8 The autonomic nervous system
Organisation of the autonomic nervous system
The autonomic nervous system comprises the major autonomous or non-volitional efferent outflow to all organs and tissues of the body with the exception of skeletal muscle. Anatomically, the autonomic outflow from the spinal cord to the end organ occurs through a chain of two neurons consisting of a pre- and postganglionic component. The preganglionic component neurons live in the grey matter of the spinal cord. The postganglionic component neurons vary in location with some living in the paraspinal or sympathetic ganglia, and others in ganglia distant from the cord, known as stellate ganglia. Although historically only the efferent connections were considered, all of the projections of the autonomic nervous system are reciprocal in nature and involve both afferent and efferent components. The autonomic system can be divided into three functionally and histologically distinct components: the parasympathetic, sympathetic, and enteric systems. All three systems are modulated by projections from the hypothalamus. Hypothalamic projections that originate mainly from the paraventricular and dorsal medial nuclei influence the parasympathetic and sympathetic divisions as well as the enteric division of the autonomic nervous system. These descending fibres initially travel in the medial forebrain bundle and then divide to travel in both the periaqueductal grey areas and the dorsal lateral areas of the brainstem and spinal cord. They finally terminate on the neurons of the parasympathetic preganglionic nuclei of the brainstem, the neurons in the intermediate grey areas of the sacral spinal cord, and the neurons in the intermediolateral cell column of the thoracolumbar spinal cord. Descending autonomic modulatory pathways also arise from the nucleus solitarius, noradrenergic nuclei of the locus ceruleus, raphe nuclei, and the pontomedullary reticular formation (PMRF).
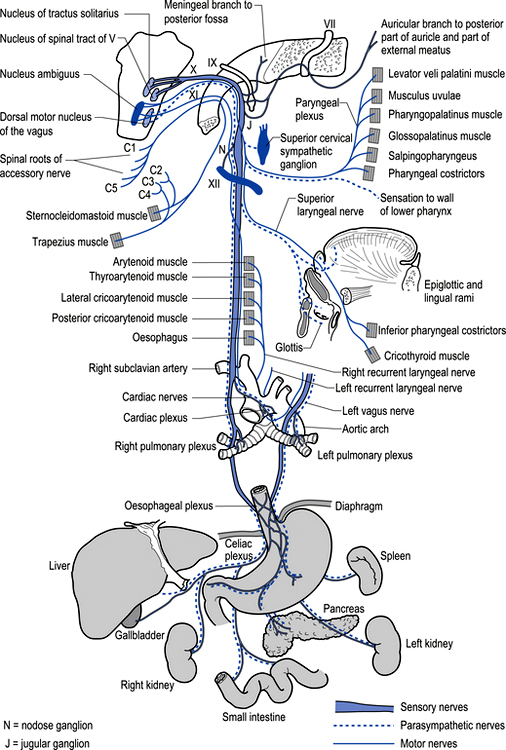
The parasympathetic system communicates via both efferent and afferent projections within several cranial nerves including the oculomotor (CN III) nerve, the trigeminal (CNV) nerve, the facial (CNVII) nerve, the glossopharyngeal nerve, and the vagus (CN X) and accessory (CN XI) nerves (Fig. 8.1). The vagus nerve and sacral nerve roots compose the major output route of parasympathetic enteric system control (Furness & Costa 1980). Axons of the preganglionic nerves of the parasympathetic system tend to be long, myelinated, type II fibres and the postganglionic axons tend to be somewhat shorter, unmyelinated, C fibres (see Chapter 7). The cell bodies of parasympathetic preganglionic neurons are located in discrete nuclei at various levels of the brainstem as described above and in the intermediolateral cell column of levels S2–4 in the spinal cord or vertebral level L1–2. In contrast to the sympathetic system, the preganglionic parasympathetic neurons are generally longer than the postganglionic neurons as they synapse in ganglia that are further from their origin and closer to the effector than the postganglionic neurons innervate.
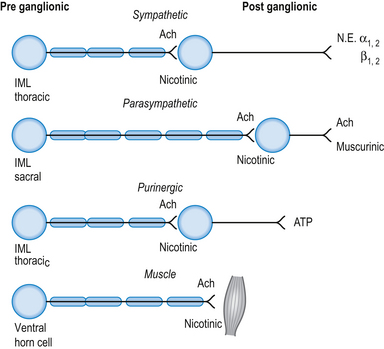
The neurotransmitter released both pre- and postsynaptically is acetylcholine. Cholinergic transmission can occur through G-protein coupled mechanisms via muscarinic receptors or through inotropic nicotinic receptors. The activity of ACh is terminated by the enzyme acetylcholinesterase, which is located in the synaptic clefts of cholinergic neurons. To date, seventeen different subtypes of nicotinic receptors and five different subtypes of muscarinic receptors have been identified (Nadler et al. 1999; Picciotto et al. 2000).
Cholinergic, nicotinic receptors are present on the postsynaptic neurons in the autonomic ganglia of both sympathetic and parasympathetic systems. Cholinergic, muscarinic receptors are present on the end organs of postsynaptic parasympathetic neurons (Fig. 8.2).
The neurological output from the parasympathetic system is the integrated end product of a complex interactive network of neurons spread throughout the mesencephalon, pons, and medulla. The outputs of the cranial nerve nuclei including the Edinger–Westphal nucleus, the nucleus tractus solitarius, the dorsal motor nucleus, and nucleus ambiguus are modulated via the mesencephalic reticular formation (MRF) and PMRF. This complex interactive network receives modulatory input from wide areas of the neuraxis including all areas of cortex, limbic system, hypothalamus, cerebellum, thalamus, vestibular nuclei, basal ganglia, and spinal cord (Walberg 1960; Angaut & Brodal 1967; Brodal 1969; Brown 1974; Webster 1978). The relationship of the parasympathetic outflow to the immune system has received very little study to date and as a consequence very little is known about the influence of the parasympathetic or the enteric system on immune function.
Supraspinal modulation of autonomic output
Monosynaptic connections between two structures suggest an important functional relationship between the two structures in question. Polysynaptic connections may be important as well but are not as well understood as monosynaptic connections. Monosynaptic connections have been demonstrated to exist between a variety of nuclei in the medulla, pons, diencephalon, and the preganglionic neurons of the IML (Smith & DeVito 1984; Natelson 1985). Nuclei with monosynaptic connections with the neurons of the IML include:
• Areas of the ventral lateral reticular formation including neuron pools in the ventral pons and ventral lateral medulla;
• Neuron pools in the locus ceruleus of the dorsal rostral pons;
• Serotonergic neurons of the raphe nucleus;
• Epinephrine-producing neurons of the caudal ventrolateral medulla;
• Neuron pools of the parabrachial complex;
• Neuron pools of the central grey area and the zona incerta; and
• Neuron pools in the paraventricular and dorsal medial nuclei of the hypothalamus.
The projections from the cerebral cortex and their role in modulation of autonomic function are not well understood. However, existence of direct projections from the cortex to subcortical structures regulating autonomic function has been established (Cechetto & Saper 1990). Neurophysiological studies demonstrating autonomic changes with stimulation and inhibition of the areas of cortex also suggest a regulatory role. The following outlines the established areas of cortex and their projection areas:
1. Medial prefrontal cortex has direct projections to the amygdala, hypothalamus, brainstem, and spinal cord areas involved in autonomic control.
2. The cingulate gyrus has direct projections to the amygdala, hypothalamus, brainstem, and spinal cord areas involved in autonomic control.
3. The insular and temporal pole areas of cortex also demonstrate direct projections to the amygdala, hypothalamus, brainstem, and spinal cord areas involved in autonomic control.
4. Primary sensory and motor cortex are thought not to control autonomic activity directly but to coordinate autonomic outflow with higher mental functions, emotional overlay, and holistic homeostatic necessities of the system.
Most areas modulating the autonomic systems are bilateral structures
It is worth noting at this point that with the exception of a few midline structures in the brainstem, the locus ceruleus, and the raphe nuclei, all other structures that modulate the autonomic output are bilateral structures. This presents the possibility that asymmetric activation or inhibition lateralised to one side or the other may translate to the activity of the end organs and produce asymmetries of function from one side of the body to the other (Lane & Jennings 1995). Accurately assessing the asymmetric functional output of the autonomic nervous system is a valuable clinical tool in evaluating asymmetrical activity levels of cortical or supraspinal structures that project to the output neurons of the autonomic system.
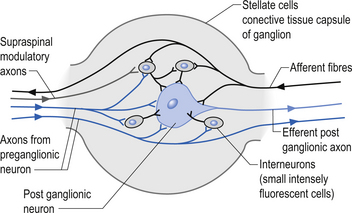
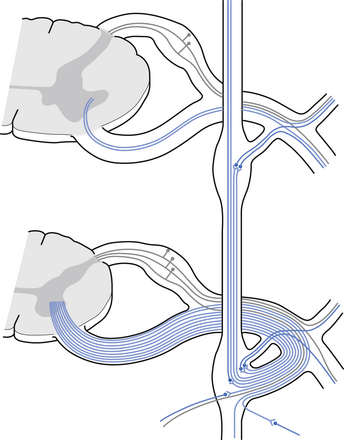
The autonomic ganglion
Incoming and outgoing nerve bundles are attached to the ganglion (Fig. 8.3). The incoming bundles contain afferent fibres from the periphery returning to the spinal cord, preganglionic axons that synapse on the postganglionic neurons in the ganglion, preganglionic axons that pass through the ganglion giving off collateral axons to the interneurons as they do so, and descending axons from cholinergic neurons in the spinal cord that modulate the activity of the interneurons in the ganglion. The interneurons in the ganglion are referred to as small intensely fluorescent cells and they are thought to be dopaminergic in nature. The outgoing bundle contains postganglionic axons, and afferent fibres from the periphery entering the ganglion (Snell 2001). The presence of such a complex structure in the ganglion has led to the suspicion that the ganglion is not just a relay point but an integration station along the pathway of the autonomic projections.
Parasympathetic efferent projections
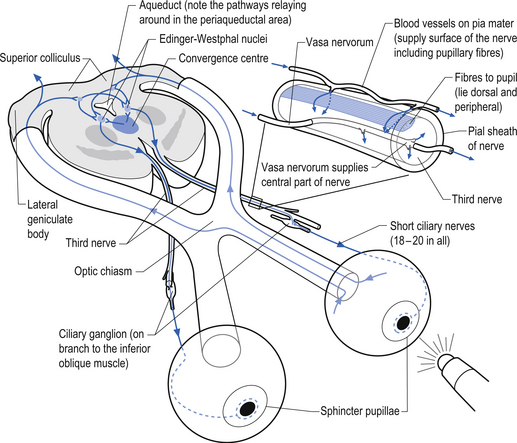
The oculomotor parasympathetic fibres commence in the midbrain. These fibres are the axon projections of neurons located in the Edinger–Westphal nucleus (EWN) or accessory oculomotor nuclei. The parasympathetic projections travel with the ipsilateral oculomotor nerve and exit with the nerve branch to the inferior oblique muscle and enter the ciliary ganglion where they synapse with the postganglionic neurons. The axons of the postganglionic neurons then exit the ganglion via the short ciliary nerves and supply the ciliary muscle and the sphincter pupillae. Activation of the postganglionic neurons causes contraction of both the ciliary muscle, resulting in relaxation of the lens, and the sphincter pupillae muscle, resulting in constriction of the pupil. These actions can be stimulated separately or simultaneously as in the accommodation reflex (Fig. 8.4).
The parasympathetic efferent projections of the facial nerve involve motor axons to the submandibular gland and the lacrimal gland. The motor fibres project in two different pathways and to two different ganglia. The motor projections to the submandibular gland arise from neurons in the superior salivatory nucleus in the medulla. The axons of these neurons emerge from the brainstem in the nervous intermedius and join the facial nerve until the stylomastoid foramen where they separate as the chorda tympani, which traverse the tympanic cavity until they reach and join with the lingual nerve. They travel with the lingual nerve until they reach and synapse on the postganglionic neurons of the submandibular ganglion. The axons from these neurons project to the submandibular glands via the lingual nerve supplying the secretomotor fibres to the gland. Activation of the postganglionic neurons results in dilatation of the arterioles of the gland and increased production of saliva (Fig. 8.5).
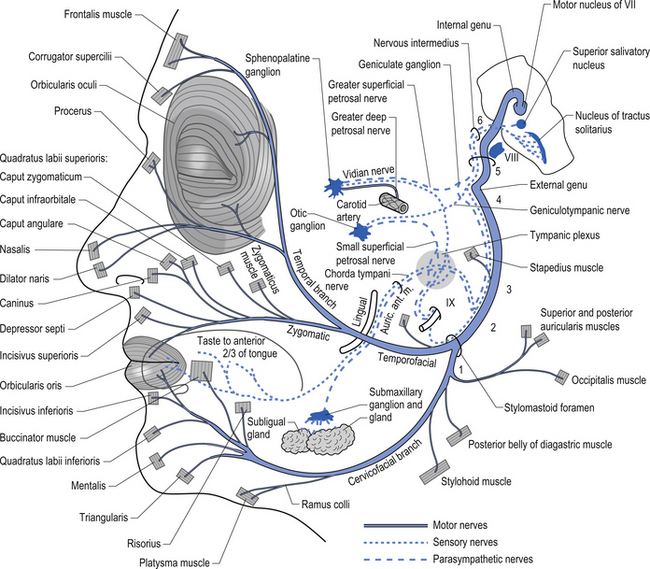
Figure 8.5 This figure outlines the motor and parasympathetic projections of the facial nerve (CN VII).
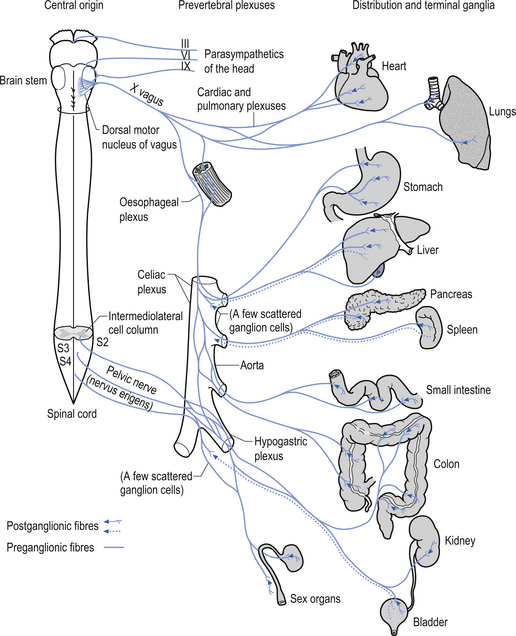
The motor projections of the vagus nerve arise from the neurons of the dorsal motor nucleus and the nucleus ambiguus of the medulla. The cardiac branches are inhibitory, and in the heart they act to slow the rate of the heartbeat. The pulmonary branch is excitatory and in the lungs they act as a bronchoconstrictor as they cause the contraction of the non-striate muscles of the bronchi. The gastric branch is excitatory to the glands and muscles of the stomach but inhibitory to the pyloric sphincter. The intestinal branches, which arise from the postsynaptic neurons of the mesenteric plexus or Auerbach’s plexus and the plexus of the submucosa or Meissner’s plexus, are excitatory to the glands and muscles of the intestine, caecum, vermiform appendix, ascending colon, right colic flexure, and most of the transverse colon but inhibitory to the ileocaecal sphincter (Fig. 8.6).
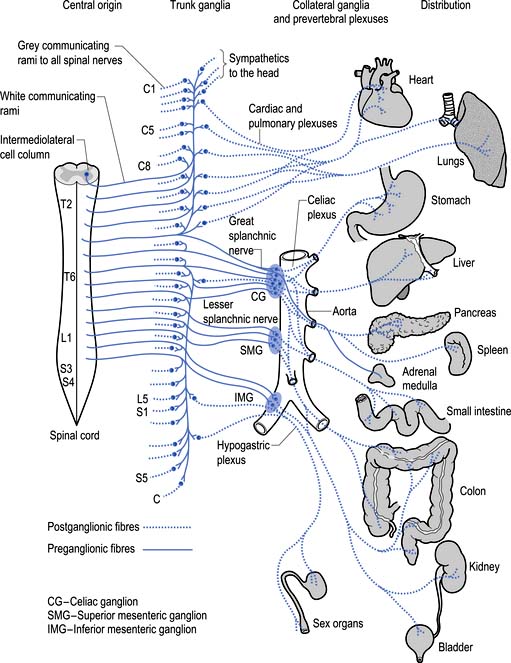
The sympathetic system enjoys a wide-ranging distribution to virtually every tissue of the body (Fig. 8.7). The presynaptic neurons live in a region of the grey matter of the spinal cord called the intermediomedial and intermediolateral cell columns located in lamina VII. Axons of these neurons exit the spinal cord via the ventral rami where they further divide to form the white rami communicantes. The fibres then follow one of several pathways (Fig. 8.8):
1. They synapse in the paravertebral or prevertebral ganglia segmentally.
2. They synapse in segmental regions of the paravertebral or prevertebral ganglia other than those at which they exited.
3. They do not synapse in the prevertebral or paravertebral ganglia and continue as presynaptic myelinated fibres into the periphery (Williams & Warwick 1984).