Temporal Lobe Epilepsy
Epilepsy is defined as recurrent, unprovoked seizures and is present in up to 0.5 to 1% of the population.1,2 The incidence of epilepsy is age-dependent, with approximately 50 or so new pediatric cases diagnosed per 100,000 per year.2–5 In up to one-third of young patients, epilepsy becomes intractable,6 and the majority of seizures remain of unknown origin. Nevertheless, a significant portion of the seizures do have a temporal lobe onset, and these patients are candidates for surgical treatment with the resection of temporal lobe foci. Up to 10% of cases of new-onset childhood epilepsy may have lesions,7 many in the temporal lobe. Most temporal lobe seizures are of unclear etiology, although genetic syndromes have been described.8 This chapter focuses on the evaluation and surgical management of this population.
Temporal lobectomy is the only surgery with level 1 evidence to support its use9 in an adult population. There is no such equivalent study for children. The evidence is very strong to support that surgical outcomes are more favorable than the natural history,10–23 and in fact, a randomized study in children would likely not be undertaken, given the strength of current data.24
Most cases of epilepsy are treated successfully with medication. A single agent can control epilepsy in the majority of patients.5,25 However, once the first agent has failed, successive drugs or combinations of drugs have a rapidly diminishing rate of success. After two failed drugs, the chance of complete seizure freedom with any medical combination is poor.5,25 The rate of intractability in patients with lesions and new-onset seizures has been much higher than in patients without lesions.6,7,26 In fact, the majority of those presenting with lesions do ultimately have recurrent seizures when followed for many years. Thus, early surgery should be strongly considered for children with difficult-to-control seizures and a lesion congruent with other evaluations.
72.1 Temporal Lobe Seizures: Semiology and Electroencephalographic Findings
Most temporal lobe seizures are complex partial seizures.27 The characteristic temporal lobe seizure semiology in adolescents may be similar to that in adults. Epigastric rising, ictal vomiting (especially on the nondominant side), and experiential feelings (déjà vu, an abnormal feeling of familiarity; jamais vu, a lack of familiarity with an item that should be familiar; other feelings of altered time or flow of time) may resemble adult semiology. Speech alterations are common postictally after seizures in the dominant temporal lobe. The memory of temporal lobe seizures and surrounding events can be impaired. The recollection of seizure frequency may be an underestimate, especially if caregivers are not always present to witness any seizures.
In infants and young children, the semiology may be quite different. Hypomotor, motor, and epileptic spasms may all be seen.2,28,29 Dystonic posturing begins to be seen in young children.2 Apnea can be the presenting form of temporal lobe epilepsy in the very young.30,31
72.2 Natural History
Before the widespread use of surgical intervention, the natural history of poorly treated temporal lobe epilepsy was documented.32,33 Ongoing seizures can lead to a poor neurocognitive, behavioral, and psychosocial outcome.34 A lack of seizure freedom, unemployment, and dependence, all contributing to social isolation, are common long-term findings.
The negative impact on cognitive function can be multifactorial. All antiepileptic drugs have side effects and, even at therapeutic levels, can adversely affect cognitive and behavioral function in children.35 Even newer drugs, which may be better tolerated, have clear side effects. Leveteracitam is associated with behavioral disturbances and topiramate with language deficits, for example. Seizures may exist in association (causal or otherwise) with an underlying brain disorder. These disorders may primarily drive cognitive dysfunction. Lower measures of intelligence36 and behavioral, schooling, and learning difficulties33,37 are all seen in this population, with adverse effects on quality of life.38
Mortality is increased in children with uncontrolled epilepsy,39 although the underlying neurologic disorder may contribute significantly to this finding.2
72.3 Evaluation
Diagnostic electroencephalography (EEG) is typically performed in the evaluation of children suspected to have epilepsy. Routine EEG can be normal in up to 50% of patients with epilepsy40 thus, a single negative study does not exclude the diagnosis. Similarly, in a patient who clearly has temporal lobe epilepsy, a routine EEG may be normal, especially if the seizures are rare. However, a longer-term EEG, such as video EEG, which captures longer periods of time and ideally specific events, is typically sought as part of the evaluation. In temporal lobe epilepsy, anterior temporal interictal discharges are common. In fact, the absence of interictal discharges calls into question the diagnosis of medial temporal lobe seizures. The presence of lateralized interictal spikes is quite significant in the localization of seizures to one temporal lobe.
Auras may not have EEG changes, but full seizures would be expected to show EEG changes. Typical changes may include anterior temporal lobe spike–wave complexes, focal temporal lobe slowing, and temporal intermittent rhythmic delta activity (TIRDA). These may have a tendency in younger children to appear less focal.2 Ictal recordings may rapidly spread and appear bilateral or even generalized.2
Magnetic resonance (MR) imaging arguably provides the most important piece of information in determining candidacy for temporal lobectomy.41 As in adults, hippocampal signal change, including hippocampal sclerosis, may be identified (▶ Fig. 72.1). However, the findings may be more subtle than in adults, and MR imaging findings may be absent in many cases, even cases of histologically proven sclerosis.14 More importantly, other pathologies are prevalent in pediatric series, including dysplasia of the hippocampus, parahippocampus, and/or anterior temporal cortex. The concept of dual pathology is critical to pediatric epilepsy surgery. The presence of both a neocortical and a medial temporal lobe pathology is well described (▶ Fig. 72.2).15,42–45 Although the exact mechanism is unknown, the development of hippocampal pathology is presumed to be secondary to the primary lesion, becoming a second focus. Alternatively, the medial temporal changes can be part of the same underlying process(es), especially in the case of abnormalities (e.g., dysplasia) of the adjacent temporal lobe neocortex (▶ Fig. 72.3).
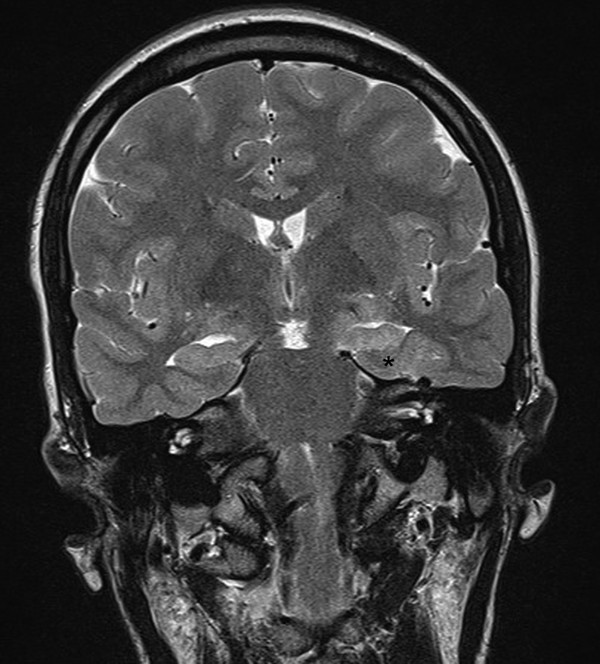
Fig. 72.1 Coronal T2-weighted magnetic resonance image showing left hippocampal sclerosis in a 3-year-old. The hippocampus (*) is small, with increased signal and absence of the internal architecture (see the dentate gyrus on the normal, right side).
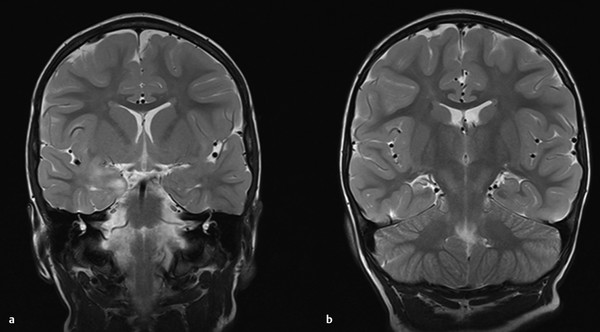
Fig. 72.2 (a) Lesion of the amygdala. (b) Hippocampal sclerosis co-presenting with the lesion in (a).
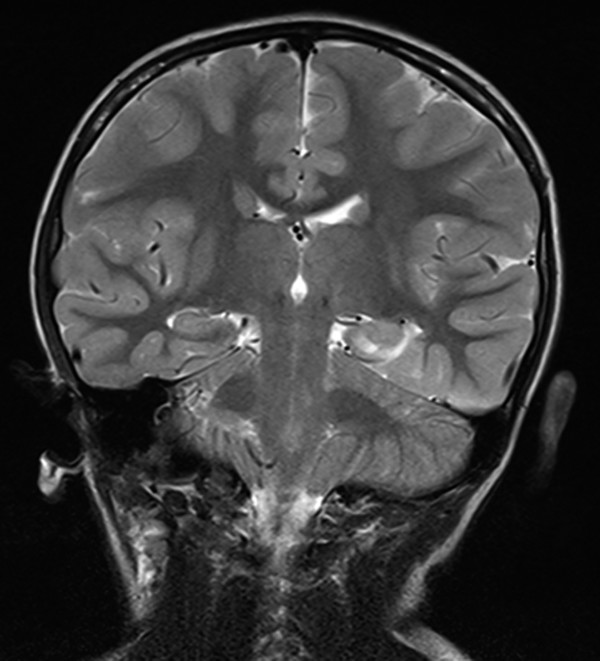
Fig. 72.3 Dysplastic lesion involving the parahippocampus and hippocampus on the left side.
As in adults, MR imaging signal changes on the T2 and FLAIR (fluid-attenuated inversion recovery) sequences can help establish the diagnosis. However, volume loss may be less common. In fact, a dysplastic hippocampus can look thickened on coronal imaging, along with a fattened amygdala. Loss of the internal architecture of the hippocampus is seen in many cases, including those in which the hippocampus does not have volume loss (▶ Fig. 72.4). Other subtleties, such as abnormal organization of the basal temporal gyri,46 may be best seen on three-dimensional reconstructions, some of which are not part of widely available imaging software packages. However, hippocampal sclerosis may be difficult to detect, and MR imaging may be normal in up to one-half of cases.14 Nevertheless, surgery for patients with negative MR results may still be successful.47
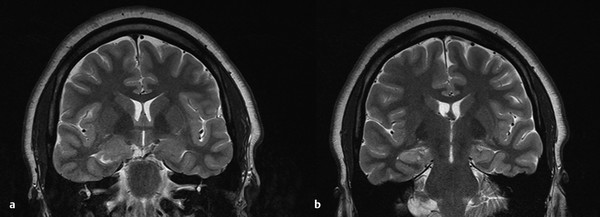
Fig. 72.4 (a) Anterior hippocampus and amygdala showing dysplastic lesion. (b) Extension of the abnormality into the hippocampus, with thickening of the hippocampus and loss of normal internal architecture.
Other MR imaging findings that can argue for epilepsy surgery are medial or lateral temporal lobe tumors48 (▶ Fig. 72.5). These are typically low-grade tumors, such as gangliogliomas, dysembryoplastic neuroepithelial tumors (DNETs), or pilocytic tumors; however, malignant tumors, especially supratentorial primitive neuroectodermal tumors (PNETs), can present with seizures in the medial temporal lobe.
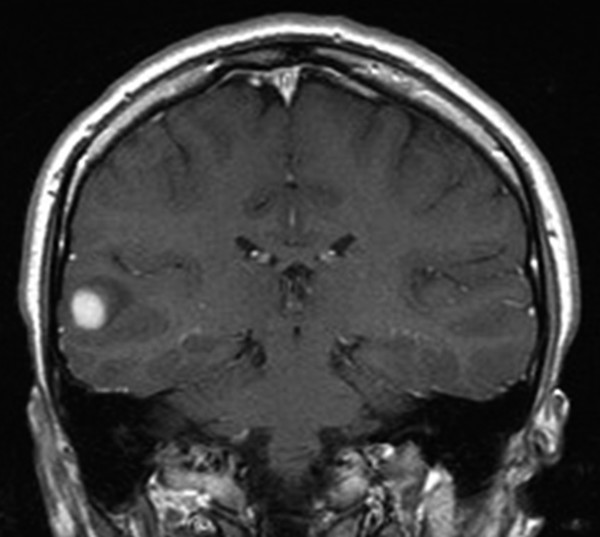
Fig. 72.5 Lateral temporal lobe lesion presenting with epilepsy. Resection showed a pilocytic astrocytoma.
In the case of tumors, it may advisable to proceed with surgery for oncologic reasons as well as for epilepsy control.49 Although a benign-appearing lesion may not grow for some time, the epileptogenic nature of such a lesion can be established much more quickly than in nonlesional cases.26 Given the frequent progression to intractability and the better prognosis with surgery, resection of a tumor is typically indicated, just as a headache-causing tumor, even if likely benign, would typically be removed. Furthermore, resection allows a more accurate diagnosis by limiting the risk for undersampling. In the case of more aggressive lesions (▶ Fig. 72.6), early diagnosis and gross total resection may increase survival.
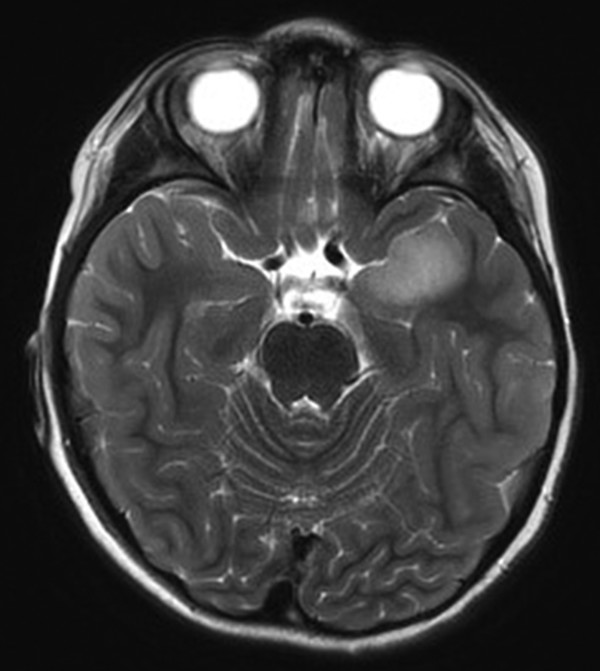
Fig. 72.6 Axial T2-weighted magnetic resonance image. Although it was not enhancing and presented with some hippocampal signal change, the lesion was malignant, a primitive neuroectodermal tumor.
In the case of normal MR imaging findings or of EEG and MR imaging data that are not congruent, other imaging modalities may be employed.50 Positron emission tomography (PET) with fluorodeoxyglucose (FDG) allows an assessment of the metabolic status of the cortex and can reveal temporal lobe foci.51,52 Epileptic foci are typically hypometabolic during the interictal phase (▶ Fig. 72.7). After a morning fast, radioactively labeled FDG is injected over 45 minutes. Cortical uptake allows measurement of the regional glucose metabolism. The period during injection should be quiet, without sensory stimulation or significant spontaneous movement; otherwise, those parts of the brain involved in, say, vision, somatosensory experience, or movement will show increased activation. During seizures, or even frequent interictal discharges, the local regional metabolism may increase. This can give a mixed picture, and the interpretation is often helped by recording the EEG during the PET to assess the interictal versus the ictal state.
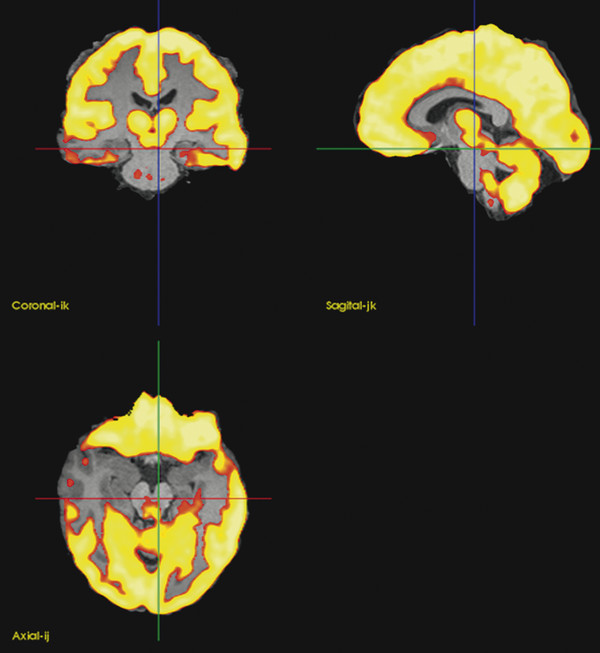
Fig. 72.7 The medial temporal hypometabolism on the positron emission tomographic (PET) scan (color scale) is superimposed on the magnetic resonance image. This co-registration aids in localization of the abnormality.
Typically, the interpretation is made by comparing the two hemispheres and commenting on relative hypometabolism on one side. Statistical parametric maps can compare glucose metabolism (normalized to overall brain metabolism) with a database of PET scans from persons without epilepsy. This may better identify foci. The lack of large databases of pediatric PET scans has limited this approach, however.
Single photon emission tomography (SPECT) studies53 are obtained by injecting during the beginning of a seizure and during the interictal phase. This is typically done on the telemetry floor, so that the onset of a seizure can be determined both by the early clinical manifestations and/or by early changes in the EEG if it is being monitored by a knowledgeable technician. Then, the injection must be performed quickly to maintain the ability to obtain a valid localization. Because the logistical requirements (e.g., radiation handling, need for an EEG technician, qualifications of the technician, bedside nursing needs) are significant, these studies are done mostly at larger centers and/or are reserved for more challenging localization situations.
Subtraction of the ictal and interictal studies can show the blood flow change associated with seizure onset. The onset zone will show increased blood flow relative to the interictal baseline. The method is quite sensitive to late injection, early spread, and other factors that may lead to incorrect localization.
Other modalities that are employed less often50 include magnetoencephalography (MEG), high-density EEG, and MR spectroscopy. MEG measures the magnetic field of brain activity and may help localize temporal lobe interictal activity.54–56 Movement is poorly tolerated, and primarily interictal studies are recorded. High-density EEG involves the placement of up to 256 electrodes to improve spatial sampling and may provide additional information.57 Other sophisticated EEG mapping tools hold promise in increasing localization power.58 Other MR imaging methods, such as MR spectroscopy, which looks at the chemical composition of the temporal lobe,59 and functional connectivity MR imaging,60 which looks at cross-region interactions, are emerging technologies that show promise in the diagnosis of temporal lobe epilepsy.
The extent of resection, and certainly the preoperative consultation, will be influenced by whether surgery is to be performed on the dominant or nondominant temporal lobe. Dominance is typically in reference to language function. Historically, dominance was established by the cerebral Amytal (or Wada) test. In this test, each hemisphere is independently injected. A short-acting anesthetic, such as amobarbital (Amytal; Marathon Pharmaceuticals, Deerfield, IL) or an alternative, is administered through a selective carotid artery injection. The injected hemisphere is temporarily disabled. Injection into the dominant hemisphere characteristically leads to the inability to speak. Memory dominance is indicated by the inability to remember items presented during the hemispheric anesthesia.61 The test is limited by the distribution of anesthetic, which does not well cover the hippocampus or posterior–medial temporal lobe, although it does cover frontal as well as temporal areas. Left dominance is extremely common in right-handed individuals with a normal developmental history.62 Functional MR imaging has increasingly been used as an alternative mechanism to establish dominance.63–65 Furthermore, it is suspected that children can better tolerate resection of the dominant temporal lobe; however, the evidence for this is minimal, and verbal memory deficits can be seen,66 as in adults.67 Functional MR imaging (and the Wada test) require a cooperative patient and can, by a person with significant training, be done in children as young as 5 years of age.68,69
Surgical resection is often guided both by the preoperative evaluation, including imaging, and also by direct brain recordings, or electrocorticography (ECoG). ECoG can be acquired intraoperatively70–73 or over longer periods by implanting electrodes for several days.74 The latter approach is used when diagnostic localization is unclear despite noninvasive tests (▶ Fig. 72.8). Typically, the questions relate to the extent of medial versus lateral involvement in temporal lobe epilepsy (beyond what can be addressed in a standard resection), the evaluation of orbitofrontal and/or insular involvement, the inclusion of the temporal lobe in a case in which multiple regions may be involved based on evaluation, and the localization of function (e.g., speech) before a resection.75
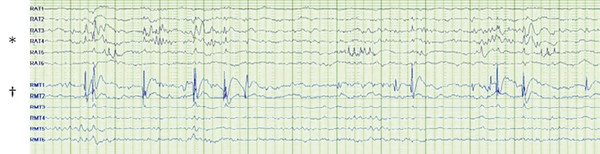
Fig. 72.8 Dual pathology on invasive monitoring. Independent interictal discharges are seen in basal temporal electrodes (rows RAT3, RAT4, and RAT5 [*]) and in medial temporal structures (RMT1 [†]).
Intraoperative ECoG76 is limited by the length of recording and is typically interictal, although a seizure may be fortuitously recorded, especially in an active case. There is no standard for duration, and the required length of time of recording may vary depending on the question at hand. If ECoG is being used to limit a resection, say, of the hippocampus,70,77 then frequent interictal activity in just a few minutes is sufficient to exclude the null hypothesis78 and therefore justify resection of the involved area (provided the area was suspected to some degree ahead of surgery). The use of intraoperative ECoG to augment lesionectomy is a common strategy in temporal lobe surgery,79 both to ensure adequate neocortical resection79 and also to assess the role of the inclusion of medial temporal (e.g., hippocampal) structures. This approach is supported by meta-analyses that suggest superior outcomes80 and other comparison studies,81 but a direct comparison has not been done, and lesionectomy alone may be a successful strategy in some cases.82,83 Intraoperative ECoG can be influenced by anesthetics84 propofol (Diprivan; Pfizer, New York, NY), dexmedetomidine,85 and sevoflurane are typically used, although minimization of anesthesia as tolerated during actual recordings may be indicated.
Implanted ECoG may use subdural grid and strip electrodes, depth electrodes, foramen ovale electrodes, or a combination of some or all of these configurations.74,86,87 Medial temporal lobe coverage should include the hippocampal and parahippocampal regions.88 Electrode leads are tunneled through the scalp after the dura is closed and the bone flap reapproximated. Alternatives for closure include loose reapproximation of the bone flap, use of an osteoplastic flap, and sterile storage of the bone flap until removal of the electrodes and resection, as indicated by the results of the monitoring. Electrode placement is generally well tolerated but does carry a higher risk for infection compared with other surgeries, and neurological deficits, especially transient, can occur.89–91
72.4 Surgical Approach: Lesionectomy
When a lesion has a benign appearance, such as of dysplasia, a cavernoma, or a hamartomatous lesion (e.g., DNET), then the decision to operate is usually driven by seizure control. EEG evidence of congruent interictal or ictal activity further supports surgery, but the absence of EEG abnormalities should not preclude surgery if other aspects of the evaluation (e.g., seizure type, neuropsychology) are congruent with the MR imaging appearance of the lesion. As discussed above, there is weak evidence to support the use of ECoG to enhance seizure resection. This is particularly true when adjacent structures are suspected, as in the case of hippocampal involvement in anterior–medial tumors.
The surgical steps of temporal lobe lesionectomy depend on whether the target is medial, lateral, or some combination thereof. Lateral lesions (▶ Fig. 72.9) are readily approached through a standard craniotomy. For many lesions, neuronavigation will be helpful in planning the incision and bone flap. Epidural hemostasis is achieved and a dural opening made in the usual fashion. Normal end-tidal CO2 along with attention to head positioning is usually adequate. Cerebrospinal fluid aspiration can be used to achieve brain relaxation without the need for diuretics or hypoventilation. Elevation of the head is also unnecessary and increases the risk for air embolus. Steroids may be considered before surgery and may aid recovery.
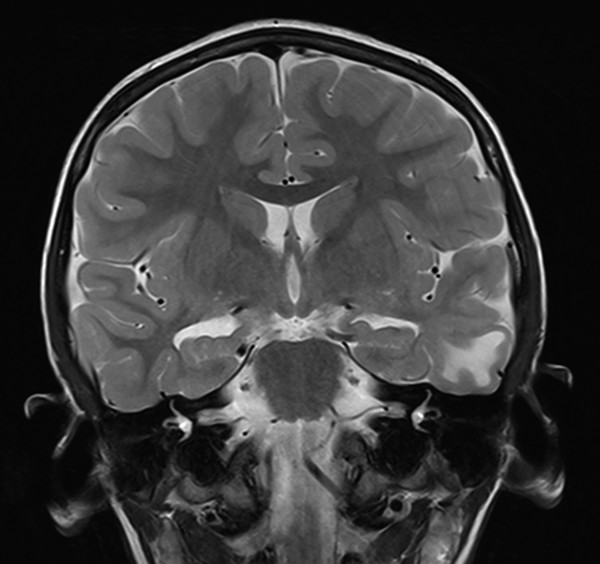
Fig. 72.9 Type IIB dysplasia of the lateral temporal lobe. The medial structures were spared, with seizure freedom on long-term follow-up.
Navigation may be especially important in cases of intrinsic brain tumors that may have a visual appearance similar to that of normal cortex. Dysplastic tissue may be firm to gentle palpation and will aspirate differently from surrounding cortex; it often has a foamy appearance when the ultrasonic aspirator is used on lower settings. Dysplasia is particularly common in association with mesial temporal lobe pathology,42,45 although in an adult series, anterior gray–white abnormalities did not have to be completely resected.92 If EcoG is performed before lesion removal, the extent of resection can be planned.
Functional considerations often determine the practical limit of resection. Tissue that is disconnected or can be undermined by resection into the white matter of the temporal lobe need not be spared. However, resection into speech areas will increase morbidity. As discussed before, language dominance may be determined with cerebral amobarbital or imaging, but language dominance is often inferred by side and clinical presentation. Many dominant temporal lesions are well anterior and/or medial to suspected speech areas. However, for more lateral and posterior lesions, speech mapping may be necessary if the boundaries of a lesion are not distinct or approach language areas. Speech mapping can be performed with an awake surgery even in a preadolescent patient,85 or invasive monitoring can be performed to determine language localization before a definitive resection. Speech may be different in children, with temporal lobe sites less common in one series.63
On the nondominant side, resections of the lateral surface are not reliably associated with clinically obvious deficits. Thus, a major consideration is extensive resection of the basal temporal lobe that could lead posteriorly to impairments of face processing, although the anatomical correlation with clinically relevant deficits remains unclear. Visual field deficits will be limited to quadrantanopsia unless the resection is taken so far back that inferior field fibers are injured where they come out of the geniculate. Of note, these fibers can be injured with a far posterior–lateral temporal resection. The vascular supply coming off the depth of a lateral temporal-occipital sulcus can be a tenuous supply to geniculocalcarine radiations, and hemianopsia following posterior–lateral temporal resection can be seen.
Once the extent of resection is determined, the corticectomy can begin with coagulation of the pia. Dividing the pia sharply allows ultrasonic aspiration of the lesion. Many lesions will respect adjacent sulci, and the subpial approach93 allows a nice delineation of the resection boundaries. If gyral vessels are inadequately coagulated, they may retract, and coagulation of the cut pial edge and deeper coagulation when a sulcus is approached is usually sufficient. The operative microscope aids in discerning lesion margins and preserving the tissue and vascularity of surrounding brain.
For lesions that are less superficial, the temporal horn of the lateral ventricle provides a critical landmark to avoid extension of the resection through the temporal stem or into the subcortical structures. Inferior and anterior to the ventricle, the medial pia is a reliable boundary between major vascular structures, the brainstem, and cranial nerves. Some pathologies will cause the pia–tissue interface to be more adherent; however, the plane can usually be identified at the edge of the tentorium and followed medially.
Above the ventricle, no such barrier exists, and resection must not violate the basal ganglia or perforators from the middle cerebral artery. Thus, for deeper lesions, identification of the lateral horn should be an early step. By taking the resection anteriorly and inferiorly first, and then resecting superiorly and posteriorly at the same depth, it is guaranteed that one will enter the ventricle before violating the temporal stem. If the superior and posterior resections of temporal cortex are not kept as superficial as the anterior and inferior extent, this relationship is lost, and other means of keeping the resection safe will be required.
72.4.1 Medial Resections
The appropriate extent of surgery and the approach to medial regions are controversial.94–96 So-called “selective” approaches advocate minimal resection of the lateral cortex, “standard” temporal lobectomy with aggressive resection of the lateral temporal lobe cortex (classically 4 cm on the dominant side and 6 cm on the nondominant side), and “tailoring” with ECoG and sometimes functional (speech) considerations to determine the lateral extent. Although in adults selective procedures seem to be adequate,97 several studies suggest that a highly selective approach in children will miss significant temporal lobe pathologies and should be used with caution.98,99
The entry to the ventricle is the key first step in approaching medial temporal structures. Of the myriad of so-called selective approaches, all but the transsylvian approach100 require entry into the ventricle as part of the resection before removal of the medial temporal lobe. A standard temporal lobectomy involving resection of the lateral cortex can be limited to the middle temporal gyrus, often as little as 3 cm, and can be tailored based on the presence or absence of recorded epileptiform activity by ECoG or imaging or on other concerns for anterolateral temporal involvement. Because the ventricle typically sits under the superior temporal sulcus, extensive resection of the superior temporal gyrus is usually unnecessary solely for the purpose of reaching the ventricle. An approach through the middle temporal gyrus or through the inferior temporal gyrus or upward retraction and resection of the fusiform gyrus,101 will allow an approach to the ventricular wall. A more posterior approach can also be used.102 Once the ventricle is entered, the lateral amygdala, choroid, and hippocampus are typically visible, even in the setting of mass lesions. The ventricular wall can be opened along the extent of its exposure. Early in the process, a cotton patty can be placed in the ventricle to avoid bleeding into the remainder of the ventricular system. Additional ECoG can now be performed directly on the hippocampus. The lateral amygdala can be resected flush with the roof of the ventricle; however, there is no pia to provide a superior border. The mesial pia will be encountered from any of several maneuvers—resection of the lateral amygdala, superior extension of the resection of the basal temporal lobe, resection of the temporal lobe off the sylvian fissure anterior to the ventricle, and resection of the hippocampus anteriorly off the choroidal fissure. The order of these steps is arbitrary and can be modified according to the surgeon’s preference.
If the superior temporal gyrus anterior to the ventricle is removed early in the resection, the sylvian fissure can be followed laterally to medially. With the use of operative microscopy, the resection is taken anteriorly to the middle cerebral artery, which can often be seen through the intact pia. As the resection is taken in the inferomedial direction, the mesial pia will be encountered. The lateral amygdala resection can be completed here as the most medial part is taken off the pia. At this point, the hippocampal head will remain. By looking at the choroidal point, the most anterior portion of the temporal horn, the remainder of the hippocampal head can be seen. Tissue can be taken off the choroidal fissure, starting anterior to the choroid plexus. The tissue is very thin, and only a small amount needs to be aspirated off the fissure. As the resection moves anteriorly and sharply medially, it is taken in front of the midbrain and the medial pia is encountered. The third nerve often will be visible, along with the carotid artery and posterior communicating artery, through the pia (▶ Fig. 72.10). Coagulation of the mesial pia is rarely necessary, and simple application of a cotton patty is usually sufficient to stop the venous bleeding. This also avoids any coagulation injury to perforators off the carotid or posterior communicating artery that may be just under the mesial pia.
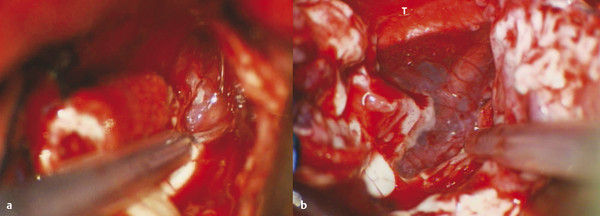
Fig. 72.10 Mesial subpial resection during a left temporal lobectomy. (a) Just medial to the head of the hippocampus is the intact pia, with neurovascular structures evident underneath. (b) After resection of the tissue off the pia with the ultrasonic aspirator, the third nerve, carotid artery, choroidal artery, and branches are all protected. T, tentorial edge.









