Syringomyelia and Hydromyelia
Syringomyelia, or cystic cavitation of the spinal cord, is caused by various congenital, inflammatory, or traumatic problems of the brain and spine. Progressive syringomyelia causes neurologic deficits requiring surgical intervention. Although the cyst is often treated with direct drainage or shunting, treatment should ideally be directed at the etiology of the cyst. In this chapter, we review the incidence, pathophysiology, treatment options, and prognosis of syringomyelia as it relates to its various etiologies.
31.1 Definition
Spinal cord cavitation was first described in 1546 by Esteine in a treatise entitled La Dissection du Corps Humain.1 The term syringomyelia was coined by D’Angers nearly 300 years later, in 1827.1 In Greek mythology, Syrinx was “a nymph who was changed into a reed to save her from the amorous pursuit of Pan. From this reed, Pan then fashioned his musical pipes.”2 Today, the term syringomyelia is used broadly to define a fluid-filled cavity (Greek surinx, “pipe”) within the parenchyma of the spinal cord (Greek muelos, “marrow”) that extends across more than one spinal segment. It is associated with a variety of pathologic conditions but is most frequently seen with hindbrain abnormalities such as the Chiari 1 malformation. It rarely causes death unless associated with bulbar symptoms.
Because syringomyelia comprises a family of disorders with different etiologies and potentially diverse pathophysiologic mechanisms, attempts have been made to classify it according to various criteria. Unfortunately, no classification scheme has satisfactorily illustrated all the major differences between the various forms of spinal cord cavitation. For instance, syringomyelia is divided into communicating and noncommunicating categories. A communicating syringomyelia consists of a cavity that communicates with the central canal and thus the fourth ventricle. Such a cavity is ordinarily surrounded by an ependymal lining and is located within the central canal, a condition also termed hydromyelia (▶ Fig. 31.1). In contrast, a syringomyelia is noncommunicating when the syrinx cavity does not involve the central canal. However, for the sake of simplicity in nomenclature, we use the term syringomyelia in all cases throughout this chapter.
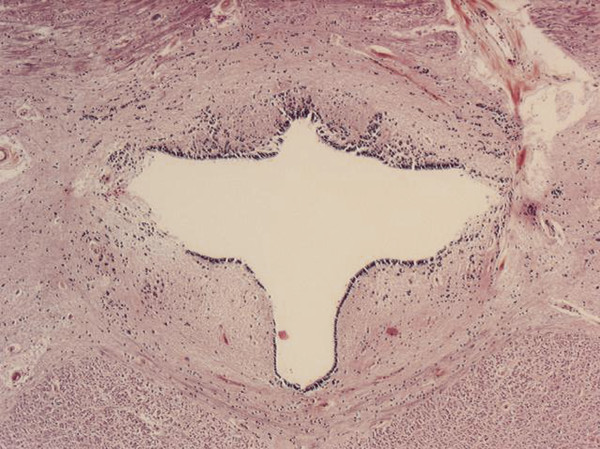
Fig. 31.1 Cross section of the cervical spinal cord showing a cystic cavity surrounded entirely by ependymal lining: “classic” hydromyelia.
31.2 Pathogenesis
The exact pathogenesis of syringomyelia is poorly understood. There is some evidence that in cases of direct compression of the spinal cord resulting from a mass, tight bony canal, tethering, or arachnoiditis, spinal cord edema may develop.3,4 Concomitantly, the patient may develop signs and symptoms of cord dysfunction that may return to normal or at least improve if the pathology is treated swiftly. Edema has been described as part of a “presyrinx” state and is well visualized on T2-weighted magnetic resonance (MR) imaging.3,4 When syringomyelia eventually develops, it can occur at any level, but it usually appears in close proximity to the area of known pathology and extends from there. Nonetheless, most of the literature that attempts to dissect the pathophysiologic causes tends to argue a specific type of syringomyelia that results from hindbrain herniation. A discussion of these theories in detail is beyond the purpose of this chapter. Instead, readers are referred to the chapter on Chiari malformations and an excellent review of the subject,5 in which the hydrodynamic theory, the perivascular cerebrospinal fluid (CSF) dissection theory, the mechanical stress theory, and the intramedullary pulse pressure theory are described. Theories that relate more exclusively to other etiologies are discussed individually under the various etiologic subheadings.
31.3 Classification and Management of Syringomyelia Based on Etiology
31.3.1 Classification Scheme Based on Etiology
Conceptually, syringomyelia is a secondary abnormality caused by other spinal or cranial pathology. It is doubtful if primary syringomyelia actually exists, and it may be only a matter of time and improved technology before idiopathic syringomyelia can be explained and placed into previously undetected etiologic categories. One obvious example is that of the Chiari 0 abnormality, in which a pathophysiologic posterior fossa abnormality is assumed to be present (because the syrinx resolves with posterior fossa decompression) but which remains elusive anatomically (no posterior fossa abnormality is evident on diagnostic imaging). The anatomical problems currently recognized to cause syringomyelia include, but are not limited to, Chiari 1 and 2 malformations, congenital and acquired tethered spinal cord (terminal syringomyelia), tumor, trauma, vascular malformation, degenerative stenosis and disk herniation, arachnoiditis, and infection. In this section, we classify syringomyelia based on these associated pathologies.
31.3.2 Management Scheme Based on Etiology
In general, the management of symptomatic syringomyelia is best approached by identifying and treating the associated condition thought to have caused the syrinx. The decision to treat syringomyelia surgically is straightforward when the patient is symptomatic, but treatment of the asymptomatic patient is more controversial. Small, asymptomatic syrinx cavities without any other obvious pathology may and often should be observed over time with serial imaging examinations. Conversely, an enlarging syrinx associated with progressive neurologic compromise merits timely surgical intervention. Difficulties arise in the large number of cases in which clinical or radiographic progression is not obvious, especially when no etiology is recognized (idiopathic syringomyelia) or when many potential etiologies coexist (e.g., spina bifida). Therefore, as with clinical presentation and pathophysiology, we discuss the general recommendations for surgical intervention according to etiology. The management algorithms presented after each section are guided by the literature but mainly reflect practice patterns garnered by the senior author’s clinical experience.
31.3.3 Chiari 1
The Chiari 1 malformation consists of caudal displacement of the cerebellar tonsils into the upper cervical spinal canal approximately 5 mm below the plane of the foramen magnum. The vast majority of these malformations are congenital; however, acquired tonsillar herniation related to prolonged lumbar drainage or increased intracranial pressure has also been reported. Although the occurrence of acquired hindbrain hernias only complicates the pathogenesis theories reported, it is reasonable to assume that syringomyelia in such cases is caused by a relative obstruction to the flow of CSF at the foramen magnum (▶ Fig. 31.2). Treatment almost uniformly consists of posterior fossa decompression. For data on the incidence, presentation, and treatment of syringomyelia associated with the Chiari 1 malformation, readers are referred to the relevant chapter in this textbook as well as the Chiari 1 Algorithm (▶ Fig. 31.3
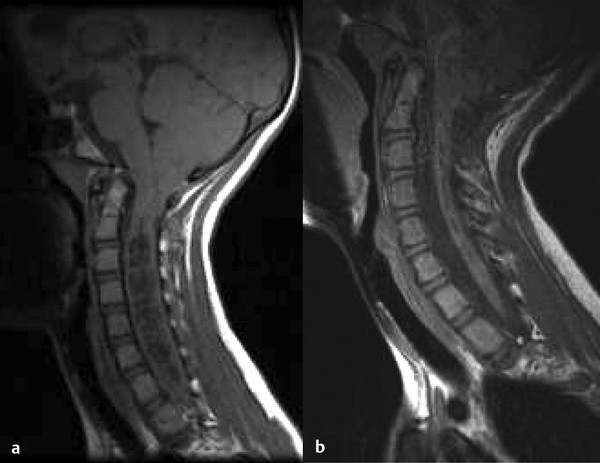
Fig. 31.2 (a) Sagittal magnetic resonance image of a 14-year-old girl with a Chiari 1 malformation and holocord syringomyelia. (b) Note the syrinx resolution after posterior fossa decompression.
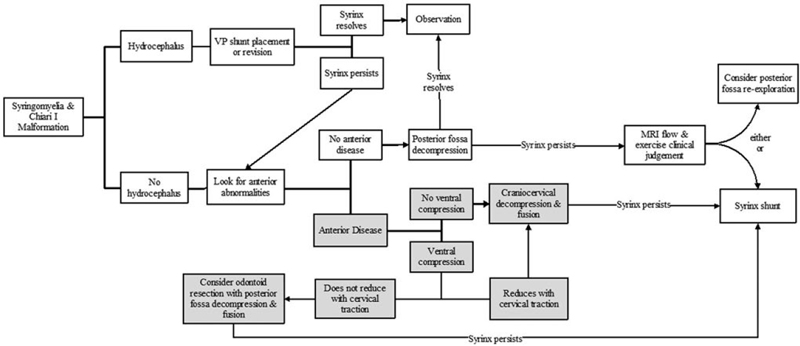
Fig. 31.3 Chiari I algorithm
31.3.4 Chiari 2 (Spina Bifida)
The Chiari 2 malformation (or Arnold-Chiari malformation) occurs solely in patients with spina bifida and consists of caudal displacement of the cerebellar vermis, fourth ventricle, and brainstem.6 It is associated with hydrocephalus in more than 90% of patients and syringomyelia in 40 to 95%. The complicating factor in managing syringomyelia in patients with spina bifida is the fact that it is often difficult—if not impossible—to determine whether the syrinx is caused by hydrocephalus and shunt malfunction, Chiari 2, or a tethered spinal cord at the myelomeningocele repair site (▶ Fig. 31.4). However, it is increasingly apparent that regardless of the type of symptoms or imaging findings, the first treatment option to be considered should be cerebrospinal shunt revision (▶ Fig. 31.4).Readers are referred to the relevant chapter in this textbook and the Chiari 2 Algorithm (▶ Fig. 31.5 for more detailed information regarding this anomaly and its association with syringomyelia.
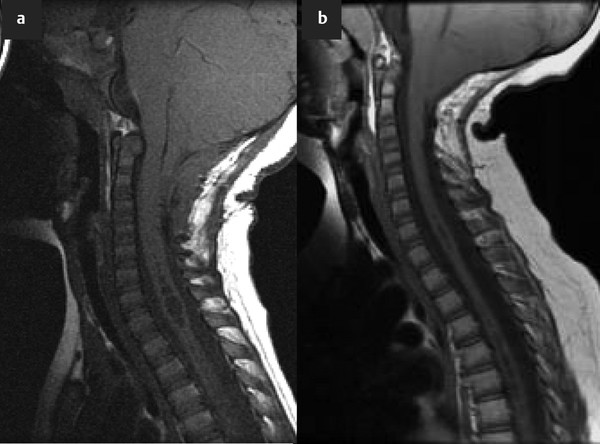
Fig. 31.4 (a) Sagittal magnetic resonance image of a patient with spina bifida showing a Chiari 2 malformation and syringomyelia. (b) The syrinx resolved after ventriculoperitoneal shunt revision.
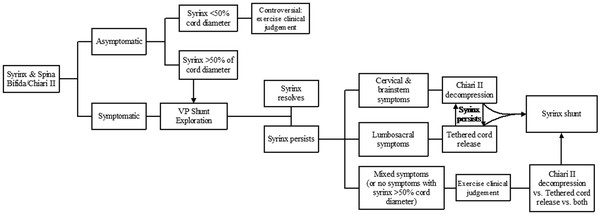
Fig. 31.5 Chiari II algorithm
31.3.5 Chiari 0
Described in 1997, Chiari 0 defines idiopathic syringomyelia that improves in response to suboccipital decompression.7 Even though patients with Chiari 0 do not have tonsillar herniation on MR imaging, evidence suggests that Chiari 0 and Chiari 1 share clinical, radiographic, and genetic characteristics, as detailed in the relevant chapter in this textbook.8,9 In general, however, the foramen magnum appears anatomically normal on imaging and intraoperative examination. This suggests that patients with Chiari 0 may have functional rather than anatomical obstructions at the foramen magnum,7,8,10 which one hopes can ultimately be identified with functional imaging modalities, such as cine flow MR imaging (see later section on imaging studies). It has further been hypothesized that Chiari 0 and Chiari 1 malformations are variants of the same disorder and have a common underlying pathophysiology. Regardless, our knowledge of Chiari 0 remains inadequate to determine the best treatment options (see Idiopathic and Chiari 0 Algorithm, ▶ Fig. 31.6). It is clear that some patients with idiopathic syringomyelia respond to posterior fossa decompression; 10 of 15 patients showed improved symptoms and signs after posterior fossa decompression in the largest series published to date.10 However, it is still unclear how these patients are different from other patients with idiopathic syringomyelia. Therefore, it is imperative to evaluate them thoroughly for other causative problems, such as tumors, arachnoiditis, and tethered cord, before any plan for posterior fossa decompression is made.
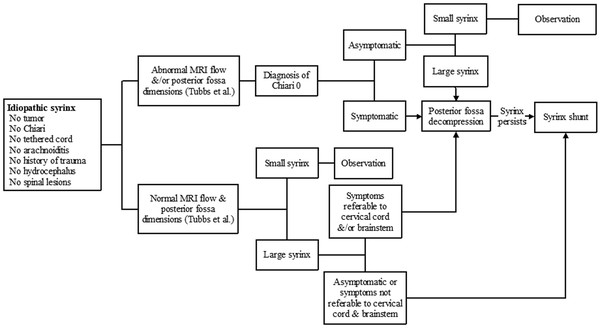
Fig. 31.6 Idiopathic and Chiari 0 algorithm
31.3.6 Tethered Cord (Terminal Syringomyelia)
Syringomyelia that occurs in the distal spinal cord, termed terminal syringomyelia, is often associated with spinal cord tethering. Tethered cord can be congenital or acquired. Acquired tethering usually occurs because of scarring, years after myelomeningocele repair. These patients are discussed in some detail in the earlier section on Chiari 2, and their cases are complicated by the fact that the Chiari 2 malformation and hydrocephalus may play a significant role in symptomatology and/or syrinx development. Congenital tethered cord, also known as spina bifida occulta, is associated with syringomyelia in 6 to 45% of cases (▶ Fig. 31.7). The spectrum of malformations that make up spina bifida occulta include tight filum terminale, split-cord malformation types 1 and 2, lipomyelomeningocele, dermal inclusion cyst with sinus tract, meningocele manqué, and neurenteric cyst.11 Terminal syringomyelia associated with spina bifida occulta occurs in the distal third of the spinal cord12 (▶ Fig. 31.7). These patients do not have hydrocephalus, Chiari malformation, or other brain anomalies. The epicenter of the syrinx is usually located in close proximity to the tethering structure in the lumbosacral region. Patients with terminal syringomyelia often present with progressive bladder and lower extremity dysfunction. However, it is often impossible to distinguish between the symptoms associated with the syrinx and those associated with the cord tethering.12 Releasing the tethered cord is essential in all patients with occult spinal dysraphism in order to prevent neurologic and urologic deficits, or the progression of already-existing deficits (see Congenital tethered cord algorithm ▶ Fig. 31.8).13 Older studies have suggested that when the associated syrinx is large in cross-sectional diameter or extends for two or more vertebral levels in longitudinal dimension, it is preferable to place a shunt in addition to untethering.12 However, more recent large studies have shown that terminal syringomyelia does indeed improve in response to untethering alone.14,15 Therefore, like syringomyelia due to foramen magnum pathology, syrinxes associated with tethered cord lesions appear to respond to correction of the underlying pathology, with a minority requiring direct shunting or drainage.
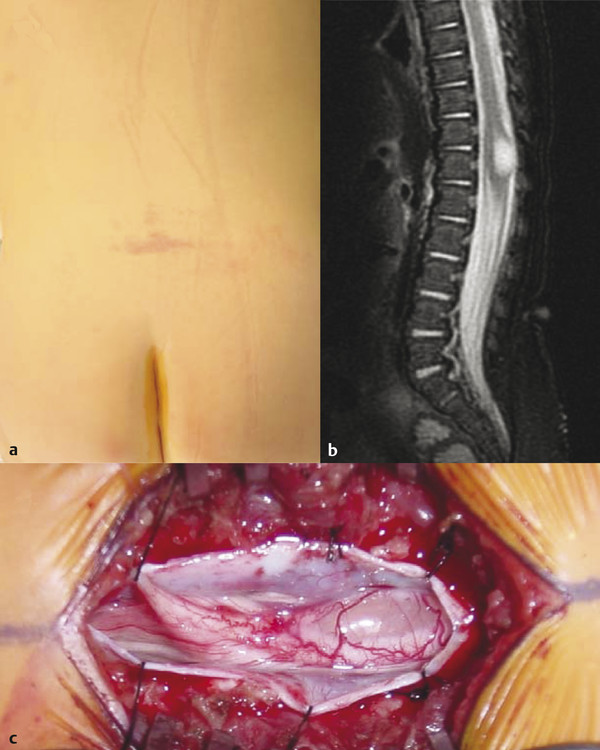
Fig. 31.7 (a) Terminal syrinx in a patient with a congenital tethered cord who presented with a lumbar hemangioma and (b) a conus in normal position. (c) Note the glistening surface of the thinned-out dorsal columns in the intraoperative photograph.
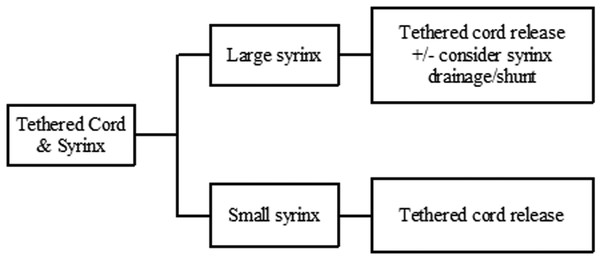
Fig. 31.8 Congenital tethered cord algorithm (terminal syringomyelia)
31.3.7 Arachnoiditis
Definition and Epidemiology
Spinal arachnoiditis can occur for a number of reasons, including infection (tuberculosis, bacterial or viral infection), chemical meningitis secondary to drugs and surgical interventions, and tumor (carcinomatous meningitis); even a familial form of spinal arachnoiditis has been described. Most commonly, however, it is associated with trauma. Imaging often reveals deformation of the spinal cord at the site of arachnoidal adhesions, blurring of a part of the syrinx wall (corresponding to the adhesive sites), flow void within the syrinx, and lack of abnormal contrast enhancement.16 In the series of Klekamp et al, the syrinx occurred above the level of arachnoiditis in approximately 40% of patients, below the level of arachnoiditis in 20 to 25%, and both above and below in 30 to 40%.17 Older studies had reported the relative success of syringoperitoneal shunting in treating patients with syringomyelia secondary to adhesive arachnoiditis.18 Since then, it has become obvious that most syrinx shunts are likely to fail, and once they fail, revisions are fraught with complications.17,19 Subsequently, the more recent literature has reported improvement in outcomes when shunting procedures were abandoned in favor of microsurgical dissection and untethering of the cord.19,20 In such cases, it is hypothesized that decompressing the part of the spinal cord that is tethered by the arachnoiditis leads to the re-establishment of subarachnoid patency and physiologic CSF flow.17,21,22 Regardless of the treatment modality, however, these patients continue to experience unpredictable rates of syrinx recurrence (see Arachnoiditis algorithm, ▶ Fig. 31.9).17,22
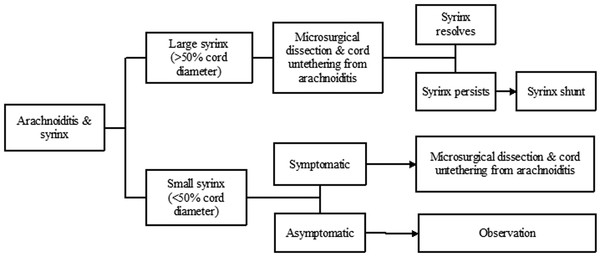
Fig. 31.9 Arachnoiditis algorithm
31.3.8 Trauma
Although uncommon, syringomyelia is a well-known sequela of spinal cord trauma.23,24 Syringomyelia secondary to trauma is of clinical importance because of both the potential for progressive neurologic deterioration and the opportunity for successful surgical management when it is diagnosed. The exact incidence of symptomatic syringomyelia after trauma is unknown but is estimated to be 2 to 5%; however, the incidence of symptomatic syringomyelia in patients with spinal cord injury has been reported to exceed 50% in some series.25,26 Additionally, the clinical picture of this condition is often complicated by the extreme temporal variability in the initial presentation of the syringomyelia, ranging between 2 months and 36 years after the initial injury.27,28 Posttraumatic syringomyelia can be caused by arachnoiditis, as described in the previous section; alternatively, spinal cord contusion or infarction with hematoma may evolve into necrosis and cavitation.32 Although most small posttraumatic syrinxes remain stable, focal arachnoiditis can cause further injury to the spinal cord, perpetuating syrinx evolution. Regardless of the mechanism, once the initial cyst forms, subsequent events must take place that result in further enlargement and development of the syrinx. Perhaps surprising is the documentation that most patients seeking medical attention for posttraumatic syringomyelia report relatively minor trauma. Despite these epidemiologic data, studies of posttraumatic syringomyelia most often involve patients with either complete or incomplete spinal cord injury who have been rendered paraplegic or quadriplegic as a result. Bonfield et al33 identified 22 studies that met inclusion criteria and determined that all of the studies had evidence of poor or very poor quality, with no randomized controlled trials or multicenter series. Despite the poor quality of the evidence, these investigators were able to conclude that patients with complete spinal cord injury have a higher risk for syringomyelia than those with incomplete injury. Pain is by far the most commonly reported presenting symptom in this group of patients with syringomyelia.17,34,35 Additionally, patients have reported sensory loss, paresthesias, progressive weakness, and less commonly ataxia, spasticity, sphincter disturbance, swallowing dysfunction, and autonomic dysfunction. It should also be noted that in numerous studies, the severity of symptoms did not correlate with the size of the syrinx cavity.36,37
Treatment of an Enlarging Syrinx or Progressive Symptoms
When there is progression of neurologic deficits, surgical treatment of the syrinx may become important (see Trauma algorithm, ▶ Fig. 31.10). However, spinal cord trauma often causes progressive cord atrophy leading to neurologic deterioration that does not respond to any surgical intervention.40 Therefore, before the patient is committed to a surgical procedure, careful review of the MR images should differentiate between a syrinx that expands the spinal cord versus an ex vacuo cyst with associated cord atrophy.38,39,40 Furthermore, although it is well established that a progressively enlarging syrinx should be treated surgically, controversy remains regarding the most effective type of treatment. Few authors recommend draining the syrinx with a stent or shunt, with the distal end of the shunt placed in the subarachnoid space, pleura, or peritoneum. Although early reports of these techniques showed encouraging results, long-term outcomes have been disappointing at best, with an 80 to 100% syrinx recurrence rate in one series.17 Based on a systematic review of the literature, the preferred surgical technique for posttraumatic syringomyelia is spinal cord untethering with expansile duraplasty, although it must be reiterated that this recommendation is based on case series only.33
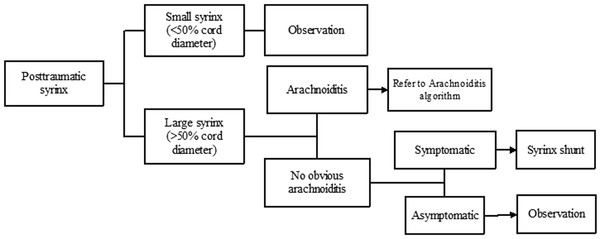
Fig. 31.10 Trauma algorithm
Treatment of a Stable Syrinx with Associated Pain
Posttraumatic syringomyelia is occasionally related to ex vacuo cavitation from a hemorrhagic contusion rather than from cystic expansion secondary to obstruction, and the pain associated with such syringomyelia does not ordinarily respond to surgical treatment of the syrinx. In these cases, other methods of treatment for the pain have been attempted, including spinal cord stimulation, intrathecal morphine pump, thalamotomy, dorsal root entry zone (DREZ) procedures, and others, all of which have shown variable but usually poor results. Outcome reporting in the population with trauma is difficult because the end points of success and failure are not well demarcated. In addition, survival in this group is often decreased, preventing adequate long-term follow-up and assessment.
31.3.9 Tumors
Tumors pose an interesting diagnostic dilemma in patients who have a cyst within the spinal cord parenchyma. Of course, syringomyelia can be associated with posterior fossa tumors,41,42 as well as spinal tumors that are extradural; in such cases, the syrinx almost always responds to treatment that is directed primarily at the tumor. However, our interest in this chapter is to present the association between syringomyelia and tumors arising in the spinal cord proper. These cystic structures can be seen in 25 to 60% of all patients with intrinsic spinal cord tumors (refer to the chapters on spinal cord tumors.43,44,45,46 The tumor types most likely to cause syringomyelia are ependymomas and hemangioblastomas (▶ Fig. 31.11) and, less frequently, astrocytomas of any grade. Syrinxes associated with spinal tumors can be classified into two main types: cysts caused by an obstructive phenomenon that interrupts CSF flow, akin to syrinxes with other etiologies; and tumor cysts caused by the secretion of fluid by tumor cells, similar to the cysts that accompany intracranial gliomas. Whereas obstructive syrinxes contain fluid identical to CSF, true tumor cysts contain fluid with a high protein concentration. Certainly, as in the brain, true syrinxes and proteinaceous cystic cavities should be distinguished from tumor necrosis. A necrotic cavity, by definition, carries a worse outcome because it may denote a higher-grade tumor; in addition, such a cavity is ordinarily lined with tumor cells, which may influence the type and extent of resection needed. The main treatment option in patients with tumor-associated cysts is tumor resection, with little need for direct syrinx drainage (see relevant chapters in this textbook and Tumor algorithm, ▶ Fig. 31.12). In their review of 44 patients who had von Hippel-Lindau disease with spinal hemangioblastomas, Lonser et al45 showed that the syrinx resolved in all patients after tumor removal, regardless of whether or not the syrinx cavity was entered.
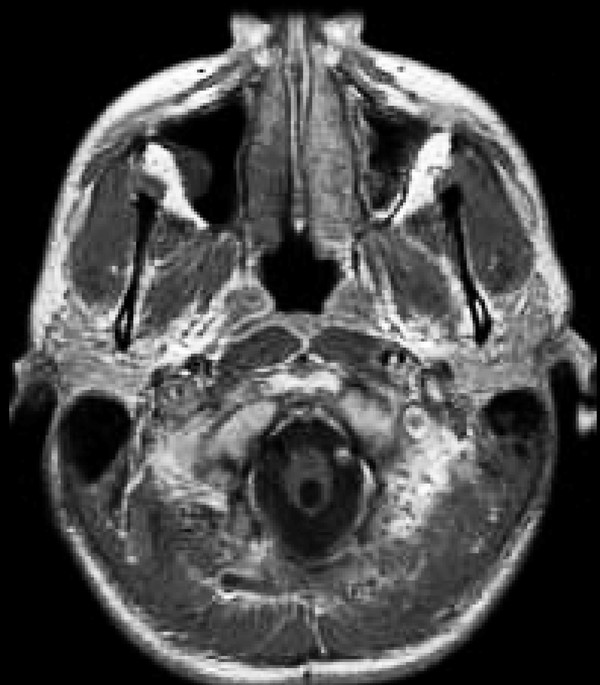
Fig. 31.11 Syringobulbia secondary to a cervicomedullary hemangioblastoma. The cyst decreased significantly in size within a year of tumor resection.









