Chapter 28 Syringomyelia
• The presence of syringomyelia requires lifelong neurological follow-up with repeat magnetic resonance imaging scans.
• When a patient with Chiari I or Chiari II malformation, with hydrocephalus and syringomyelia, has deteriorated radiologically, the shunt should be investigated first before any treatment for syringomyelia is considered.
• Chiari I patients with syringomyelia require decompression of the cerebrospinal fluid (CSF) obstruction at the craniovertebral junction.
• Treatment of syringomyelia caused by trauma requires decompression of the CSF at the level of the deformity or block followed by spinal stabilization, when clinically indicated. Treatment with a syringosubarachnoid or syringopleural shunt may stop the progression of the syrinx and neurological deterioration but the long-term prognosis remains guarded.
• Chiari II malformation is very different from that of Chiari I with respect to syringomyelia management.
The condition, syringomyelia, was described in 1876 by Leyden1 and in 1891 by Abbe and Coley.2 Although several theories had been proposed in the 1960s and 1970s on the pathophysiology surrounding the formation and propagation of syringomyelia, the real progress came after the advent of magnetic resonance imaging (MRI) in the past decade, which facilitated noninvasive high-definition imaging of the condition, and later with the development of dynamic in vivo imaging of CSF movement.
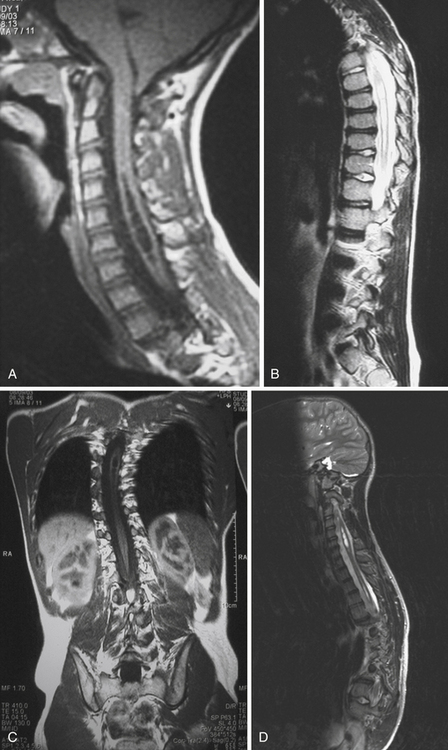
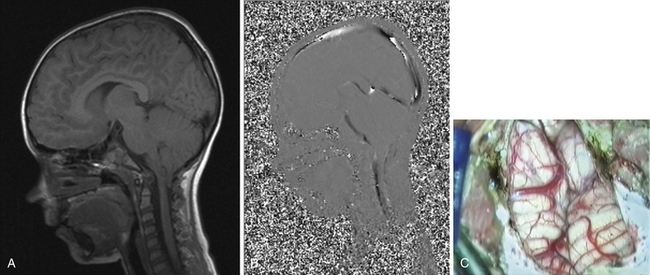
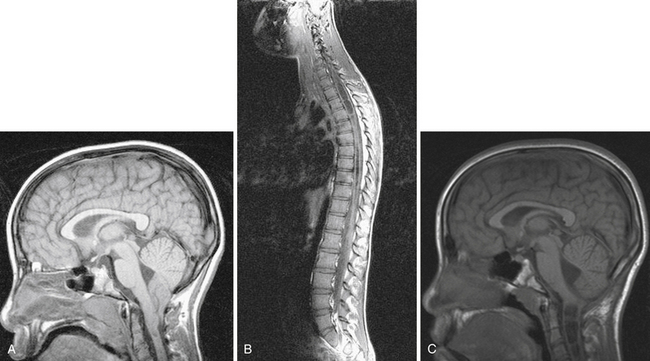
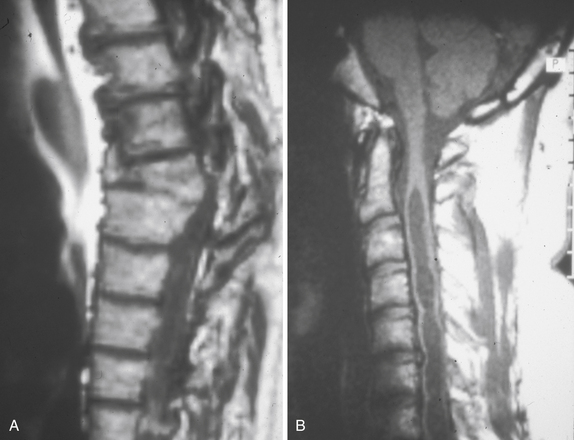
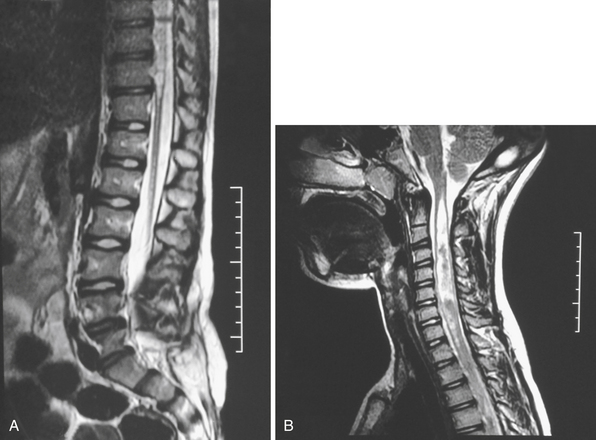
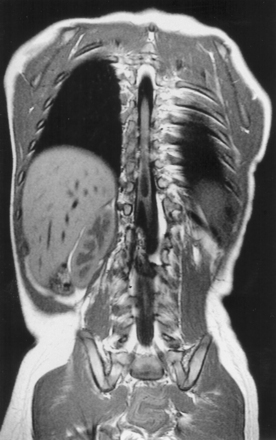
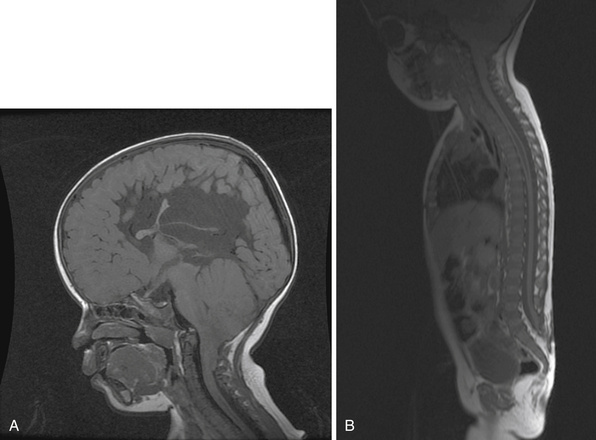
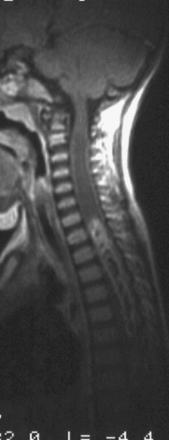
Syringomyelia is most frequently associated with hindbrain herniation. Hindbrain herniation, or Chiari I malformation, is the herniation of the cerebellar tonsils for 5 mm or more beyond the foramen magnum below a line connecting the basion and opisthion (Figs. 28.1 to 28.3). In large clinical series 50% to 80% of patients with symptomatic Chiari I malformation (CMI) have syringomyelia. However, because these series contained only symptomatic patients it is difficult to know the precise incidence of syringomyelia among all patients with Chiari I malformation. It is unclear also if syringomyelia represents an evolutionary stage in the natural history of the Chiari I malformation or it starts ab initio, from the beginning of the creation of the malformation. In most patients, Chiari I malformation is regarded as congenital. It is unclear if the malformation changes with time, if the cerebellar herniation increases. Certainly, from studies in asymptomatic patients and from the author’s unpublished experience of following over 20 asymptomatic children for many years with yearly MRI scans, in most patients the cerebellar herniation does not increase with time.3,4 Very few occasions of spontaneous resolution of Chiari I malformation have been reported.5 On the other hand, syringomyelia frequently changes with time. Acquired Chiari I malformation can develop following lumbar shunt placement or failure of a ventricular shunt, if the hindbrain is pulled down through the foramen magnum.6,7 Syringomyelia may be associated also with various other conditions such as spinal arachnoid cysts; traumatic paraplegia (Fig. 28.4); postinfectious, postinflammatory, or tuberculous meningitis; chemical insults following use of intrathecal agents, antibiotics, or myelographic contrast agent Myodil-Pantopaque; epidural abscess; Pott’s disease; and idiopathic meningeal fibrosis. In addition, various forms of spinal dysraphism such as spinal lipoma (Fig. 28.5), diastematomyelia (Fig. 28.6), recurrent tethering after myelomeningocele repair (Fig. 28.7), thickened filum terminale, rare malformations affecting the foramen magnum region such as achondroplasia, posterior fossa arachnoid cysts, Chiari II malformation, syndromic craniosynostosis, osteogenesis imperfecta, mucopolysaccharidosis, and spinal cord tumors can also be associated with syringomyelia (Fig. 28.8).
Syringomyelia affects commonly the cervical and upper thoracic spinal cord, although it can extend throughout the entire spinal cord, creating a holocord syringomyelia (see Figs. 28.3B and 28.5). If untreated, it follows a progressive and often relentless course over a number of years. Usually the neurological deterioration is gradual, but sudden deterioration has been described. The neurological function of arms and legs is at risk and patients in whom, despite treatment, the syringomyelia cavity continues to extend end up quadriplegic. The cavity may extend both proximally and distally and the tension of the fluid inside it continues to increase. Longstanding syringomyelia provokes gliosis formation in the cavity walls and the surrounding cord. Gliosis formation has been seen in postmortem examinations, in which the cavity walls are seen to be clearly lined by gliotic tissue.8 A drain inserted in the syringomyelia cavity may be encased by gliotic tissue.8 Even though surgical treatment may lead to radiological collapse of the syringomyelia cavity, sometimes this is not associated with clinical improvement. Gliosis inside the cord may lead to progressive neurological loss even after successful surgery and collapse of the cavity.9 The syringomyelia cavity can reach the upper cervical cord and medulla, becoming syringobulbia (Fig. 28.3C); it may cause deterioration of neurological function in the arms and eventually affect the function of the lower brainstem, producing bulbar palsy, respiratory failure, and death.
Epidemiology
The incidence of Chiari I malformation in the general population is estimated at 7%, but not all subjects are symptomatic. The increasing use of MRI scanning in the investigation of headaches has led to an increase in the incidence of this diagnosis. It is unclear, though, what percentage of patients with radiological evidence of Chiari I malformation have symptoms attributable to it. Syringomyelia has a prevalence of 8.4 cases per 100,000 population. It is estimated that in the United States, 21,000 Americans suffer from syringomyelia. More accurately, the figure is likely to be considerably higher, as even today, despite the wide availability of MRI scanning, the condition is underdiagnosed. The incidence of post-traumatic syringomyelia is around 1% to 3% of cases of paraplegia with a male predominance and relatively young age, and parallel to the incidence or trauma for age and gender.10–12 Why so few patients develop syringomyelia following spinal cord injury is not known. The average time between spinal injury and symptomatic presentation is 7 years (range: 3 months to 30 years). Post-traumatic syringomyelia is rare in children.
Pathophysiology of Syringomyelia
The mechanism of formation of syringomyelia has not been adequately explained yet. Most of the studies that attempt to explain the development of syringomyelia focus on the model of hindbrain hernia in Chiari I malformation. In experimental studies, dye injected in the spinal subarachnoid space has been found in the central canal, believed to have traveled there along the Virchow-Robin spaces beside the vessels of the cord. It is unclear when and how the creation of the cavity commences.13 There are reports of a presyringomyelia state seen in MRI scans of patients with Chiari I malformation, with upper cervical spinal cord swelling, which subsequently developed into syringomyelia.14–16 These reports are very few, in contrast to the very high numbers of patients with established syringomyelia that have been reported and are seen in clinical practice. This by implication may signify that the presence of a presyringomyelia state may be the exception rather than the rule. Several theories have been proposed on the formation and propagation of syringomyelia. The most notable ones, in chronological order, are Gardner’s hydrodynamic theory, Williams’ theory of “pressure dissociation,” and Oldfield’s theory of increased subarachnoid CSF pressure wave:
 In the 1960s Gardner observed that in patients with hindbrain hernia the foramen of Magendie was partially obstructed by a membrane. He believed that fluid entered the central canal in the obex at the floor of the fourth ventricle due to the “water hammer” effect of the arterial pulsations on CSF flow. Fluid accumulated inside the central canal creating the syringomyelia cavity—communicating syringomyelia or hydromyelia.17,18 This led to the surgical concept of obex plugging, where a piece of muscle or cotton wool was used to plug the obex. Syringomyelia not communicating with the central canal was called noncommunicating. MRI studies have shown that in the majority of the patients there is no direct communication between the obex and the syringomyelia cavity. Cardiac gated cine phase contrast MRI (cine PC-MRI) studies have shown that during systole the syrinx cavity contracts, whereas if Gardner’s theory was correct it should expand under the force of inflowing CSF. Gardner’s theory is regarded as imperfect and the term communicating syringomyelia has been used with less frequency. The technique of obex plugging has fallen out of favor, if for no other reason than the material used to plug the obex caused significant arachnoid fibrosis and adhesions, with subsequent recurrence of the syringomyelia.
In the 1960s Gardner observed that in patients with hindbrain hernia the foramen of Magendie was partially obstructed by a membrane. He believed that fluid entered the central canal in the obex at the floor of the fourth ventricle due to the “water hammer” effect of the arterial pulsations on CSF flow. Fluid accumulated inside the central canal creating the syringomyelia cavity—communicating syringomyelia or hydromyelia.17,18 This led to the surgical concept of obex plugging, where a piece of muscle or cotton wool was used to plug the obex. Syringomyelia not communicating with the central canal was called noncommunicating. MRI studies have shown that in the majority of the patients there is no direct communication between the obex and the syringomyelia cavity. Cardiac gated cine phase contrast MRI (cine PC-MRI) studies have shown that during systole the syrinx cavity contracts, whereas if Gardner’s theory was correct it should expand under the force of inflowing CSF. Gardner’s theory is regarded as imperfect and the term communicating syringomyelia has been used with less frequency. The technique of obex plugging has fallen out of favor, if for no other reason than the material used to plug the obex caused significant arachnoid fibrosis and adhesions, with subsequent recurrence of the syringomyelia.
 In the early 1980s Williams described the concept of “pressure dissociation” between the subarachnoid CSF spaces of the head and the spine secondary to the block of hindbrain hernia, based on in vivo CSF pressure measurements and in vitro biomechanical studies, which he performed.19 Every time a Valsalva maneuver takes place (e.g., coughing) the pressure from the chest and abdomen is transmitted to the spinal canal through the valveless venous plexus around the vertebral bodies. Normally, pressures are equalized within the spine rapidly. In the presence of a partial subarachnoid block, fluid is forced upward past the block more efficiently than it can run down again. This leads to a collapsed theca below the block, which exerts a suction effect on the spinal cord (the “suck” mechanism). Fluid that has entered the cord cavity can extend the cavity (the “slosh” mechanism).9,20,21 Williams’ theories were partly verified by MRI scan observations, and Williams’ demonstrations of his “suck and slosh” theory with models were impressive. Using cine PC-MRI it has been shown that in the presence of hindbrain hernia CSF movement in the region of the foramen magnum differs between systole and diastole. However, Williams’ observations of pressure differential were not confirmed by other studies of intraoperative CSF pressure measurements.22 It is possible that the sitting position he used to obtain his readings contributed to exaggerated cranial-spinal pressure difference readings, owing to CSF leakage from the site of the spinal measuring needle.
In the early 1980s Williams described the concept of “pressure dissociation” between the subarachnoid CSF spaces of the head and the spine secondary to the block of hindbrain hernia, based on in vivo CSF pressure measurements and in vitro biomechanical studies, which he performed.19 Every time a Valsalva maneuver takes place (e.g., coughing) the pressure from the chest and abdomen is transmitted to the spinal canal through the valveless venous plexus around the vertebral bodies. Normally, pressures are equalized within the spine rapidly. In the presence of a partial subarachnoid block, fluid is forced upward past the block more efficiently than it can run down again. This leads to a collapsed theca below the block, which exerts a suction effect on the spinal cord (the “suck” mechanism). Fluid that has entered the cord cavity can extend the cavity (the “slosh” mechanism).9,20,21 Williams’ theories were partly verified by MRI scan observations, and Williams’ demonstrations of his “suck and slosh” theory with models were impressive. Using cine PC-MRI it has been shown that in the presence of hindbrain hernia CSF movement in the region of the foramen magnum differs between systole and diastole. However, Williams’ observations of pressure differential were not confirmed by other studies of intraoperative CSF pressure measurements.22 It is possible that the sitting position he used to obtain his readings contributed to exaggerated cranial-spinal pressure difference readings, owing to CSF leakage from the site of the spinal measuring needle.
 Using preoperative PC-MRI scanning and intraoperative CSF pressure measurements in patients with syringomyelia and Chiari I malformation, Oldfield’s group observed that CSF velocity was increased at the foramen magnum but CSF flow was decreased. Cervical subarachnoid CSF pressure was increased, spinal CSF compliance was decreased, and syrinx CSF flowed caudally during systole and cranially during diastole.22,23 They surmised that the prolapsed cerebellar tonsils act like a piston, partially occluding the subarachnoid space, and create a state of increased subarachnoid pressure waves, which compress the spinal cord from without and force CSF into the syrinx cavity and propagate it with each heartbeat.
Using preoperative PC-MRI scanning and intraoperative CSF pressure measurements in patients with syringomyelia and Chiari I malformation, Oldfield’s group observed that CSF velocity was increased at the foramen magnum but CSF flow was decreased. Cervical subarachnoid CSF pressure was increased, spinal CSF compliance was decreased, and syrinx CSF flowed caudally during systole and cranially during diastole.22,23 They surmised that the prolapsed cerebellar tonsils act like a piston, partially occluding the subarachnoid space, and create a state of increased subarachnoid pressure waves, which compress the spinal cord from without and force CSF into the syrinx cavity and propagate it with each heartbeat.
All these theories do not explain precisely how and where CSF enters the cord and the mechanism of formation of septations inside the syringomyelia cavity. In the past decade, research with in vitro computer simulations using parameters gained from MRI has focused on the hydrodynamic properties of CSF, how they are altered in the presence of a subarachnoid block, and how the spinal cord viscoelastic properties relate to these changes.24–31 It is hoped that as our noninvasive imaging of CSF flow evolves, more insights will be gained on the formation and propagation of syringomyelia.
In patients who suffer spinal cord trauma, cord contusion leads to edema, blood effusion, and subsequent cord liquefaction during the first few months after injury in up to 50% of patients. Subsequently, cavity formation results and may be referred to as the primary cyst.32–34 Commonly, the cyst at the site of the injury is separated from the syringomyelia cavity by an intact septum. The syringomyelia cavity may decompress above and below the injury site following treatment, but the primary cysts may remain unchanged.35,36 As the acute injury settles, inflammation is followed by adhesions in the subarachnoid space and gliosis in the cord. Obstruction of the CSF pathways may be compounded by narrowing of the bony spinal canal from the spinal fracture. Fluid may enter the cord at the fracture site, which is often thin-walled.
Posterior Fossa Volume
The pathophysiology of syringomyelia and Chiari I malformation is often studied together, as the latter is the most common coexisting pathological condition with the former. The theory that a small posterior fossa is implicated in the cause of hindbrain hernia has been pursued for many years. It is a very obvious suggestion, as it is unlikely that Chiari I patients have “excess” cerebellar tissue compared to normal control subjects. It follows logically that some patients may have a smaller posterior fossa, which is unable to accommodate the normal volume of cerebellum. Early two-dimensional studies on lateral skull radiographs demonstrated that the height or the area of the posterior fossa is small in patients with Chiari I malformation.37,38 Recent studies used advanced image analysis techniques to calculate posterior fossa volume in computed tomography (CT) or MRI scans.39–41 The researchers conclude that the posterior fossa is smaller than normal in patients with Chiari I malformation due to presumed maldevelopment of the occipital enchondrium. This finding supports to a large extent the widespread philosophy of surgical treatment of hindbrain hernia with craniovertebral decompression to provide the cerebellum with more room, that has evolved in the past two decades.
Careful appraisal of all these studies demonstrates that none has separated patients with Chiari I according to the presence of syringomyelia. In fact, in most studies the patients with syringomyelia form the majority of the sample. A study that focused on the pediatric age group demonstrated that patients with Chiari I alone have posterior fossa of normal volume, whereas patients with Chiari I and syringomyelia have posterior fossa volume significantly smaller than normal.42 The difference in posterior fossa volume ratio according to the presence of syringomyelia was more pronounced in children who presented before the age of 10 years and smaller in those presenting after the age of 10 years. After the age of 10 years there is no significant change in the growth of the cranium overall, and little change in the growth of the skull base.43 These findings challenge the view that the development of syringomyelia represents an evolutionary stage of the Chiari I malformation. From personal observations, although new development of syringomyelia can be seen after craniovertebral decompression as a result of arachnoiditis, in patients who did not have syringomyelia at presentation, such development has not been seen in nonoperated patients with Chiari I malformation alone. Currently, there are no sizeable reports in the literature of patients with nonoperated Chiari I malformation, who have been followed for a very long time, to clearly define the risk of developing new syringomyelia. On the other hand, the phenomenon of acquired Chiari I malformation in different circumstances (e.g., following lumbar or ventricular shunting) is well recognized.6,7
There is increasing evidence that the mechanism of symptoms creation in Chiari I malformation is probably not volume related. Even in patients with syringomyelia, it has been noticed that in a small proportion of patients symptoms and radiological signs have not responded following suboccipital craniectomy, leading certain authors to conclude that the underlying mechanism causing the syringomyelia is not volume related. The presence of normal posterior fossa in Chiari I alone does not explain the development of tonsillar herniation. It could be postulated that localized venous hypertension and possible altered geometry of the posterior fossa43,44 could initiate the downward migration, which subsequently is perpetuated by CSF movement and impaction. Personal observations during foramen magnum decompression operations for Chiari only in the absence of syringomyelia indicate that soon after dural opening and division of arachnoidal adhesions that link the impacted tonsils to the surrounding structures, the cerebellar tonsils usually ascend to their natural position, without the need for further coagulation or resection. Thus, it appears that foramen magnum decompression probably works by allowing disimpaction of the tonsils from the craniovertebral junction, rather than “enlarging” the posterior fossa, as the tonsils never move to occupy the newly enlarged cisterna magna, but ascend to a normal location. In that line, there is a universal tendency for reduction of the size of the craniectomy in the foramen magnum.
Clinical Features
Presenting Symptoms
Symptoms relevant to the presence of syringomyelia include weakness or clumsiness from arms and legs, which may not be following a myotomal distribution; paresthesias, dysesthesias, and pain, which may not be following a dermatomal distribution; unsteady gait; muscle atrophy; and spasticity. Pain is commonly a prominent feature. In a small percentage of patients there may be urinary and fecal incontinence and impotence in male patients. The location of symptoms may not be correlated with radiological findings. They can be strikingly unilateral even though the syrinx may appear to occupy the central part of the cord on imaging. Syringobulbia can be life threatening. The commonest symptom of syringobulbia is spreading facial numbness. Swallowing and voice may also be affected, then vision, hearing, and finally respiratory function. Nocturnal hypoventilation has been described in the presence of syringomyelia not extending to the medulla.45
It should be emphasized that a significant proportion of patients with radiologically identified Chiari I malformation do not experience any symptoms and their malformation was identified incidentally during investigations for other reasons.3,4 A large study of 22,591 MRI scans identified 175 patients with Chiari I malformation and 14% of them were completely asymptomatic.
Clinical Examination Findings
In patients with Chiari I malformation or syringobulbia clinical examination findings include lower cranial nerves and cerebellar signs. In patients with syringomyelia long tract signs are common. Neuro-ophthalmological findings include impaired visual acuity, extraocular muscle palsy, nystagmus, and papilledema in a small minority.46 Ophthalmological assessment is of paramount importance in patients with Chiari I malformation. Other signs from lower cranial nerves include facial sensory loss, sensorineural or conductive hearing loss, impaired vestibular function, vocal cord palsy, and impaired gag reflex. Cerebellar signs such as dysmetria or truncal ataxia are not uncommon.
Clinical examination findings associated with syringomyelia include weakness of arms and legs, not always following a myotomal distribution, and sensory deficits over arms and legs, not always following dermatomal distribution. Unilateral weakness of one upper limb, not infrequently associated with a sudden strain and associated pain, is commonly seen. The small muscles of the hand are often affected first and wasting of the first dorsal interosseous muscle between the fingers and the thumb may be an early finding. The triceps or the shoulder musculature could be affected before the hand. In advanced cases it is not uncommon to find claw deformity in the hand with wasting of all the forearm and arm musculature. Sensory loss can be of spinothalamic or dorsal column type, occasionally in patchy distribution. It is usually unilateral and tends to involve the upper limb early, progressing by an increase of the density of the deficit more often than by anatomical extension. It can even be variable throughout the course of the day, and is presumably related to a variable state of filling of the syrinx. The traditionally described pain/temperature dissociation that is loss of pain and temperature sensation with preserved light touch and joint position sense is rare. Muscular atrophy and fasciculation can be seen as well as dystrophic (Charcot) joints. Hyper- or hyporeflexia can be present depending on the level of syringomyelia.47,48 Asymmetry is not uncommon and certain reflexes may be unexpectedly spared. Spasticity can affect upper and lower limbs. Scoliosis is a common finding with syringomyelia in young children, and is seen often in previously undiagnosed young adults as well.49–53 Short neck and low hairline are seen in patients with basilar invagination. Horner syndrome is seen infrequently. Gross disturbance of sweating can occur.54 Dry skin or hyperhidrosis can be observed, usually symmetrically in the lower half of the body, although the upper limbs may be affected in an asymmetrical distribution with facial involvement at times, and unequal pupils may be the only feature.
In patients with spinal cord injury incomplete paraplegia that deteriorates or the development of new autonomic features may be associated with syringomyelia. The most important features are those that ascend. Descending syringomyelia is rarely diagnosed. In complete paraplegia, improvement of leg spasms could imply downward extension of the syringomyelia. So also could alteration of sweating patterns and impairment of other autonomic functions such as bladder and bowel control and sexual function. Pain is common in post-traumatic syringomyelia, and a paraplegic complaining of severe pain has syringomyelia until proved otherwise.9–12,35,36,55–59 It is frequently associated with straining and progression of neurological deficit. Although it is commonly experienced at the level of the deficit, it can affect sites above or below that level and can be misleading. It can be confused with chest or abdominal disease. Not infrequently it is soon replaced by dysesthetic sensory loss.
Diagnosis
Radiology
MRI scanning shows the Chiari malformation and the extent of the syringomyelia cavity and haustrations, especially in T2-weighted sequences.34,60,61 Around 15% of patients have hydrocephalus. Other pathological conditions are clearly demonstrated. The syrinx in children with profound scoliosis can be difficult to visualize in MRI scanning (see Fig. 28.1). Because the curvature of the spine can be extensive it may not be possible to have the entire spinal cord in a single sagittal view, and it may be difficult to correctly count the vertebral levels and identify the full extent of the syrinx (see Fig. 28.1B and C). Children with significant scoliosis can have syringomyelia in the absence of Chiari I malformation. Post-traumatic syringomyelia and its relation with primary cysts shows well in MRI scans (see Fig. 28.4), although in some patients with extensive spinal fractures it may be difficult to identify the complexity of the bony injury. Spinal lipomas (see Fig. 28.5) and diastematomyelia (see Fig. 28.6) are clearly seen. In patients with spinal lipoma there may be the need to obtain fat suppression sequences to visualize the relation between lipoma and spinal cord better, in preparation for a cord untethering. In patients with diastematomyelia, commonly the syringomyelia cavity is not extensive. Spinal cord tumors are clearly identified in contrast-enhanced MRI sequences (see Fig. 28.8). Whenever a patient is found to have syringomyelia in the absence of a clearly identifiable associated lesion, contrast-enhanced sequences should be obtained to exclude the presence of spinal cord tumor. In a small minority of patients no associated lesion is identified. Information relating to the CSF circulation can be obtained with cardiac gated cine PC-MRI (see Fig. 28.2B). In current clinical practice the value of cine-MRI is limited in the influence it may have in clinical decisions. The utility of this tool is evolving in the study of CSF movement and its correlation with clinical symptoms and outcome.62–68 Sequential MRI is useful in assessing the results of treatment or in observing the evolution of the symptoms and deciding when radiological progression may indicate a need for surgical intervention.69,70
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree