig. 5.3F). An anterior pedicled Palva flap should then be raised directly off the mastoid bone. With that, the posterior ear canal lumen becomes visible. Here, the spine of Henle serves as a landmark. Subsequently, the posterior canal skin should be carefully elevated to avoid tearing. When using a vascular strip incision, the previously made canal incisions should be identified from posteriorly and extended as needed. When using a standard flap, careful incision of the canal skin provides access to the ear canal. During closure, the Palva flap should be sewn back into place. Then, the postauricular skin may be closed in a standard fashion using either absorbable or nonabsorbable suture material. A head wrap or Glasscock dressing should be applied and left in place for 2–3 days. This approach is used for more extensive surgeries of the lateral skull base and for neurotologic procedures of the cerebellopontine angle where full exposure of the sigmoid sinus and beyond is needed. The incision is performed about two to three finger breads behind the postauricular fold. The superior part of the neck should also be included into the surgical prepping to allow for emergency access to the internal jugular vein (in case of uncontrollable bleeding through a hole in the sigmoid sinus). The temporoparietal fascia should be identified as with a standard postauricular incision and an extended version of a Palva flap can be used. Several tissue types have been used as grafting materials in otologic surgery. Currently, most surgeons use either autologous cartilage or temporalis fascia for chronic ear surgery. Only a few centers use cadaver-harvested homografts for tympanic membrane replacement. Due to infectious concerns mainly focusing on the possible transmission of prion disease, homografts will not be discussed in greater detail in this book. Xenografts such as AlloDerm and other products have also been used to reconstruct the tympanic membrane. The selection of the specific material depends on various factors: the size and location of the perforation, the status of the eustachian tube, previous procedures, and probably most importantly, surgeon’s preference. Fig. 5.3 G–L Temporalis fascia is the most commonly used grafting material in chronic ear surgery. Typically, the fascia graft is obtained during the surgical approach relatively early in the procedure. Both the postauricular (Fig. 5.3G) and endaural (Fig. 5.3C) approaches allow for easy access to the temporalis muscle and its overlying fascia. When using a transmeatal route of access, an additional postauricular incision becomes necessary. This should be placed in the hairline relatively superior and posterior to the auricle (similarly to the superior extension of a standard postauricular approach). Once a large enough piece of temporalis fascia has been obtained, a tissue press can be used to flatten and extract the moisture from the graft. After a few minutes, the tissue press may be opened to allow the graft to dry. Once dry, the graft can be cut into shape. To place the graft, most clinicians prefer to dip it into saline immediately before introducing it into the ear. Several pathologies require structural reinforcement of the new tympanic membrane or a specific area of it. Such pathologies include a collapsed middle ear space and atelectatic drum and permanent eustachian tube dysfunction. Cartilage can be obtained from various areas of the auricular cartilage. The most common sites are the tragus (tragal cartilage, Fig 5.3E) and the cartilage of the concha (concha cartilage, Fig. 5.3F). Tragal cartilage is very straight and therefore not preshaped. It is easily accessible via a small skin incision of the tragal skin. This incision can be made on the posterior surface and thus remains concealed for cosmetic purposes. When leaving the lateral-most cartilage untouched, the outer shape of the tragus remains unchanged. Conchal cartilage, in contrast, is larger and thus more material is available. The cartilage itself, however, is slightly curved and preshaped and has to be weakened to obtain a straight piece. Also, harvesting conchal cartilage requires a postauricular incision. Care must be taken not to injure the skin of the conchal bowl. Perichondrium or combined grafts (cartilage–perichondrium grafts) can be obtained from both sites. Again, the conchal cartilage area provides more graft material and a larger area. Some surgeons prefer perichondrium as an alternative to temporalis fascia. With multiple previous surgeries, it might be somewhat more complicated to obtain enough tissue for grafting. Temporalis fascia has usually been removed during a previous procedure. As a substitute, large pieces of perichondrium can be obtained from the conchal area. Also, periosteum from the posterior occipitomastoid area can be harvested as a replacement. However, it is often more difficult to establish the correct tissue planes and the tissue is usually much thicker. Several flaps have been described for access to the middle ear. Common to all flaps is that traditional surgical principles such as hemostasis and gentle tissue handling should be applied; general values of surgical exposure and access should also be taken into consideration. Most flap systems are modifications of a standard tympanomeatal flap designed to achieve specific tasks. Prior to flap elevation, careful injection of a vasoconstrictor should be performed. Injections should be performed under the endosteum of the canal. This way, elevating the flap with traditional instruments is easier and less traumatic. The fibrocartilaginous tympanic annulus should be elevated out of its groove to preserve the epithelial continuity of the meatal skin with the tympanic membrane and to avoid an iatrogenic perforation. The standard tympanomeatal flap can be used for most tympanoplasties since most pathologies require access only to the posterior tympanum. The meatal incision can be made parallel roughly 6–8 mm lateral to the tympanic annulus (Fig. 5.3I). A triangular-shaped flap (gothic arch) is preferred by many clinicians and typically provides similar access. The canal incisions for the standard flap can be extended inferiorly (Fig. 5.3K) as well as superiorly (Fig. 5.3L) to gain access to the anterior-superior quadrant for congenital cholesteatomas or to the hypotympanum for intratympanic glomus tympanicum tumors. In any case, the flap should be extended to fit the requirements of the specific exposure. The remaining tympanic membrane can be left pedicled on the manubrium of the malleus. When extensive bone removal of the posterior superior canal wall (scutum) might be required such as in stapes surgery, the corresponding area of the flap can be extended laterally (Fig. 5.3K). Thus, the initial skin incision should be moved laterally along the posterior canal wall. By definition, the vascular strip is not a tympanomeatal flap but a flap to move soft tissues of the EAC to provide exposure of the tympanic membrane. It is primarily used for postauricular underlay tympanoplasty, for lateral grafting, and for tympanomastoid procedures. The basic principle of this flap is to move the posterior superior ear canal skin (the vascular strip) laterally into a protected area and to bring it back at the end of the procedure. This flap requires careful vasoconstrictor injections and careful cleaning of the ear canal through a transcanal route. Then, the two vertical incisions, one roughly in line with the tympanomastoid and one with the tympanosquamous suture line should be connected with an incision very close to the tympanic annulus (Fig. 5.3H). The created box remains pedicled laterally (Fig. 5.3I). The postauricular approach can then be completed and the previously created canal incisions can be found from behind. Then the entire vascular strip can be elevated out of the canal. The flap can be held in place with a retractor or by using a Penrose drain. Balkany TJ, Telischi FF, Angeli SI, Eshraghi AA. Tympanomeatal flaps. Laryngoscope 2003;113(7):1266–1268 Downey TJ, Champeaux AL, Silva AB. AlloDerm tympanoplasty of tympanic membrane perforations. Am J Otolaryngol 2003;24(1):6–13 Linder TE, Fisch U. A checklist for surgical exposure in stapes surgery: how to avoid misapprehension. Adv Otorhinolaryngol 2007;65:158–163 Pediatric otology presents unique challenges. The patho-physiology of disease and specific anatomical differences in children make certain aspects of medical and surgical management of pediatric ear disease unique. • Computed tomography (CT)—see p. 95 • Chronic suppurative otitis media (CSOM)—see p. 130 • Complications of otitis media—see p. 141 • Early acquired hearing loss and auditory neuropathy—see p. 162 The epidemiology of hearing loss in children is different from that in adults. Many pediatric patients with hearing loss have conductive hearing loss from acute and chronic otitis media or sensorineural hearing loss that is congenital. Other entities that present at birth or in early childhood include syndromic and nonsyndromic genetic causes. Rarely, congenital loss is a result of infection, such as cytomegalovirus (CMV) or rubella during pregnancy, or neonatal meningitis. There are also iatrogenic causes, such as ototoxicity through gentamicin administration (Fig. 5.4A). The anatomy of the pediatric temporal bone partially explains why pediatric patients are at higher risk for certain problems, such as acute and chronic otitis, and why their presentation and management may be different from those in adults. Eustachian tube dysfunction is very common in young children for many reasons, including a smaller tube lumen and a more horizontal orientation. In some populations such as patients with cleft palate or Down syndrome, eustachian tube dysfunction is nearly universal (see p. 9). Early treatment is important to prevent complications. For multifactorial reasons, children are more likely to demonstrate complications of acute otitis media, such as acute mastoiditis, facial nerve paralysis, meningitis, and brain abscess. Many children each year undergo pressure equalization tube placement (see p. 291). Eustachian tube obstruction may also be a result of adenoid hypertrophy, which is also more common in children. Adenoidectomy should be considered for children requiring repeat tympanostomy tube placement. As the eustachian tube grows and changes, the immune system matures, and the adenoids become smaller relative to the nasopharynx, and most children will outgrow eustachian tube dysfunction. The temporal bone anatomy in children is also different from that of an adult. The mastoid does not develop until late in fetal life and it continues to develop until 2 years of age (Fig. 5.4B, C). The mastoid grows laterally. Accordingly, the mastoid segment of the facial nerve is more lateral in children. Care must be taken during periauricular skin incisions, soft-tissue dissection, and periosteal elevation, particularly at the mastoid tip, which may not overhang and protect the facial nerve as in the adult. The cochlea is near adult size at birth, and probably of adult size by 25 weeks of gestation. The facial recess and mastoid antrum are adequately formed by 1 year of age. Therefore, cochlear implants can be performed at a very young age. The auricle is nearly adult size by age 4 years and is adult size by 9 years of age. Therefore, microtia reconstructive surgeries can be performed at ~7 years old, when the auricle is nearly 90% of its adult size. The external auditory canal is shorter in children than in adults and grows until ~9 years of age. Acquired cholesteatoma (see p. 136) develops from squamous epithelium that enters the middle ear through a retraction pocket or a perforation. The etiology of the more rare congenital cholesteatoma is quite different. Specifically, it is hypothesized that epidermoid (ectodermal origin) remnants become entrapped medial to the tympanic membrane during embryologic development. Classically, congenital cholesteatoma has been described as originating from the anterosuperior quadrant, although many are found in other locations as well. There can be an inclusion cholesteatoma in the tympanic membrane or there may be no connection to the tympanic membrane, which is often found intact. They are usually encapsulated and limited, but if they do extend posteriorly they are considered aggressive. Some congenital cholesteatomas are found in the medial temporal bone and they can present with facial palsy. Congenital cholesteatoma has been reported to carry a higher rate of postoperative recurrence than acquired cholesteatoma. Fig. 5.4 A–E There are aspects of the natural course of cholesteatoma in children that are different from adults. Preoperative complications, such as labyrinthine fistula, facial weakness, and sensorineural hearing loss, are lower in children than adults. However, cholesteatoma, whether congenital or acquired, is generally more aggressive, grows faster, and has a higher rate of recidivism in children (rates vary, but are likely around 40%–67%). Recidivism occurs when epithelium is left behind or when the mastoid is not well aerated and retraction recurs and keratin debris reaccumulates. The mastoid is usually well aerated in congenital cholesteatoma. However, temporal bones are less sclerotic in children and possibly have lower mineral content than the adult bone. Pediatric temporal bones may therefore be more easily eroded by cholesteatoma. In addition, the biology of congenital cholesteatoma may be different, with possibly more osteoclastic activity. Tympanoplasty is generally less successful in children, likely because of frequent upper respiratory infections and poor eustachian tube function resulting in an increased propensity for otitis media. Regardless of the etiology of a tympanic membrane perforation, such as acute otitis media, chronic otitis media, iatrogenic from pressure-equalization tubes, or trauma, the timing of repair of perforations in children is controversial. Tympanoplasty success or take rates are between 70% and 90% for the first surgery, even if a child develops further otitis media. However, it seems that if eustachian tube dysfunction is present, re-perforation would be more likely. Generally, a child should be at least of school age and the ear should be dry for at least 1 year prior to closure of a tympanic membrane perforation. Evaluation of the adenoids is advised in children undergoing tympanoplasty, but previous adenoidectomy does not change the likelihood of success of tympanoplasty. Management of allergic or infectious rhinosinusitis is also advised. Myringosclerosis is associated with poorer hearing outcomes and higher failure rates than normal tympanic membranes. The timing of ossicular chain reconstruction in the pediatric population remains controversial. Children are more prone to upper respiratory infections, eustachian tube dysfunction, and otitis media. However, good results for ossicular chain reconstruction in children have been reported. Mobilization for stapes fixation should be considered initially for children who are still at risk for otitis media, meningitis, and other complications of stapedotomy or stapedectomy with placement of a prosthesis. Mobilization may be successful in a significant number of children. Care must be taken to rule out an inner ear malformation causing a third-window effect and a pseudoconductive hearing loss with present acoustic reflexes. Computed tomography is usually diagnostic. Cochlear implants are indicated in children with sensorineural hearing loss who do not benefit from hearing aids. While most deaf children are born to hearing parents, cultural sensitivity is necessary when considering implantation of children. Communication by sign language is one option, and there may be parents, usually deaf, who choose not to use assistive devices. However, cochlear implants are safe for children. In general, the earlier cochlear implantation is performed, the better the speech and language results. Ideally, cochlear implantation should be performed as close to 12 months as possible for congenitally deaf children (Fig. 5.4E). As a prelingually deafened patient gets older, the potential benefit from implantation decreases. Prelingually deafened adults are poorer candidates for cochlear implants as they have not obtained open-set speech; however, other benefits may be achieved with an implant. For optimal results, implanted children should be in a program to promote auditory skills. Children or adolescents who are postlingually deafened are also good candidates for a cochlear implant. While the cochlea, mastoid antrum, and facial recess are of adult size at about 1 year of age, the cochlear implant surgeon must consider congenital abnormalities of the mastoid, middle ear, and inner ear. Insults early in gestation can cause deformity of the inner ear (see p. 123). Twenty percent of children with congenital sensorineural hearing loss have some form of cochlear dysplasia (Fig. 5.4D). Patients with an enlarged vestibular aqueduct alone or patients with other anomalies such as Mondini deformity can be good cochlear implant candidates. A perilymphatic gusher or leak may be encountered with the cochleostomy. After insertion, the cochleostomy site should be packed with fascia or muscle. Only partial insertion of the cochlear implant may be possible. There should be special consideration for electrode array selection (non–modiolar-hugging, circumferential, or compressed) depending on the anomaly encountered. Cochlear implantation should not be undertaken in the setting of otitis media or a tympanic membrane perforation. In addition, it is not possible in patients with Michel’s deformity, where there is congenital agenesis of the inner ear. It should also be considered carefully in cases of radiographic cochlear nerve deficiency. With a completely absent cochlear nerve, auditory brainstem implantation may be an option in the future (a non–FDA-approved indication). Additional assistive listening devices may be considered for appropriate patients. Hearing aids may be appropriate for most children with hearing loss. For microtia-atresia patients, BAHA devices or active middle ear implants may be options (see Chapter 5, pp. 331 and 334). Frenzel H, Hanke F, Beltrame M, Steffen A, Schönweiler R, Wollenberg B. Application of the Vibrant Sound-bridge to unilateral osseous atresia cases. Laryngo-scope 2009;119(1):67–74 Nikolopoulos TP, O’Donoghue GM, Archbold S. Age at implantation: its importance in pediatric cochlear implantation. Laryngoscope 1999;109(4):595–599 Shirazi MA, Muzaffar K, Leonetti JP, Marzo S; Surgical Treatment of Pediatric Cholesteatomas. Surgical treatment of pediatric cholesteatomas. Laryngoscope 2006;116(9):1603–1607 Shohet JA, de Jong AL. The management of pediatric cholesteatoma. Otolaryngol Clin North Am 2002;35: 841–851 Enlargement of the external auditory canal is often warranted during chronic ear surgery or lateral grafting. Canaloplasty alone is the treatment of choice for exostoses and other lesions of the external auditory canal. Preservation of the skin of the auditory canal is essential to provide optimal results and avoid random scarring. • Chronic suppurative otitis media (CSOM)—see p. 130 • Lateral graft tympanoplasty—see p. 303 • Management of middle ear trauma—see p. 287 Canaloplasty of the external auditory canal describes all otologic procedures that attempt to restore a patent canal lumen. Canaloplasty can be part of other otologic procedures such as lateral grafting or it can be used alone for conditions affecting the external auditory canal. Thus, indications for canaloplasty include exostoses or osteomata of the external auditory canal (see p. 117) as well as other procedures that require a canaloplasty by default. Congenital external canal stenosis and its management will be discussed elsewhere (see Chapter 5, p. 283). It is typically caused by a failure of canalization of the epithelial plug portion of the first bran-chial cleft. Persistence of the tympanic ring results in a bony atretic plate at the level of the tympanic membrane. External auditory canal stenosis may also be combined with aural microtia. Acquired external canal stenosis may occur following trauma, infection, or dermatologic disorders. Stenosis can be secondary to previous otologic surgery in which the skin and periosteum of the external auditory canal are elevated and resected. Secondary healing occurs with the formation of granulation and scar tissue. A similar situation can occur following prolonged refractory external otitis leading to ulceration and fibrosis that result in acquired canal stenosis. Less common causes include long-standing impacted foreign bodies and fracture to the tympanic bone if not properly stented. The main indication for canaloplasty is external auditory canal exostoses. The presence of exostoses alone, however, does not warrant surgical intervention. Surgery is typically indicated when a conductive hearing loss develops because of the narrowing of the canal lumen and due to frequent cerumen impactions. Also, exostoses can lead to defective reepithelialization of the canal skin and subsequent chronic or recurrent otitis externa. The patient should be prepared for a postauricular incision and the fact that the external auditory canal will be packed for several weeks following the procedure. Also, facial nerve monitoring should be used for each case since the mastoid segment of the facial nerve can be lateral to the posterior-inferior part of the tympanic annulus, where it might be at risk during posterior canal drilling. Typically, canaloplasty is performed via a postauricular approach. However, several techniques have been described that utilize a transcanal or endaural route. These techniques lack the broad exposure obtained through a postauricular incision. Most canaloplasties require removal of anterior bone, which is generally best accessed from posterior. Furthermore, protection and preservation of the canal skin and the tympanic membrane can be best achieved with adequate visualization with a more posterior angle of exposure. A standard postauricular approach is made with exposure of the posterior bony canal wall (Fig. 5.5A). The soft tissues of the canal are preserved and moved anterior through a self-retaining retractor. The spine of Henle is exposed and the posterior canal skin is further dissected medially. For posterior exostoses, the canal skin is carefully dissected from the bony exostosis. A laterally based skin flap should be raised (Fig. 5.5B). The bone is then removed predominantly by using small diamond burrs to protect the canal skin. The bone removal is continued until a normal canal contour has been restored. As a rule of thumb, bone removal should allow visualization of the entire tympanic annulus without moving the microscope (once the anterior part of the canal has been drilled as well). Medial bone removal is sometimes complicated by limited exposure. Once the tympanic annulus has been reached, care must be taken to preserve the chorda tympani and tympanic membrane. A small tympanomeatal flap can be raised to further avoid injury. When drilling on the posterior inferior bony canal wall, care must be taken not to injure the facial nerve since this might be lateral to the tympanic annulus in that region (Fig. 5.5C). Fig. 5.5 A–E As for the posterior bony canal, a flap system should be raised to protect the anterior canal skin (Fig. 5.5A). It is typically preferred to incise the canal skin over the exostosis and elevate and retract the lateral portion of the anterior canal skin. Then, the medial portion can be elevated off the exostosis as far as possible. Drilling of the excess bone can then commence. Special care must be taken not to violate the temporomandibular joint space (Fig. 5.5D). The surgeon should therefore look for bluish or grayish discolorations during drilling in that area. Generally, more bone can be removed in the superior and inferior aspects of the anterior canal bone than directly over the joint, thus creating a slightly convex shape of the bony anterior canal wall. The medial portion of the anterior canal flap can be pushed further medially and pushed onto the tympanic membrane. Some surgeons prefer to use a Silastic sheet to protect the soft tissues. Special care must be taken not to injure the anterior annulus since this might lead to blunting of the anterior tympanomeatal angle. Once all excess bone has been removed, the flaps are placed back onto the bony canal wall and the external auditory canal is packed with self-absorbable gelatin sponges. The laterally based posterior canal flap is also placed back once the packing is completed. The postauricular soft tissues are sutured back and a mastoid dressing is applied. Complications of canaloplasty are listed in Fig. 5.5E. House JW, Wilkinson EP. External auditory exostoses: evaluation and treatment. Otolaryngol Head Neck Surg 2008;138(5):672–678 Malignant lesions of the temporal bone provide a unique challenge. These tumors are often found in an advanced stage and require multimodal management including radical surgical excision. Several levels of surgical management are possible and have to be individualized for each patient. • Anatomy of the external ear and eustachian tube—see p. 9 • Imaging of the temporal bone—see p. 92 • Malignant neoplasms of the external ear—see p. 120 • Malignant neoplasms of the middle ear—see p. 151 Malignant tumors of the external auditory canal typically require a highly individualized management scheme. A careful review of CT and MRI studies and consideration of the patient’s overall health as well as the histologic classification of the tumor are necessary to conclude a management scheme. With squamous cell carcinoma involving only the skin of the external auditory canal without any bony destruction, sleeve resection of the auditory canal skin is an option. With bony destruction, on the other hand, a greater degree of surgical excision becomes necessary. However, the most important decision is whether to operate and whether extensive surgery is in the patient’s best interest. With many temporal bone malignancies, close interdisciplinary collaborations involving the neurotologist, the head and neck surgeon, the neurosurgeon, the radiation oncologist, and the medical oncologist are crucial. A sleeve resection is warranted with very small lesions of the external auditory canal without bony involvement. Excision of the involved skin with a large surgical margin is necessary. Also, adequate grafting using a split thickness skin graft usually becomes necessary to avoid stenosis of the lumen of the external auditory canal. According to onco-logic principles, surgical margins should be sent for histologic confirmation and, if they are positive for malignant tissue, the excision should be extended to a lateral temporal bone resection (Fig. 5.6A, B). Most clinicians, however, feel that sleeve resections are inadequate to manage malignant disease. A lateral temporal bone resection is indicated for lesions involving the external auditory canal without involvement of the middle ear or promontory bone. Once a lesion involves the bone of the promontory, it gains access to the lymph drainage of the carotid artery system and thus becomes less manageable. Overall, the prognosis seems to be much worsened once the cochlear promontory has been involved (see p. 151). Thus, a lateral temporal bone resection can be curative for lesions involving the external auditory canal with limited bony involvement. With a greater spread of the tumor, a subtotal temporal bone resection might be considered (Fig. 5.6A, B). In lateral temporal bone resection, the external auditory canal is removed en bloc with the tympanic membrane and the malleus. A parotidectomy or neck dissection often supplements the temporal bone specimen. Without involvement of the auricle, an extended postauricular skin incision should be performed. Then, the skin of the external auditory canal has to be separated from the auricle by performing an oval-shaped incision. The external auditory canal meatus is then closed in a layered fashion. Care must be taken to avoid in-growth of squamous epithelium and subsequent cholesteatoma formation by using a mattress suturing technique (Fig. 5.6C). Then, a cortical mastoidectomy with facial recess exposure is drilled. The sigmoid sinus and the middle fossa plate are defined. The incus is removed by rotating it posteriorly. It has to be done in a way so that the stapes tendon is holding the stapes in place and thereby avoiding inadvertent dislocation. Drilling is continued superiorly into the zygomatic arch. The bony integrity of the middle fossa plate has to be preserved. This superior trough should then be connected with the superior aspect of the glenoid fossa. The facial recess is then extended inferiorly. The entire mastoid segment of the facial recess has to be identified to the stylomastoid foramen (Fig. 5.6D). The extended facial recess is then expanded inferiorly and anteriorly. The inferior part of the tympanic annulus has to be followed anteriorly and an inferior trough is created. The jugular bulb should also be defined and the surgeon should be aware of the great amount of anatomical variations in this area. Fig. 5.6 A–E The superior and inferior troughs are both connected into the glenoid fossa. With enough bone removed in the superior and inferior troughs, the external auditory canal becomes mobile. The tendon of the tensor tympani muscle is cut and the specimen can be removed (Fig. 5.6E). The specimen as well as the surgical situs should be inspected to avoid leaving squamous epithelium or tumor remnants in place. Then, the eustachian tube can be obliterated. The defect can be reconstructed using various flap systems. A subtotal temporal bone resection is indicated with a deeply extending lesion invading the middle ear and its medial wall. In contrast to lateral temporal bone resections, subtotal temporal bone resections and en bloc resection of the specimen are difficult and probably unnecessary. It requires extensive dissection of the intrapetrous carotid artery, which is of questionable oncologic significance. Most otologists carry out a lateral temporal bone resection and expand this by removing the medial petrous bone in a piecemeal fashion. The great vessels of the neck have to be dissected. A subtotal parotidectomy has to be performed as well and the zygoma as well as the ascending ramus of the mandible are transected. The facial nerve is also cut and its distal branches should be tagged for grafting (XII–VII anastomosis or grafting with free graft from sural or greater auricular nerve). The branches of the external carotid artery are isolated and cut whenever necessary. A temporal craniotomy is performed and the dura of the temporal lobe is carefully elevated (subtemporal drop-down). This exposure is extended anteriorly into the foramen ovale. The intrapetrous portion of the internal carotid artery is identified and carefully dissected (Fig. 5.6E). If necessary, the IAC is opened. Then, the eustachian tube is opened and the specimen can be removed. Some authors have described total en-bloc temporal bone resections with transection of the intrapetrous carotid artery, the superior petrosal sinus, and the contents of the IAC. Naturally, balloon occlusion studies are necessary prior to the procedure and the overall prognosis of the disease most often does not warrant such extensive and morbid surgery. Arriaga M, Curtin H, Takahashi H, Hirsch BE, Kamerer DB. Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol 1990;99(9 Pt 1):714–721 Between 1 and 2 in 20 000 live births are affected by congenital aural atresia, whereas unilateral involvement is much more common than bilateral atresia. Surgical correction remains a challenge for otologic surgeons. The goals of surgery are to provide long-term hearing restoration and a clean and skin-lined external auditory canal. • Computed tomography of the temporal bone—see p. 95 • Congenital malformations of the external ear—see p. 108 • Congenital malformations of the middle ear—see p. 123 Congenital aural atresia exists in various degrees from a completely absent external auditory canal to a blindly ending sac to an open but stenotic canal. Aural atresia is often associated with microtia of the auricle. Both conditions provide a special challenge to both the facial plastic surgeon as well as the otologic surgeon. Many cases of aural atresia are syndromic. About half of these cases are not candidates for reconstructive surgery because of the high level of anomalous anatomy. About 75% of unilaterally affected isolated cases, on the other hand, are suitable candidates for surgical correction. As a general rule, a more developed auricle is typically associated with a more developed middle ear space and ossicular chain and thus can serve as a vague indicator for surgical suitability. Prior to the 1980s, surgeons used the mastoid to gain access to the middle ear and ossicles. Then Jahrsdoerfer described the anterior approach, avoiding mastoid opening all together. This approach has evolved into the standard in atresia surgery. The drilling procedure is essentially to follow the bone of the temporomandibular joint and the middle fossa plate to access the tympanic cavity. Every work-up prior to atresia surgery should consist of a thorough otologic and audiologic examination as well as a CT scan to classify the anomaly and rule out inner ear deformities. Also, the clinician should search for associated problems especially in syndromic cases. Most clinicians perform the CT scan relatively early around age 1 year. This can be delayed until later since atresia repair would not be indicated before age 5 years. The CT scan has special value in the preoperative work-up since it is used to classify surgical suitability according to the Jahrsdoerfer scheme (see p. 123). Also, a close collaboration with a facial plastic surgeon is essential to determine the optimal timing for repair of microtia. As a general rule, procedures for microtia should be performed prior to atresia surgery since scars from the surgery might prevent an optimal cosmetic result. Naturally, hearing should be considered of high importance, especially with bilateral involvement. With unilateral involvement, cosmetic and functional considerations should be weighed carefully. With bilateral atresia, a BAHA headband should be fitted to provide hearing until the child is old enough to undergo surgical correction. As another option, BAHA implantation remains a viable option but surgery can only be performed with adequate bone thickness of more than 4 mm. The age for this varies greatly but implantation before age 6 years is usually not possible. Another important consideration is cholesteatoma formation in extremely stenotic canals. This can be evidenced by bony erosion on serial CT scans although the true diagnosis requires exploration and surgical management. For proper postoperative follow-up including frequent canal cleanings, the child has to be able to tolerate these procedures. A complete lack of middle ear aeration and an absent cochlear function serve as absolute contra-indications for atresia repair. Multiple studies have now demonstrated that uni-lateral atresia repair is a viable option in ears with favorable anatomy as classified by the Jahrsdoerfer scheme. With bilateral atresias, however, management becomes more complicated. Specifically, next to the cosmetic issue, bilateral conductive hearing loss presents as the most substantial problem. Since atresia repair before the age of 5 years should not be attempted, a BAHA headband should be fit to ensure proper speech and language development. In contrast to many otologic disorders, the better ear should be chosen first. Reconstruction of the external ear is typically delayed until the child is roughly 5–6 years of age. Atresia repair should follow the plastic reconstructive efforts (cartilaginous framework placement, ear lobule, elevation of auricle with a skin graft). Also, the child should demonstrate a level of maturity adequate to cooperate with the planned medical treatment. After a standard postauricular incision, an anteriorly pedicled periosteal flap should be raised and drilling should start in the cribriform area, with the temporal line and the glenoid fossa as surgical landmarks (Fig. 5.7A). Care must be taken to stay anterior and superior by using the middle fossa plate as well as the bony glenoid fossa as landmarks. During drilling, as few mastoid air cells as possible should be opened. The antrum should also remain unopened to avoid graft mucosalization. The middle ear space should be entered as far superior as possible in the epitympanum. This minimizes damage to the ossicles and to the facial nerve (Fig. 5.7B). Typically, the atresia plate with the fused malleus–incus complex is encountered. Mobility should be assessed by careful palpation. The atretic bone over-lying the ossicles is carefully removed to provide maximum mobility. The manubrium of the malleus is usually absent and its neck is commonly attached firmly to the atresia plate. The fossa incudis and the anterior membranous attachments of the malleus should be kept intact. Next, the stapes and the medial portion of the incus should be inspected carefully. Both structures typically show a high degree of variation in malformed middle ears. An intact connection between the incus and the stapes is clinically important. If this is not present, a partial ossicular replacement prosthesis (PORP) should be placed onto the stapes capitulum. Also, the stapes foot-plate is palpated to ensure mobility. A temporalis fascia graft is then harvested and prepared in a standard fashion as used for tympanoplasty. The fascia is then trimmed to the desired size and placed onto the ossicles. It is draped onto the newly created bony canal wall by ~2 mm in all directions. The graft should also be draped over mastoid air cells whenever those have been exposed (Fig. 5.7C). A split-thickness skin graft is harvested next using a dermatome. Care must be taken to harvest a reasonable thickness (~0.02–0.03 mm) because if it is too thick the graft will curl and if it is cut too thin it will not withstand the natural requirements. The skin graft is cut to a size of 3 cm × 5 cm and notched at the medial edge (Fig. 5.7D). The notched edges are aligned so that the entire temporalis fascia graft is covered by squamous epithelium (Fig. 5.7E). The vertical slit faces anteriorly so that this placement reduces the risk of the free edges growing into the mastoid air cell system. The thinner the tympanic membrane, the better the hearing results. A small piece of circular Silastic sheet is cut and placed over the new tympanic membrane to hold the notched skin edges in place and to prevent blunting. The remaining external auditory canal is then packed to the level of the bony opening. Careful performance of the meatoplasty will ensure a good result. The surgeon should first make sure that the auricle and meatus align with the new canal. The auricle can be repositioned slightly by superficial soft-tissue dissection in either direction as needed. An anterior pedicled (hinged at the tragus) U-shaped skin flap is created. The underlying soft tissues including the conchal cartilage are removed and an opening to the newly created ear canal is thus created. The skin flap is then rotated into the new opening. The medial part of this flap is then sutured to the periosteum of the temporomandibular joint, which was created at the beginning of the procedure. The postauricular skin incision is closed in a layered fashion. Then, the skin graft is brought laterally and sewed to the edge of the superficial skin edge of the conchal bowl (Fig. 5.7E). Standard Merocel packing is applied. The patient is seen 1 week postoperatively for a routine check-up. Then, about 1 month after surgery, the ear canal is first cleaned. This procedure has to be continued until the result is satisfactory. Due to its aberrant course in malformed ears, the facial nerve is always at great risk. Specifically, the anterior course of the nerve in its mastoid segment poses a problem in many cases. In previous series, patients with low-set ears, canal stenosis, and cholesteatoma demonstrated higher rates of facial nerve injury. Fig. 5.7 A–E Jahrsdoerfer RA, Lambert PR. Facial nerve injury in congenital aural atresia surgery. Am J Otol 1998;19(3): 283–287 Jahrsdoerfer RA, Yeakley JW, Aguilar EA, Cole RR, Gray LC. Grading system for the selection of patients with congenital aural atresia. Am J Otol 1992;13(1):6–12 Several external influences can cause trauma to the tympanic membrane but also to the middle ear. Spontaneous healing rates for the eardrum are very good. Rarely, surgical intervention is necessary. With ossicular involvement, on the other hand, surgical exploration and ossiculoplasty is often the treatment of choice. • External ear and eustachian tube—see p. 9 • Fractures of the temporal bone—see p. 247 • Myringoplasty and underlay tympanoplasty—see p. 299 • Ossiculoplasty—see p. 306 Injury to the tympanic membrane and the middle ear is not uncommon. Several mechanisms of trauma have to be distinguished. First, penetrating trauma must be distinguished from nonpenetrating injuries. The latter are further subclassified based on the specific trauma mechanism into pressure trauma, blast injury, and thermal, electric, and caustic trauma. The most common mechanism for traumatic tympanic membrane perforations is due to pressure (overpressure). Blast injuries are less common but potentially much more serious. They can result from bomb or gasoline explosions or from deployment of a vehicle air bag. Blast injury can also affect the ossicular chain and the integrity of the otic capsule, resulting in traumatic perilymphatic fistula (see p. 171). Such an injury is commonly associated with inner ear symptoms such as fluctuant hearing loss, vertigo, and disequilibrium. With a simple pressure trauma, the most common site of the injury is the posterior aspect of the tympanic membrane (Fig. 5.8A). Such a simple injury shows a very high spontaneous healing rate of greater than 80%. Thermal injuries, for example resulting from hot debris entering the external auditory canal, can result in much more severe damage and lower spontaneous healing rates. In addition to ossicular involvement, hot slag might cause facial nerve paralysis. Thermal devascularization has been discussed as an underlying mechanism that seems to result in a much greater degree of tissue damage than simple pressure trauma. Less commonly, lightning might cause an even more serious injury of the temporal bone, including perilymphatic fistula and frank temporal bone fractures. As with other thermal injuries, perforations often do not heal. Alkaline caustics can cause liquefaction tissue necrosis and subsequent vascular occlusion. The associated injury typically extends farther than is visible during microscopic examination. The size of the perforation will likely be underestimated and the middle ear can show a massive granulomatous reaction with extensive scar tissue formation. Thus, ossicular fixation, chronic infectious processes, and blunting of the anterior tympanomeatal angle are common. Naturally, penetrating injury (Q-tip, wire) to the ear can result in tympanic membrane perforation but also injury to ossicular structures (Fig. 5.8B). Inner ear symptoms such as vertigo or sensorineural hearing loss are usually alarming and usually deserve immediate action. Less commonly, gunshot trauma is encountered. With this, angiography and further diagnostic work-up are warranted. Temporal bone fractures are often encountered and need to be managed accordingly (see p. 247). The ossicular chain might be injured both with penetrating and with nonpenetrating trauma to the ear. Several specific injuries should be distinguished: • Incudostapedial joint dislocation is the most common site of injury (Fig. 5.8C). This can be observed with both penetrating injuries as well as with longitudinal temporal bone fractures (see p. 247). • Fracture and dislocation of the stapes. The stapes superstructure can sometimes show frank fracture and dislocation. When dislocated into the vestibule (inner ear symptoms of vertigo and sensorineural hearing loss), immediate surgical exploration is warranted. • Dislocation of multiple parts of the entire chain is less often observed with penetrating trauma or gunshot injuries. • Fixation due to scarring or ossification can result as a long-term effect of substantial middle ear trauma. Fig. 5.8 A–F Tympanic membrane perforations can usually be diagnosed by routine otoscopic examination of the ear. It is important to document the exact size and location of the perforation. A central perforation does not involve the annulus, whereas a marginal one does. Marginal perforations are typically more complicated since they show lower healing rates and can result in squamous accumulation and cholesteatoma formation (Fig. 5.8D). In any case, the free edge of the perforation should be assessed for a more rugged appearance consistent with a fresh perforation (Fig. 5.8E, F). A cranial nerve evaluation should be part of the routine examination. With debris in the canal, careful cleaning should be performed with strict avoidance of irrigation or pneumatic otoscopy. The status of the middle ear should be documented as well. The presence of cerebrospinal fluid (CSF) deserves further testing including CT. If audiometric testing is unavailable, tuning-fork examination should be done. With intact inner ear function, the Weber test should lateralize to the affected ear. If possible, both bone and air conduction thresholds should be obtained. CT scanning is indicated with penetrating injuries, especially when inner ear symptoms are suspected. Special attention should be paid to the anatomical relationship of the stapes footplate and the vestibule. Angiography is indicated when vascular injury is suspected such as with gunshot trauma. MRI is only indicated with suspected CNS involvement and CSF leakage. With absent inner ear symptoms, simple perforations should be managed conservatively. Antibiotic eardrops should be applied in the acute phase. Also, oral antibiotics and steroid drops can be prescribed depending on the initial assessment and the amount of middle ear inflammation. Frequent clinical examinations should be performed to document a potential tendency for closure. With a persistent perforation after ~3–6 months, surgery for closure might be considered. Spontaneous healing rates of all types of injury are greater than 75%. Spontaneous healing rates are best with air pressure injuries, followed by water pressure and then thermal or corrosive damage. Injuries due to foreign objects also show lower spontaneous healing rates. More severe trauma deserves more immediate management. Dislocation of the stapes into the vestibule evidenced by vertigo and sensorineural hearing loss requires prompt exploratory tympanotomy as described below (ossiculoplasty). Some caustic injuries result in a chronic myringitis with a raw and weeping surface of the tympanic membrane due to granulation tissue. In such cases, combined antibiotic and steroid ear drops should be applied for several weeks. These may be supplemented by oral antibiotics. Once the infectious stage has been stabilized, surgical correction can be considered. Successful tympanoplasty requires adequate exposure, debridement, deepithelialization of the perforation, and careful graft placement. The graft has to be supported until healing has occurred sufficiently. Transcanal underlay tympanoplasty is generally used for smaller posterior perforations. Tragal perichondrium and temporalis fascia are both readily available for grafting. With larger or more anteriorly located perforations, a postauricular approach typically allows for adequate exposure of the entire perforation. Both underlay as well as overlay (lateral grafting) techniques can be used with similar success rates (see pp. 299 and 303). Dislocation of the incudostapedial joint is the most common site of injury to the ossicular chain. If it is observed during posttrauma CT, the clinician should not proceed with surgical intervention until the patient has been observed for several months. This is because formation of scar tissue often causes adherence of the dislocated ossicles to the tympanic membrane and subsequent restoration of middle ear function. Moreover, every posttraumatic ossicular discontinuity should be observed for at least 3 months. If a significant conductive component persists beyond 3 months, conventional amplification or ossiculoplasty may be offered to the patient. During surgery, the middle ear and the ossicular chain are inspected carefully (usually via a transcanal approach). If the incus has been dislocated, it can usually be removed, reshaped, and used for incus interposition grafting. If the incus cannot be utilized and the stapes is present and mobile, a PORP (partial ossicular replacement prosthesis) can be placed under a thin piece of tragal or conchal cartilage (to prevent migration of the prosthesis through the intact tympanic membrane). Some otologic surgeons prefer TORPs (total ossicular replacement prostheses) over PORPs even with the stapes superstructure present. Those surgeons commonly use the superstructure to stabilize the prosthesis and therefore avoid tilting. With a fractured or absent superstructure, however, a TORP remains as the only option. With a fractured footplate but an intact lateral chain, a regular stapes prosthesis might be placed on a fascia graft placed on the oval window niche. Also, conventional stapes surgery might be attempted in these cases. With an absent incus, malleovestibulopexy surgery can be attempted in which the stapes prosthesis is attached to the manubrium mallei (see also p. 306). Jones DT, Ogren FP, Roh LH, Moore GF. Lightning and its effects on the auditory system. Laryngoscope 1991;101(8):830–834 Kristensen S. Spontaneous healing of traumatic tympanic membrane perforations in man: a century of experience. J Laryngol Otol 1992;106(12):1037–1050 Contemporary management of chronic otitis media with Effusion (COME) includes placement of pressure equalization (PE, tympanostomy) tubes. In fact, PE tube placement is the most commonly performed procedure in the United States. Mastoidectomy, on the other hand, is a key part of many otologic procedures. The vast majority of mastoidectomy procedures, however, are performed for chronic suppurative otitis media with and without cholesteatoma. The advent of the antibiotic era and enhanced access to proper medical care have markedly reduced the need for mastoidectomy for acute infectious cases. Pressure equalization tube placement provides temporary ventilation of the tympanomastoid compartment. Especially in children, this procedure allows for proper auditory development in the setting of eustachian tube dysfunction. Tympanostomy tubes are placed in children with a 3-month or longer history of middle ear Effusion and a 20-dB (or greater) conductive hearing loss. A great variety of different types of tubes is available. • Anatomy of the middle ear—see p. 12 • Assessment of middle ear function—see p. 58 • Acute otitis media and otitis media with Effusion—see p. 126 • Complications of otitis media—see p. 141 A chronic middle ear Effusion is usually the result of eustachian tube dysfunction and subsequent poor middle ear aeration. Due to the specific anatomical configuration of the eustachian tube in children (more horizontal alignment, length; see p. 9), this problem mainly affects the pediatric population. Other risk factors for otitis media with Effusion (OME) include male gender, bottle feeding, passive smoking, crowded living circumstances, and frequent upper respiratory tract infections. Also, several syndromes including as Down, Treacher Collins, and Apert syndromes as well as certain immune system deficiencies also render affected children at greater risk for developing OME. The overall goal of PE tube placement in the pediatric population is to provide artificial ventilation of the middle ear space in the setting of eustachian tube dysfunction. As discussed earlier, the pediatric eustachian tube has certain features that render the middle ear more susceptible to developing mucosal disease such as acute otitis media (AOM) or COME. The morphologic configuration of the eustachian tube typically reaches a more effective adult stage around age 7 years. Thus, PE tubes are a temporary measure to provide middle ear ventilation until the body’s own system has reached maturity. Several studies have demonstrated adenoidectomy to be an effective adjunct treatment for eustachian tube dysfunction. Specifically, adenoidectomy typically helps to resolve COME and recurrent AOM in children older than 4 years. The Effectiveness of adenoidectomy has not been demonstrated in younger children, although the procedure is safe in this population. Thus, in the presence of other indications (such as sleep apnea), adenoidectomy should be combined with bilateral tympanostomy tube placement. Several classic scenarios serve as indications for placement of a PE/tympanostomy tube: • Intracranial or intratemporal complication of acute otitis media • Chronic otitis media with Effusion defined as persistent middle ear Effusion for at least 3 months (Fig. 5.9A) • Recurrent bouts of acute otitis media (with infection-free intervals) with or without resolution of otitis media with Effusion between acute infections (Fig. 5.9B) Other clinical scenarios sometimes require PE tube placement. These include: • Complicated course of acute otitis media in patients with underlying immune disorder or patients taking immunosuppressants • Chronic eustachian tube dysfunction with persistent retraction of the tympanic membrane to avoid development of an atelectatic ear, impending ossicular chain erosion, or imminent cholesteatoma formatio. • Certain craniofacial anomalies • Hyperbaric oxygen therapy • Low-pressure pulse generator for Ménière disease (Meniett device) • Children with otitis media and underlying sensorineural hearing loss Fig. 5.9 A–F Most PE tube placements will be performed in the pediatric population. In this case, general anesthesia is required. In contrast, most PE tubes in the adult population can be placed in the examination chair in the outpatient clinic. In any case, careful inspection of the external auditory canal, the tympanic membrane, and the underlying middle ear is critical. This scenario also allows for proper inspection, which is often difficult in the pediatric population. The patient is positioned on the examination or operating table. The ear canal is inspected with a large speculum. As a general rule, the largest speculum size that fits snugly should be used. This allows for optimal visualization and ability to clean the ear canal. When removing cerumen and debris from the EAC, the surgeon should carefully avoid any injury to the canal skin. This is especially important when PE tube placement is performed in conjunction with ABR examination for assessment of auditory function (see p. 64). When inspecting the tympanic membrane, classic landmarks might be missing due to the chronic inflammatory process. The surgeon should always look for the short process of the malleus as well as for the manubrium and the umbo. The light reflex is often quoted as a landmark but should not be used as such since it is very unreliable. PE tubes should be placed into the anterior inferior quadrant of the tympanic membrane whenever possible (Fig. 5.9C). If that is impossible (due to limited exposure), the posterior inferior quadrant might be used. This is important since inserting the tube into the posterior superior quadrant might impact the ossicular chain and thus result in a conductive hearing loss (Fig. 5.9D). Major blood vessels should be avoided to reduce the risk for bleeding. The middle ear Effusion should be at least partially evacuated using small-diameter suction tips. With some very viscous effusions, irrigation with warm saline can facilitate removal of the mucus. The tube should then be placed with an alligator forceps or a Rosen needle. When using a T-tube, both flanges should be completely recessed and unfolded within the middle ear. If necessary, the flanges can be trimmed to allow for optimal placement. With all other tubes, the lumen should be aligned within the surgeon’s line of view so postoperative care can be provided in case the tube clogs. An enormous variety of middle ear ventilation tubes is commercially available (Fig. 5.9E). Depending on the disease process, biographical data, and associated factors, the surgeon should choose a model that fits best. Usually, each surgeon uses a handful of models to fit these needs. Some basic principles should be considered: • The larger the tube lumen, the lower the water resistance (thus the larger the risk of postoperative otorrhea without precautions against water) • Standard tubes typically remain in situ for roughly 12 months. This time varies greatly, however. Some “temporary” tubes remain in situ permanently and some “permanent” tubes (such as T-tubes) extrude after only a few weeks. • T-tubes have flexible flanges (feet) that should be fully placed within the middle ear. These flanges provide resistance and consequently T-tubes remain in situ for longer. • The longer the intubation, the larger the tube, and the more often otorrhea is observed, the greater is the risk of development of a permanent perforation. Thus, with the need for only temporary intubation, shorter and smaller grommets should be used. In children with comorbidities whose eustachian tube dysfunction is less likely to resolve over time, T-tubes may be chosen. Tympanostomy tubes are available in various materials. Typically, PE tubes are very well tolerated. Postoperatively, the patient should receive eardrops twice a day for about 1–7 days. In rare cases, systemic antibiotics are necessary when purulent Effusions are encountered during the procedure. Careful parent and patient education regarding water precautions should be given. This should include counseling about different types of water (salt water with its greater surface tension versus water containing soap in the bathtub). Permanent perforations of the tympanic membrane may be observed after PE tube placement. It is believed that the longer the tympanic membrane has been intubated, and the larger the tube, the more likely a perforation will remain. Also, the number of postoperative infections evidenced by bouts of tube otorrhea seems to negatively influence the likelihood of a perforation. Tube perforations can sometimes provide a unique challenge due to their location in the anterior inferior quadrant. More sophisticated surgical techniques such as lateral grafting (see p. 303) are often necessary to provide good results. In rare cases, long intubation (mostly via T-tubes) can lead to cholesteatoma formation (see p. 136). Gates GA, Avery CA, Prihoda TJ, Cooper JC Jr. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with Effusion. N Engl J Med 1987;317(23):1444–1451 Senturia BH, Bluestone CD, Klein JO, et al. Report of the Ad Hoc Committee on Definition and Classification of Otitis Media with Effusion. Ann Otol Rhinol Laryngol 1980;89:3–4 At one time mastoidectomy for acute otitis media was the most commonly performed otologic procedure. Today, most mastoidectomies either are performed for chronic ear surgery or are part of more complex otologic procedures. Thus, the otologist should be very familiar with the anatomical relationships and the possible variations in this area. • Overview of temporal bone anatomy—see p. 6 • Computed tomography—see p. 95 • Complications of otitis media—see p. 141 • Otologic/neurotologic instrumentation—see p. 260 A cortical mastoidectomy is the most fundamental otologic procedure. Most mastoidectomies are part of otologic and neurotologic procedures to access structures of the temporal bone. In tympanomastoid surgery, for example, mastoidectomy allows access to the antrum and the air-containing spaces of the temporal bone. For many otologic procedures such as cochlear implantation, mastoidectomy provides access to the facial recess. For more complex neurotologic procedures, mastoidectomy creates a pathway for more medial drilling. Thus, mastoidectomy is usually the first step for most procedures of the lateral skull base. However, mastoidectomy can be a stand-alone procedure (simple mastoidectomy) when treating mastoid disease. In the vast majority of these cases, mastoidectomy is used to treat coalescent mastoiditis. Prior to the antibiotic era, the vast majority of mastoidectomy procedures were performed in patients with coalescent mastoiditis. Specifically, it is estimated that in that era ~1%–2% of patients with acute purulent otitis media subsequently required a mastoidectomy. Today, simple mastoidectomies for acute infections of the tympanomastoid compartment are rarely required. Recently, however, some authors have described an increasing number of cases, probably due to multiply resistant bacteria. Generally, coalescent mastoiditis describes an infectious process of the mastoid. This typically develops from an acute suppurative (purulent) otitis media. The middle ear and mastoid share the same mucosal linings and every acute otitis media demonstrates co-involvement of the mastoid. Thus, radiographic opacification of the mastoid air cell system can be seen with almost every case of AOM. Involvement of the mucoperiosteum alone does not require surgical intervention. With bony involvement, however, surgical removal of the bony septa becomes necessary. This process is termed coalescent mastoiditis and typically takes at least 10–14 days to develop. On average, AOM precedes coalescent mastoiditis by around 3–5 weeks. The coalescent process results in bone loss with subsequent osteoblastic repair. This process does not involve bony necrosis but leads to entrapment of pus in newly formed bony compartments. Mastoidectomy therefore serves to evacuate trapped pus and to remove involved bony septa. The clinical symptoms of coalescent mastoiditis are essentially the same as in AOM. The main differentiating factor is the temporal relationship of events: persistence of symptoms for at least 10–14 days after AOM, generally with fever and a high white blood cell count. This temporal relationship is fundamental since timing of surgical intervention is crucial: if done too early surgery might lead to bacterial dissemination, which carries a high incidence of intracranial and systemic complications. With delayed mastoidectomy, on the other hand, progressive bone erosion can lead to meningitis and formation of a brain abscess. Thus, exact information on the timing of events has to be obtained: prolonged or fluctuating amounts of creamy purulent otorrhea (and not mucoid) for 3 or more weeks mostly indicate a coalescent process. Basically, this ongoing process indicates impaired resolution of the acute infection, mostly due to inadequate drainage from coalescing pneumatic cells. When prolonged otorrhea suddenly subsides, a walled-off process mostly in the petrous apex should be suspected. The most common physical sign of coalescent mastoiditis is persistent postauricular tenderness (typically mild pain) several weeks after a bout of AOM (worse at night, pain behind the eye with petrous apex process). Mild recurring postauricular pain several weeks after AOM is also very alarming and should trigger further work-up. Fig. 5.10 A–F Computed tomography findings in coalescent mastoiditis are very characteristic and typically diagnostic. Opacification of the mastoid generally indicates mastoid Effusion. Coalescence, on the other hand, is characterized by a loss of bony septa (Fig. 5.10B). Typically, the distinctness of the septa is lost. The first sign is typically an erosion of the cortical plate over the sigmoid sinus. This sign was found to be quite sensitive in determining whether to proceed surgically or medically when managing mastoiditis. The basic principle is to remove infected bony septa, to drain the tympanomastoid compartment, and to provide irrigation and cleaning. Concurrent PE tube placement is often required (see p. 291). The grade of difficulty can vary considerably with the amount of pneumatization. A well-pneumatized bone may allow for a relatively straightforward procedure, whereas many temporal bones with chronic ear disease demonstrate an incomplete or almost completely absent air cell system. First, postauricular injection of lidocaine and epinephrine should be done in a standard fashion. Then, a postauricular incision ~1–2 cm behind the postauricular crease should be made (Fig. 5.10C). The subcutaneous tissue planes are dissected and bleeding should be controlled via bipolar cautery. The tissues in the superior aspect of the incision should be elevated to facilitate exposure of the temporalis fascia. Then, an anterior pedicled Palva flap is elevated off the mastoid bone (Fig. 5.10D). Care must be taken not to violate the integrity of the canal skin anteriorly. Drilling should start in the cribriform area posterior to the spine of Henle (Fig. 5.10E). This region is typically characterized by superficial mastoid air cells. Also, the surgeon may orient along the linea temporalis as the posterior extension of the zygomatic root. However, the linea temporalis must not serve as a true landmark since the middle fossa dura may lie superior or inferior to that level. A safe way to proceed medial is to follow the middle fossa dura and to thin the posterior bony canal wall. Special care must be taken not to violate the integrity of the posterior canal since this might require additional reconstructive measures or with larger holes a canal wall down procedure. Also, the middle fossa plate should be exposed but a thin bony shell should remain to protect the dura. In very pneumatized temporal bones, the plate may be found somewhat more medially. However, with absent pneumatization, the plate should be found initially and followed medially. Posteriorly, the sigmoid sinus should be identified. The sinodural angle (Citelli) should be drilled to facilitate exposure. The surgeon should try to saucerize the mastoid and to provide a funnel-shaped approach with the lateral exposure being the widest section. The antrum is found by thinning the ear canal and by following the bony middle fossa plate. Typically, a large antral air cell allows exposure of the lateral semicircular canal as a reliable landmark. In nonpneumatized bones, care must be taken not to drill into the canal since the antral air cell may be virtually nonexistent. Drilling should also be completed anterior-superior almost into the zygomatic arch. This drilling will facilitate exposure of the epitympanum and the incus. It is generally possible to see the incus by using the light reflex created by a water level in the antrum. This usually provides a hint as to when drilling is almost complete (Fig. 5.10F). Once the incus has been exposed, the mastoid tip may be drilled by following the posterior canal wall inferiorly. All diseased bony septa should be removed. The mastoid cavity may be further cleaned and irrigated. Closure is usually achieved by replacing the Palva flap and suturing the skin in a layered fashion. Some clinicians prefer to insert a small drain for a few days to avoid re-accumulation. Possible complications of mastoidectomy include dural injury with CSF leak, facial nerve injury (mostly the descending mastoid segment of thenerve in the inferior portion near the stylomastoid foramen), and injury to the sigmoid sinus, the posterior canal wall, and parts of the labyrinth such as the lateral semicircular canal. Antonelli PJ, Garside JA, Mancuso AA, Strickler ST, Kubilis PS. Computed tomography and the diagnosis of coalescent mastoiditis. Otolaryngol Head Neck Surg 1999;120(3):350–354 Zapalac JS, Billings KR, Schwade ND, Roland PS. Suppurative complications of acute otitis media in the era of antibiotic resistance. Arch Otolaryngol Head Neck Surg 2002;128(6):660–663 Zevallos JP, Vrabec JT, Williamson RA, et al. Advanced pediatric mastoiditis with and without intracranial complications. Laryngoscope 2009;119(8):1610–1615 All surgical procedures for chronic infections of the tympanomastoid compartment aim at providing a safe, dry ear. Basic principles were described by Wullstein and Zollner in the 1950s and also involve reconstruction of the tympanic membrane and restoration of the sound-conducting apparatus. The following nomenclature will be used herein: • Myringoplasty: repair of tympanic membrane (TM) defects without exploration of the middle ear space. • Tympanoplasty: repair of TM defects, eradication of disease, reconstruction of the ossicular chain, and/or exploration of the middle ear space (pp. 299, 303, 306). • Ossiculoplasty: reconstruction of the ossicular chain (p. 306). • Tympanomastoidectomy: eradication of disease in the tympanomastoid compartment (p. 310). Several surgical techniques are available for closing tympanic membrane perforations. Each approach features different grafting materials and techniques (underlay versus lateral graft, for example). Various approaches to the middle ear and tympanomeatal flaps have also been described. • Assessment of middle ear function—see p. 58 • Chronic suppurative otitis media (CSOM)—see p. 130 • Neoplasms of the middle ear—see p. 148 • Surgical access to the mastoid and middle ear, grafting materials—see p. 267 • Management of middle ear trauma—see p. 287 Perforation of the tympanic membrane can cause a variety of symptoms that include hearing loss, fullness, recurrent otorrhea, temperature sensitivity, and, rarely, pain. While most tympanic membrane (TM) perforations occur as sequelae of acute otitis media, trauma can also injure the TM. Characteristically, a blow to the external ear creates an implosive force in the external auditory canal that injures the TM indirectly. Less commonly, direct TM trauma can occur from objects such as paper clips, cotton tip applicators, and flying debris (such as metal slag), among many others. In all cases, associated ossicular and inner ear trauma should be considered prior to intervention. It is important to realize that not every perforation has to be repaired. Each case should be considered individually with the patient’s age, underlying medical condition, associated symptoms, and preferences in mind. Importantly in young children, eustachian tube functional status should be considered by assessing the frequency and severity of otitis media prior to the occurrence of the perforation as well as in the contralateral ear. It should be decidedly rare to undertake tympanoplasty in the setting of ongoing, contralateral otitis media with Effusion. In asymptomatic cases, perforation closure might be a purely prophylactic intervention. Anatomical factors should also be considered before proceeding with perforation closure. Specifically, the size of the perforation in relation to the entire surface of the TM, the location (central versus marginal, quadrant), and the status of the middle ear mucosa (dry perforation versus hyper-trophic mucosal linings) are morphologic factors that deserve consideration. Several methods for closing tympanic membrane perforations are available. The following sections list the currently most commonly used techniques and discuss the advantages and disadvantages of each technique. The term tympanoplasty has been used generically to describe surgical procedures within the middle ear that aim to eradicate disease and reconstruct the hearing mechanism, with or without tympanic membrane (TM) grafting. Myringoplasty, one type of tympanoplasty, is defined as an operation in which the reconstructive procedure is limited to repair of the TM. Tympanoplasty can further be classified according to the method used for ossicular chain reconstruction (ossiculoplasty, see p. 306) and the relationship of the TM to the remaining ossicular mass (Fig. 5.11A). This classification was initially proposed by Wullstein and Zoellner in the 1950s but still carries some clinical significance today. In their initial descriptions, focus included restoration of TM and ossicular continuity with resulting protection of the round window membrane from deleterious, phase- cancelling acoustic impulses.
Otologic/Neurotologic Instrumentation
Definition
Closely Related Topics
General Considerations (Fig. 5.1A)
Equipment for Otologic Procedures (Fig. 5.1B, C)
Equipment for Neurotologic/Lateral Skull Base Procedures (Fig. 5.1D)
Suction Irrigation (Fig. 5.1B, C)
High-Speed Drills (Fig. 5.1E)
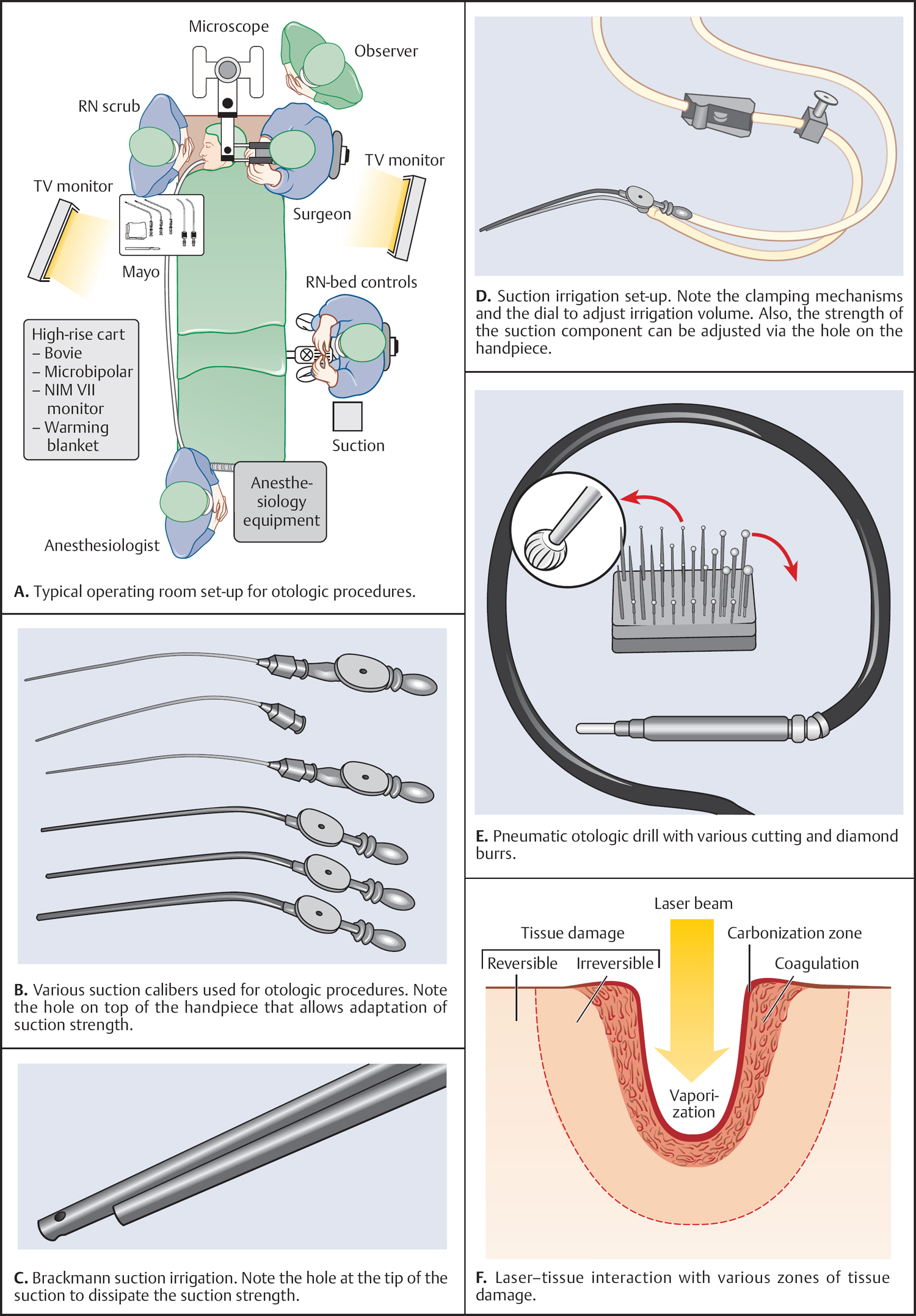
Operating Microscope
Lasers in Otology (Fig. 5.1F)
Surgical Instruments
Recommended Reading
Intraoperative Monitoring
Definition
Closely Related Topics
Facial Nerve and Lower Cranial Nerve Monitoring (Fig. 5.2A–E)
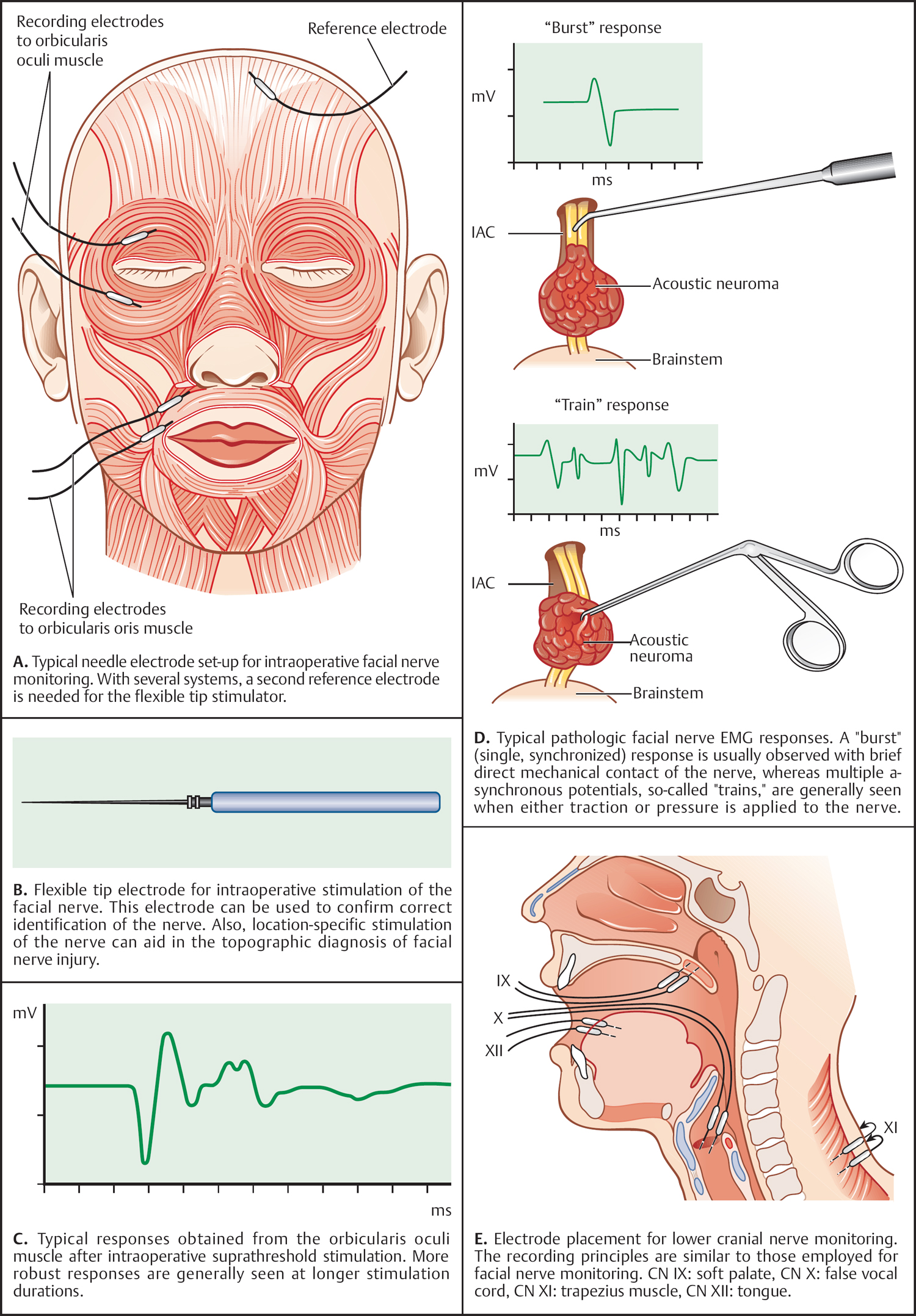
Monitoring of Auditory Function
Recommended Reading
Surgical Access to the Mastoid and Middle Ear, Grafting Materials
Definition
Closely Related Topics
Preoperative Injections (Fig. 5.3A)
Skin Incisions and Access Routes
General Considerations
Transcanal Approach (Fig. 5.3B)
Endaural Approach (Fig. 5.3C)
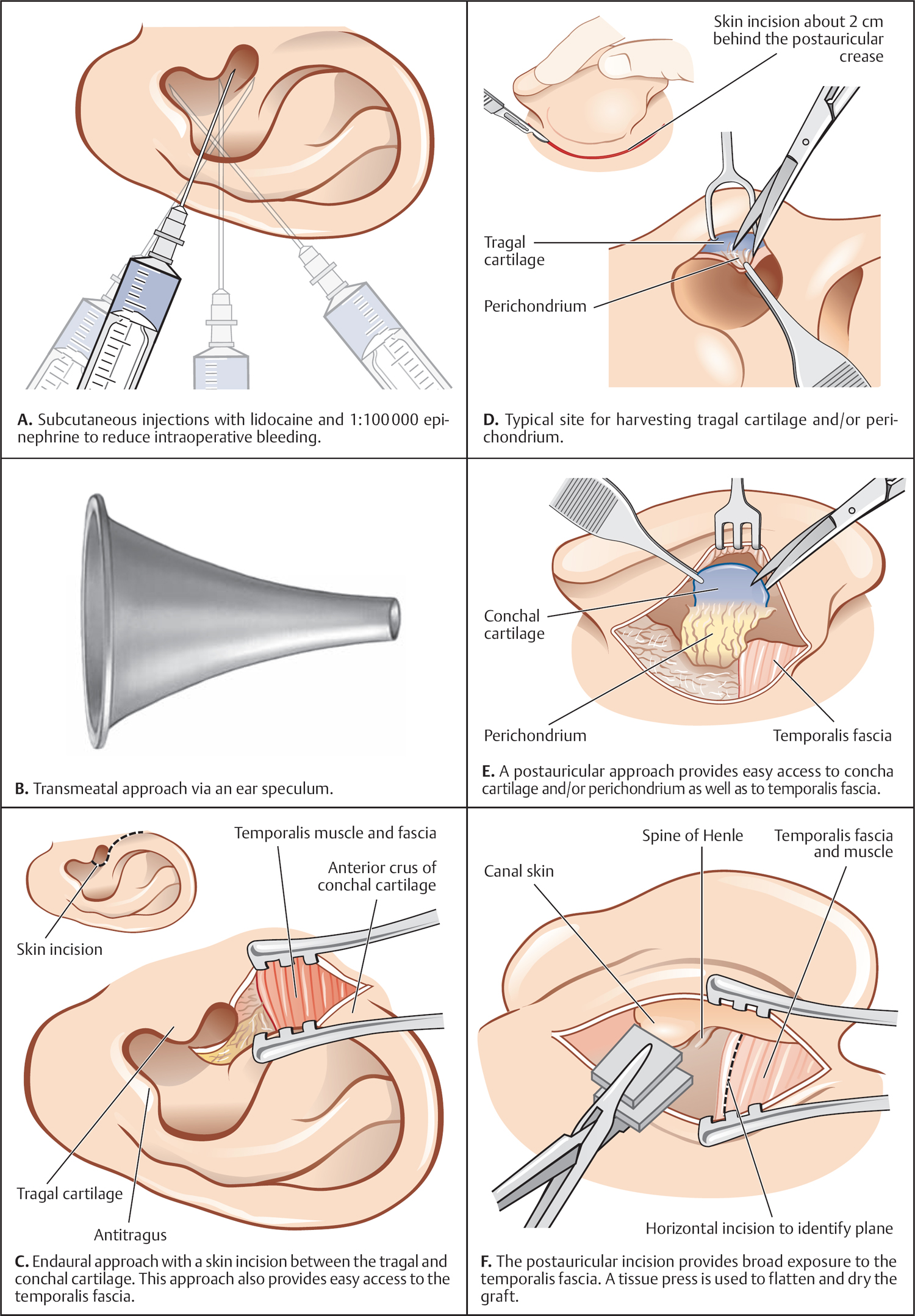
Postauricular (Retroauricular) Approach (Fig. 5.3D, F, G, I)
Extended Postauricular Approach
Grafting Materials
General Considerations
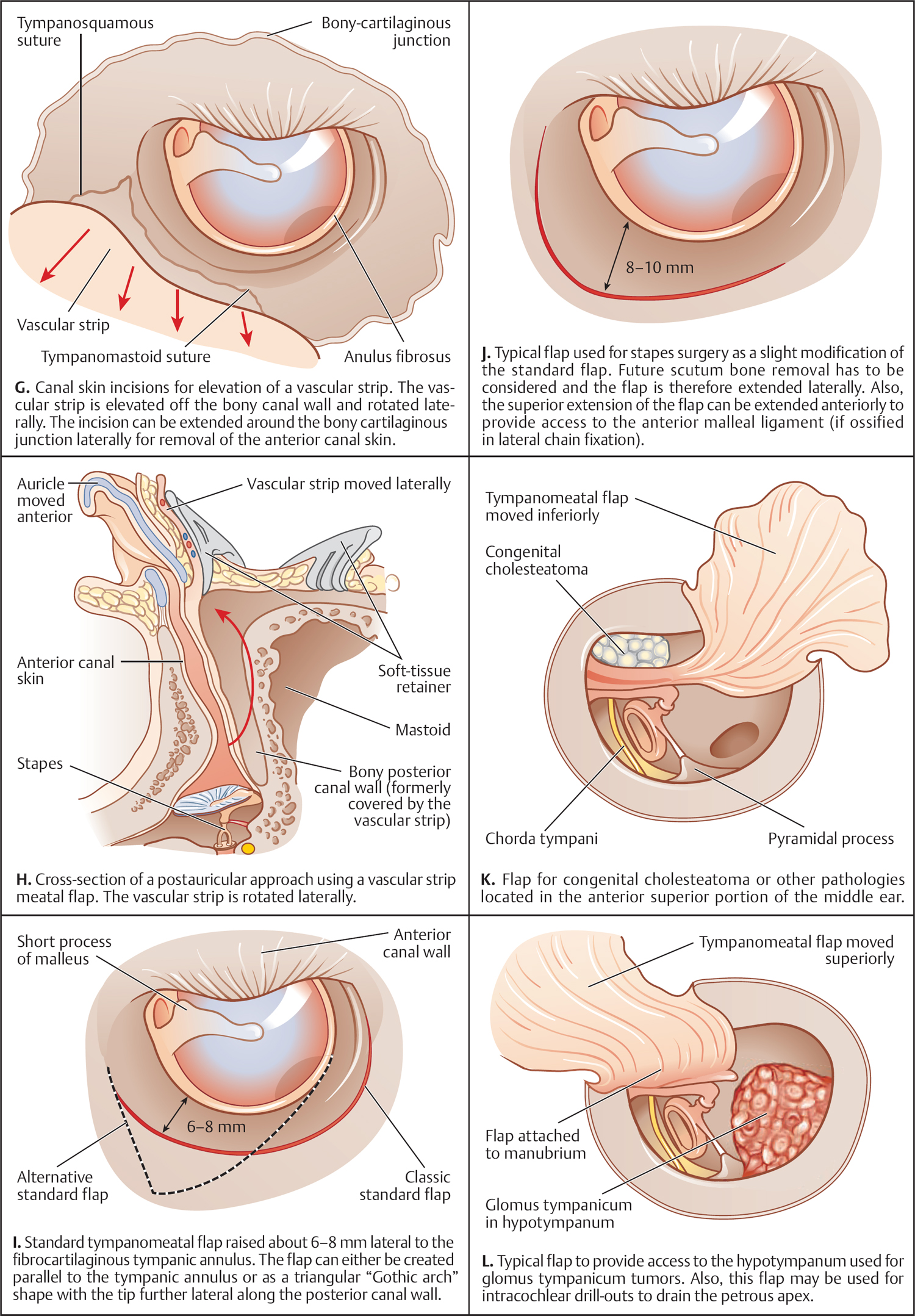
Temporalis Fascia (Fig. 5.3C, G)
Auricular Cartilage (Fig. 5.3E, F)
Potential Problems
Tympanomeatal Flaps
Standard Flap (Fig. 5.3I, J)
Extended Standard Flap (Fig. 5.3K, L)
Vascular Strip Approach (Fig. 5.3H, I)
Recommended Reading
Considerations for Pediatric Ear Surgery
Definition
Closely Related Topics
Introduction (Fig. 5.4A)
Anatomical Considerations (Fig. 5.4B, C)
Acquired and Congenital Cholesteatoma
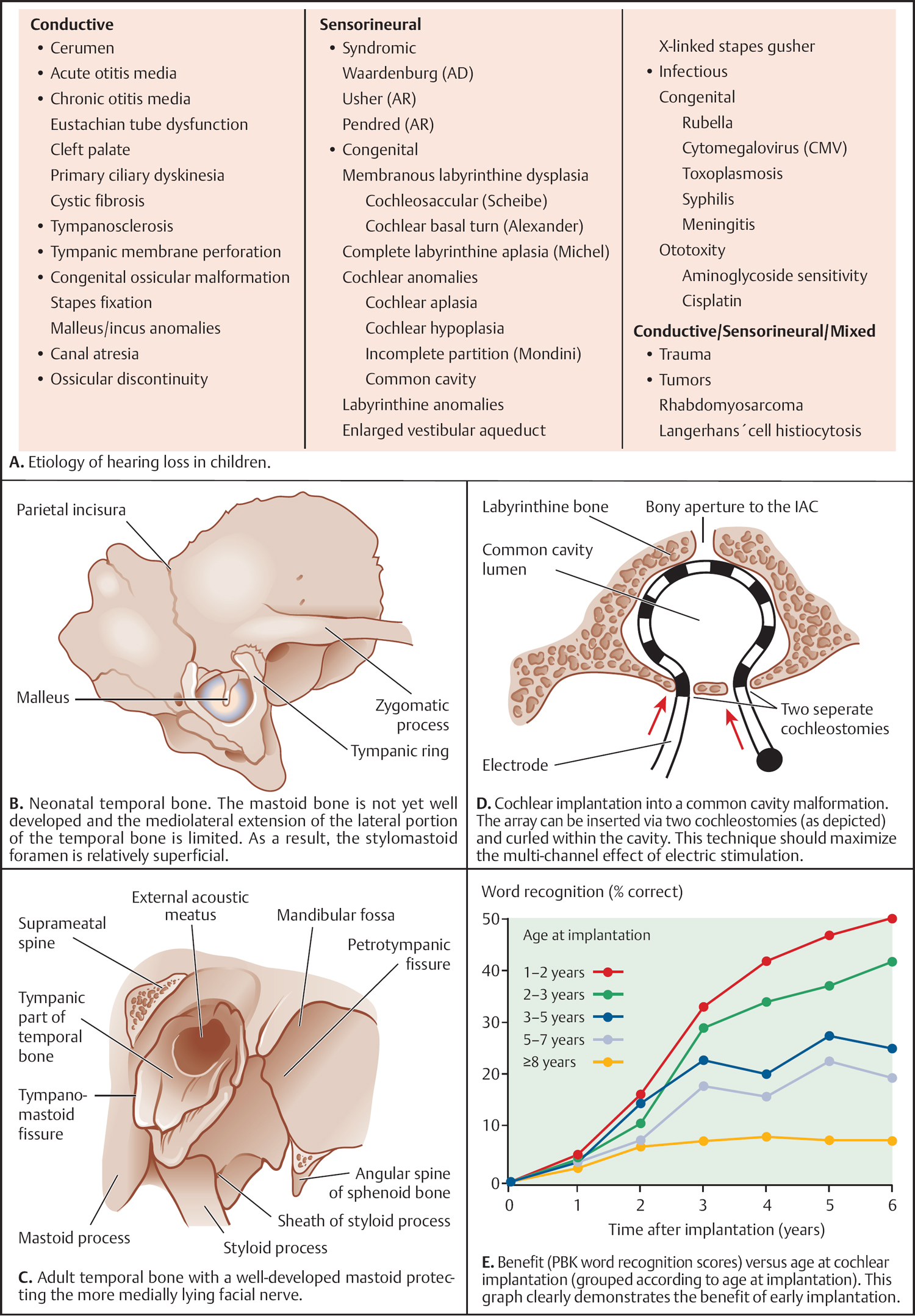
Cholesteatoma Growth Patterns
Tympanoplasty
Ossicular Chain Reconstruction
Cochlear Implantation in the Pediatric Population (Fig. 5.4D, E)
Additional Considerations
Recommended Reading
Canaloplasty of the External Ear Canal
Definition
Closely Related Topics
Introduction
Indications and Preparations
Surgical Technique (Fig. 5.5A–D)
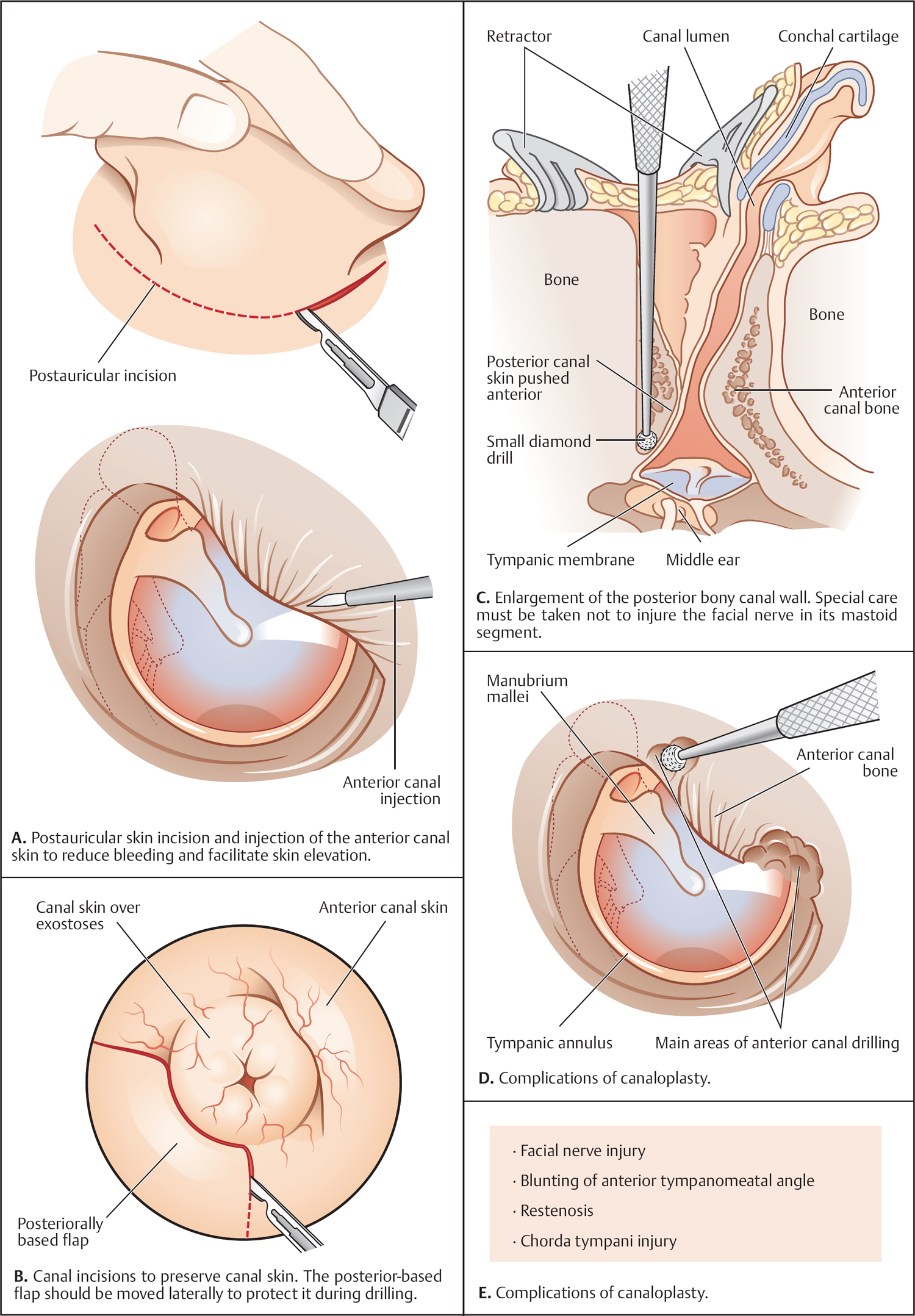
Complications
Recommended Reading
Temporal Bone Resections for Malignant Disease
Definition
Closely Related Topics
Introduction
Types of Temporal Bone Resections (Fig. 5.6A, B)
Lateral Temporal Bone Resection (Fig. 5.6C–E)
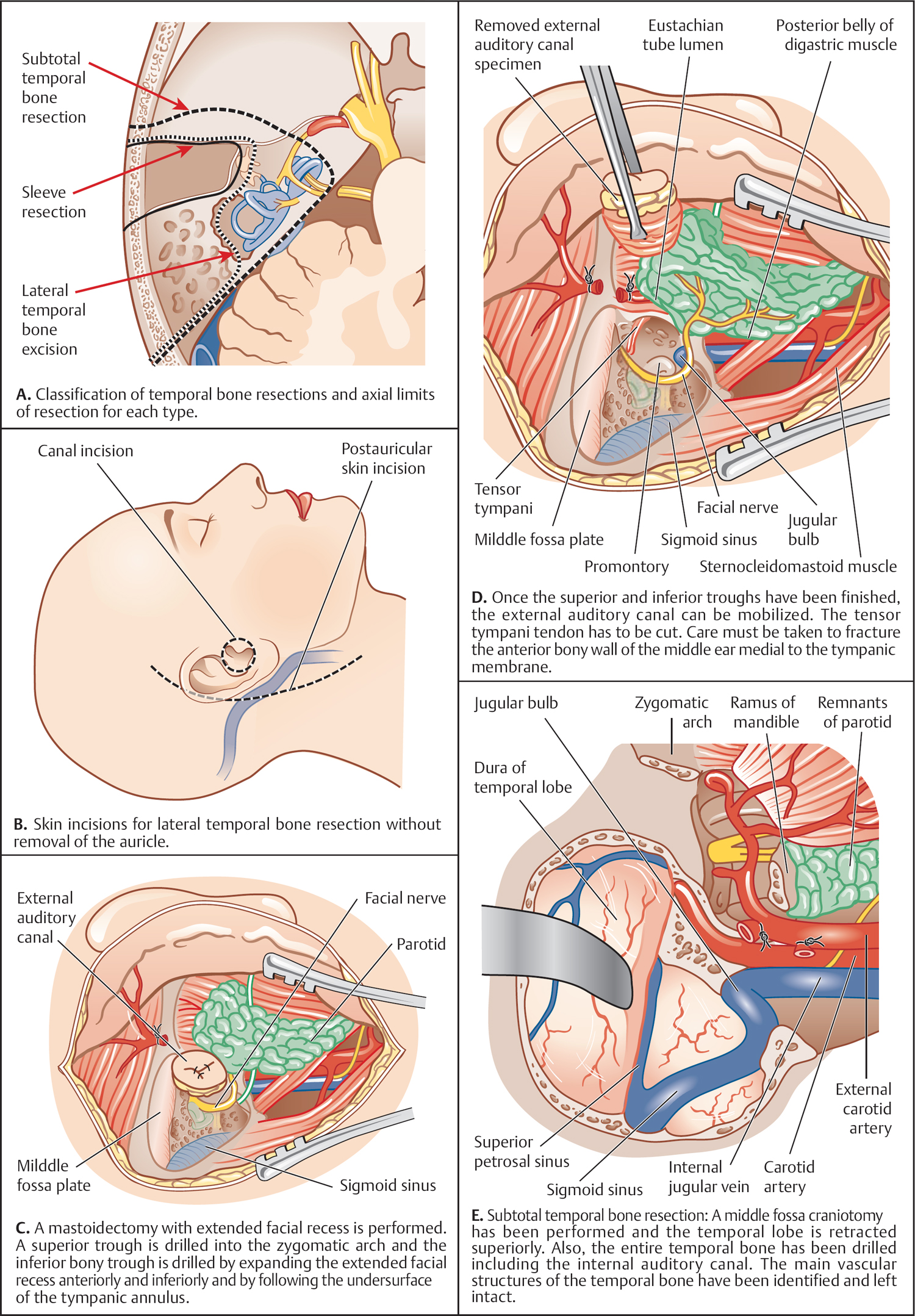
Subtotal Temporal Bone Resection (Fig. 5.6E)
Recommended Reading
Surgical Management of Aural Atresia
Definition
Closely Related Topics
Introduction
Preoperative Evaluation
Unilateral versus Bilateral Atresias
Timing of Surgery
Surgical Technique
Canal Drilling (Fig. 5.7A, B)
Grafting (Fig. 5.7C–E)
Meatoplasty and Soft-Tissue Reconstruction (Fig. 5.7E)
Complications
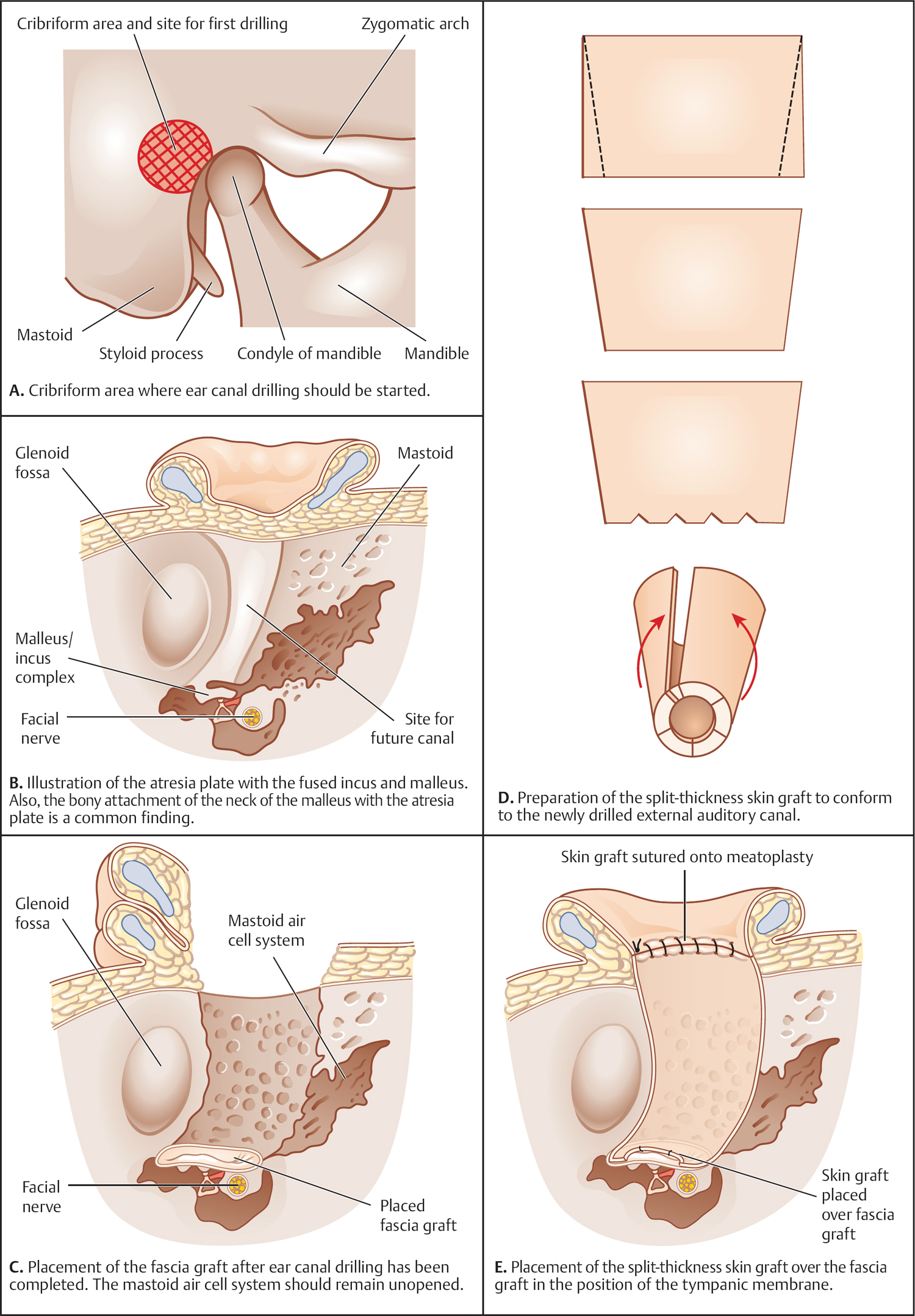
Recommended Reading
Management of Middle Ear Trauma
Definition
Closely Related Topics
Introduction
Nonpenetrating Trauma (Fig. 5.8A)
Penetrating Trauma (Fig. 5.8B)
Ossicular Chain Involvement (Fig. 5.8C)
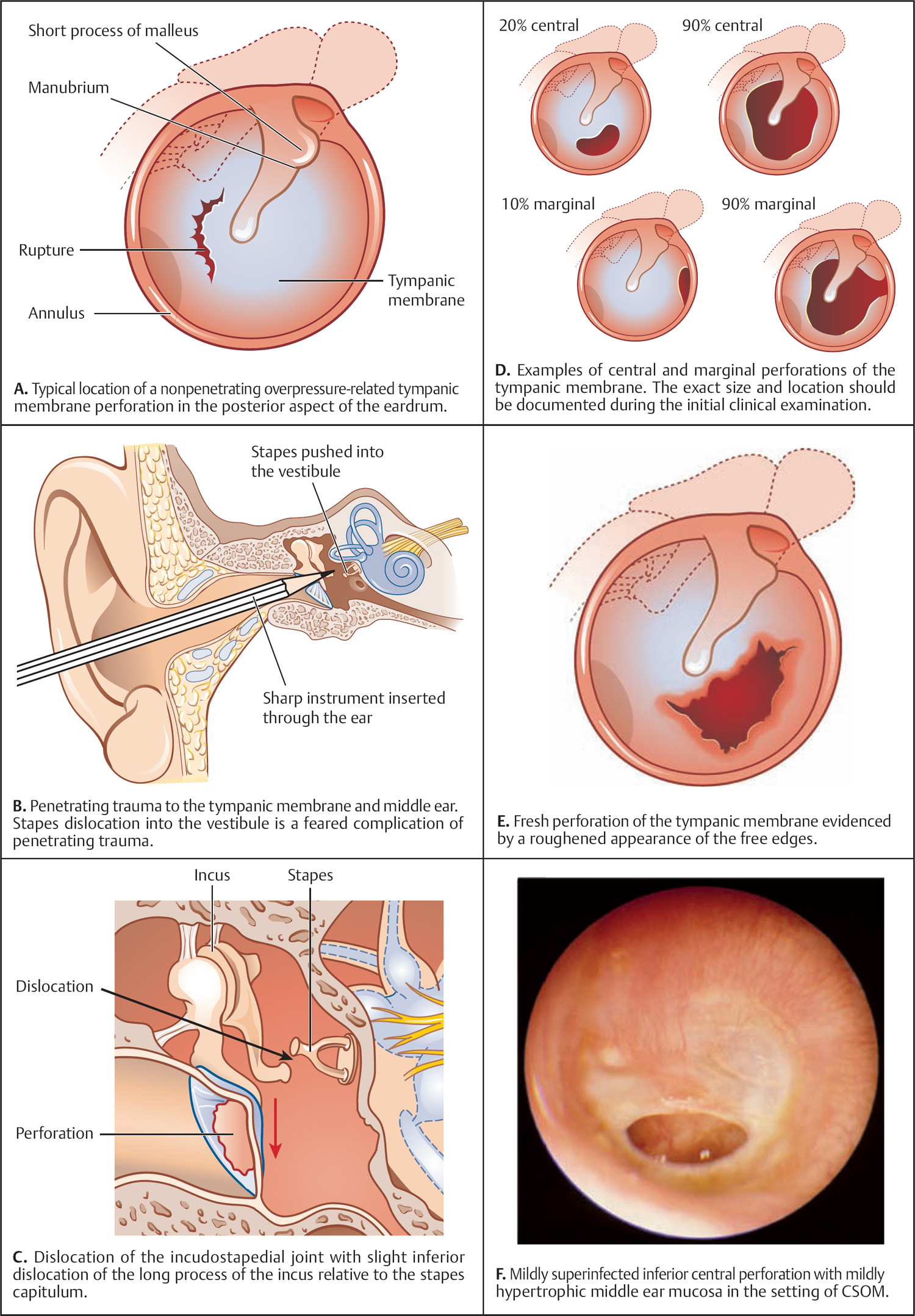
Clinical Evaluation (Fig. 5.8D–F)
Management of the Tympanic Membrane
Tympanoplasty
Management of the Ossicular Chain
Recommended Reading
Surgical Management of Acute Otitis Media and Chronic Otitis Media with Effusion
Middle Ear Ventilation Surgery
Definition
Closely Related Topics
Introduction
Principle
Adenoidectomy
Indications (Fig. 5.9A, B)
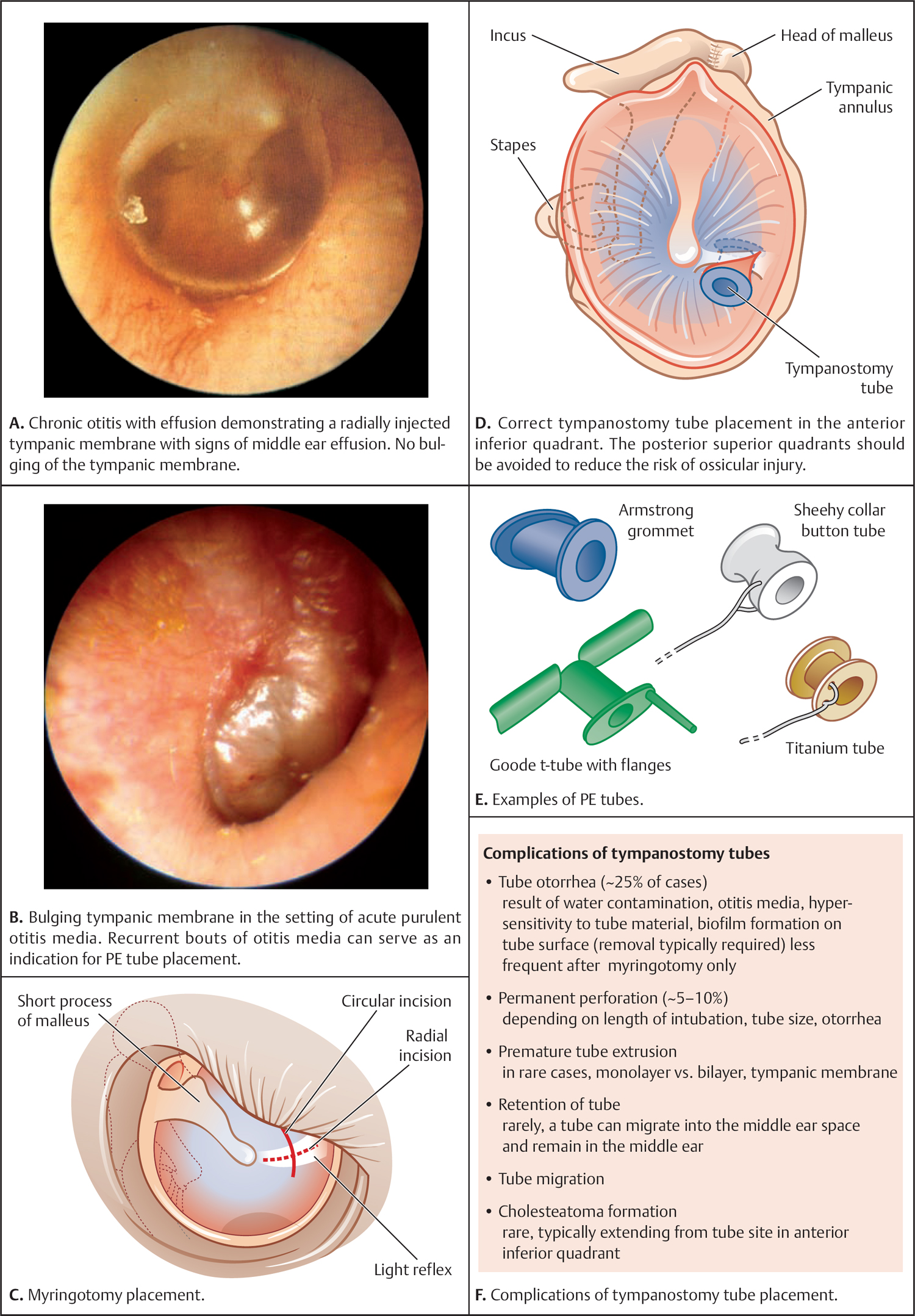
Procedure (Fig. 5.9C, D)
Tube Types (Fig. 5.9E)
Postoperative Course and Complications (Fig. 5.9F)
Recommended Reading
Mastoidectomy
Definition
Closely Related Topics
Introduction and Indication (Fig. 5.10A)
Coalescent Mastoiditis
Clinical Indication
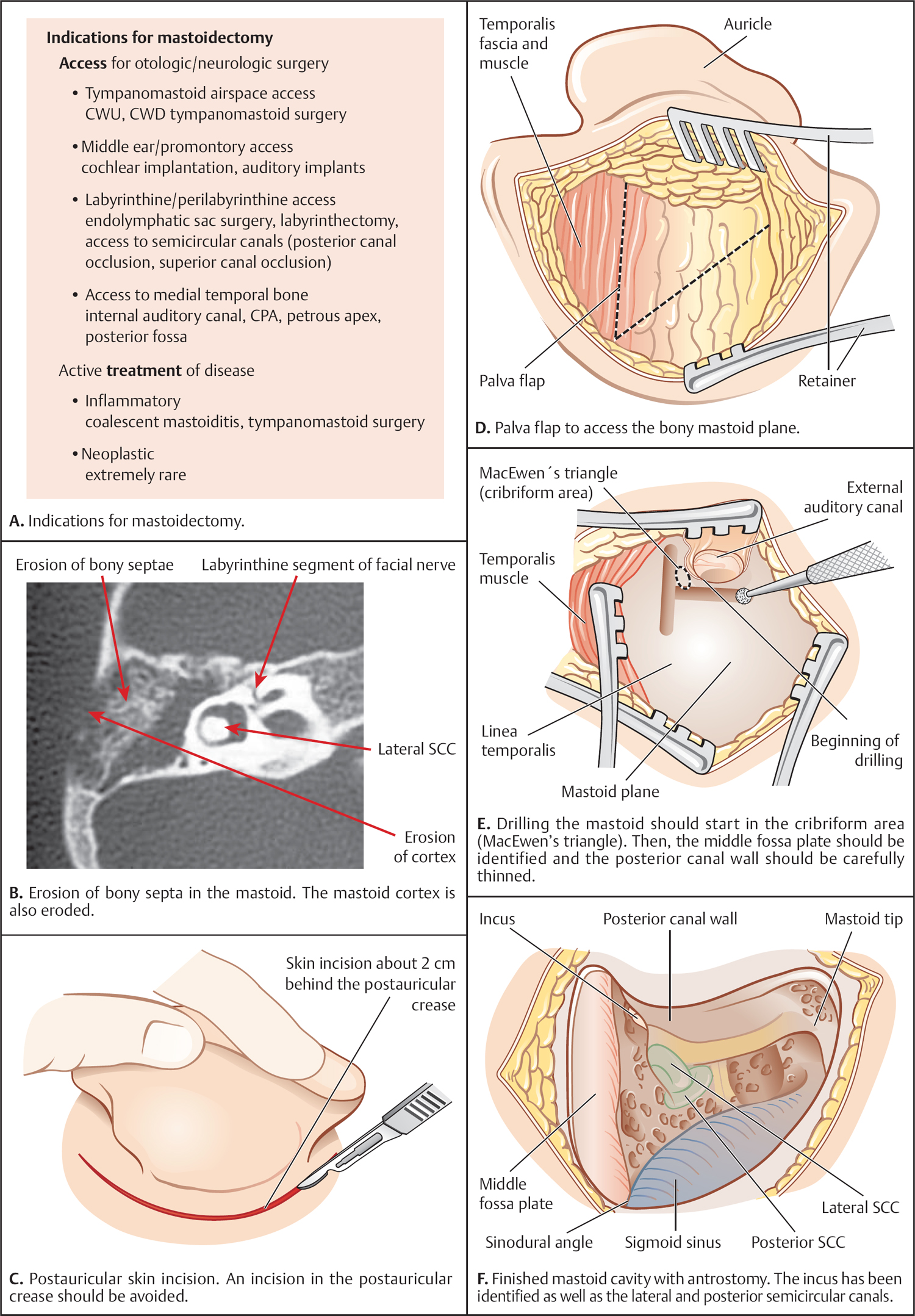
Radiographic Findings in Coalescent Mastoiditis (Fig. 5.10B)
Surgical Procedure (Fig. 5.10C–F)
Complications
Recommended Reading
Surgery for Chronic Otitis Media
Myringoplasty and Underlay Tympanoplasty
Definition
Closely Related Topics
Introduction
Principle and Classification of Tympanoplasty (Fig. 5.11A)
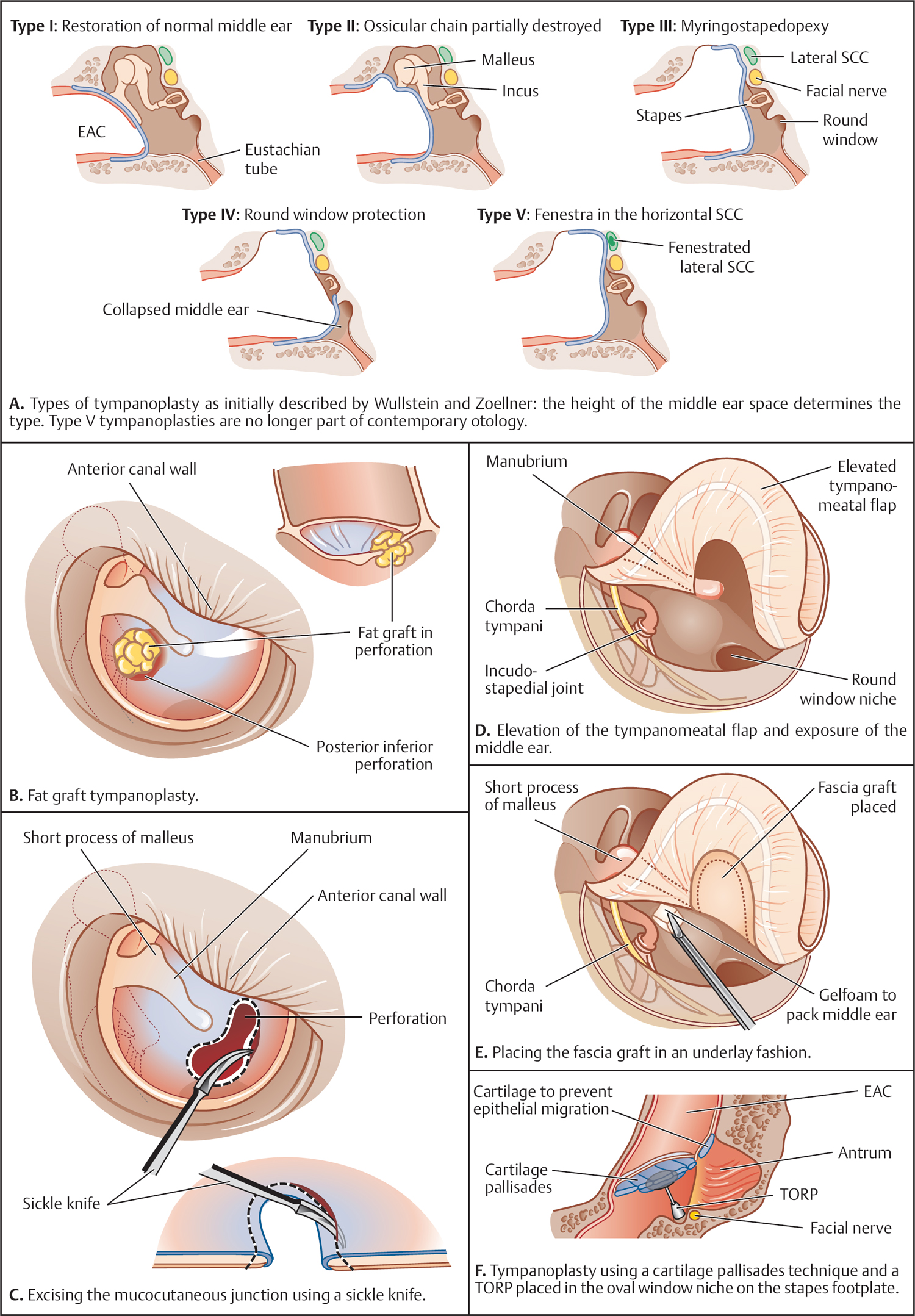
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Surgical Therapy of the Temporal Bone
Only gold members can continue reading. Log In or Register to continue





