11 The basal ganglia
Case 11.2
Questions
• 11.2.1 What are the cardinal classic symptoms of idiopathic Parkinson’s disease? Which of these does this man exhibit?
• 11.2.2 Describe the neuronal circuits of the basal ganglia thought to be responsible for hypokinetic dyskinesias.
• 11.2.3 What treatment options are available for this patient?
Case 11.3
Questions
• 11.3.1 Describe the motion of a patient with the following movement disorders: chorea, athetosis, and ballismus.
• 11.3.2 Describe the neuronal circuits of the basal ganglia thought to be responsible for hyperkinetic dyskinesias.
• 11.3.3 Describe the differences between Huntington’s disease and Sydenham’s chorea.
Introduction
Under normal conditions the inhibition and excitation of the thalamus from the basal ganglia occurs at the appropriate time and in the appropriate amounts to support the activities of the cortex. However, in certain circumstances dysfunction of the basal ganglionic circuits can result in a number of conditions that affect movement and thought processes: idiopathic Parkinson’s disease (PD), Huntington’s disease (HD), Sydenham’s chorea (SC), Tourette’s syndrome (TS), ballismus, dystonias, obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), schizophrenia, depression, substance abuse disorders, and temporal lobe epilepsy (Marsden 1984; Javoy-Agid et al. 1984; Swerdlow & Koob 1987; Reiner et al. 1988; Modell et al. 1990; Swerdlow 1996; Castellanos 1997; Van Paesschen et al. 1997; Leckman et al. 1998).
In this chapter we will consider the neurocircuitry of the cortico-thalamo-thalamic-cortico system and the disorders of movement that can arise from dysfunctions of this system. Other non-motor dysfunctions are discussed in Chapter 16.
Anatomy of the basal ganglia
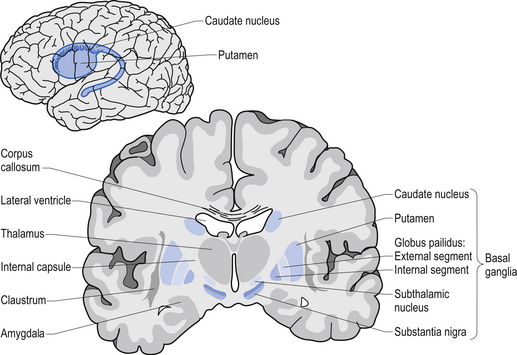
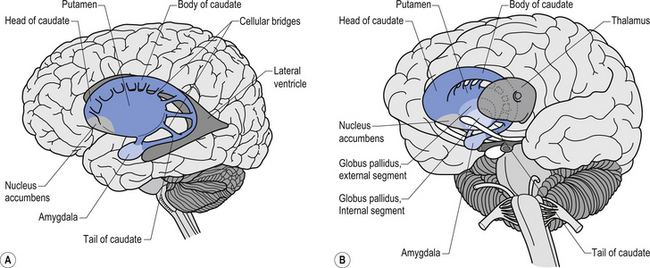
The caudate nucleus and the putamen are embryological homologues that have maintained similar morphological structure and function as they matured. For this reason these two nuclei, and the nuclei formed by the merger of these structures, the ventral striatum, are grouped into a single functional structure called the neostriatum (Kandel et al. 2000) (Fig. 11.1).
The neostriatum receives projection axons from virtually all areas of cortex and acts as the gatekeeper for all input to the basal ganglia. The caudate nucleus is a large C-shaped structure composed of a head, body, and tail that maintains a constant relationship with the lateral ventricle of the brain. Except for an area located anteriorly and ventrally where these two nuclei merge as the ventral striatum, the caudate nucleus and the putamen are separated by the fibre tracts of axons of the internal capsule. Most of the area composing the ventral striatum, which receives projections from areas of the limbic system, is taken up by the nucleus accumbens (Blumenfeld 2002) (Fig. 11.2).
The substantia nigra is a broad layer of pigmented grey substance separating the ventral portion of the mesencephalon from the tectum and extending from the upper surface of the pons to the hypothalamus. The substantia nigra can be separated into two areas which have different cell types. The most ventral area is referred to as the substantia nigra pars reticulata (SNr) and the more dorsal portion the substantia nigra pars compacta (SNc). The SNc contains a large population of dopaminergic neurons that contain a darkly pigmented grey substance, neuromelanin, which accumulates with age in dopaminergic neurons. The neuromelanin is thought to be composed of oxidised polymers of dopamine that accumulate in lysosomal storage granules in the neurons (Kandel et al. 2000).
The neostriatum is the input nucleus of the basal ganglia
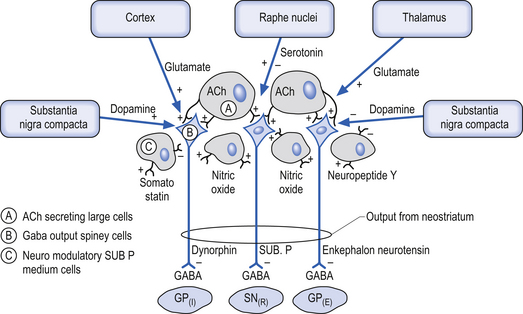
The neostriatum, which is composed of the caudate nucleus and the putamen, is the major input nucleus of the basal ganglia. The neostriatum has been estimated to contain some 110 million neurons per hemisphere (Alexander & DeLong 1992) compared to the 12 million neurons receiving cortical projections in each half of the basis pontis (Tomasch 1969). The striatum receives excitatory glutaminergic topographic projections from all areas of cortex and the intralaminar (centromedian and parafascicular) nuclei of the thalamus (Kunzle 1975, 1977; Selemon & Goldman-Rakic 1985). Dopaminergic input projections are also received from the SNc via the nigrostriatal pathway. The influence of this pathway on the neostriatal neurons involves complex interactions with various classes of dopamine receptors that result in excitation in some neurons and inhibition in others. Serotonergic axons from the raphe nuclei also project to the neostriatum.
The output neurons of the neostriatum produce spontaneous discharges in the default or resting state at about 20 spikes per second. The neurons of the globus pallidus, on the other hand, maintain a much higher spontaneous discharge rate approaching 200 spikes per second. This results in a spontaneous inhibition of the thalamus in the default or resting state. This inhibition can be modulated by the activity of the neostriatal inhibitory neurons (Kropotov 2009).
The small cell group of interneurons releases a variety of inhibitory neuroactive substances such as somatostatin, neuropeptide Y, and nitric oxide synthase (Fig. 11.3).
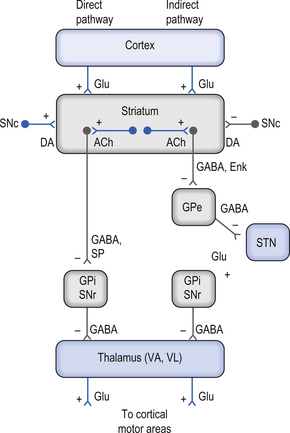
Direct and indirect pathways from the neostriatum to the GPi and SNr can modulate inhibition of the thalamus and pontomedullary reticular formation
There are two predominant pathways from the neostriatum to the output nuclei of the basal ganglia—the globus pallidus pars internus and the substantia nigra pars reticulata. Understanding the inhibition and excitation circuits involved in these two pathways will help one understand the spectrum of functional disorders ranging from hyperkinetic to hypokinetic, involving movement and thought processes caused by basal ganglia disorders.
The output neurons in the GPi and SNr are inhibitory in nature and release the neurotransmitter GABA (Fig. 11.4).
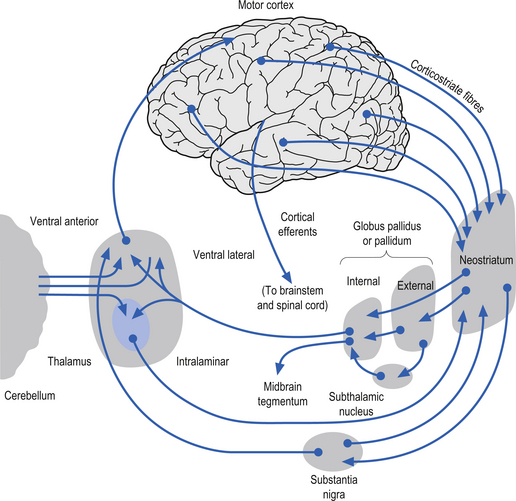
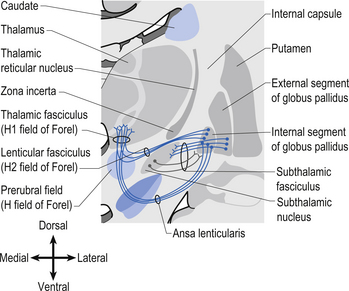
The neurons in GPi project axons via the anterior thalamic fasciculus to the ventral lateral and ventral anterior nuclei of the thalamus. These projections are mainly associated with motor control functions of the body below the head and neck. GPi neurons also project to the intralaminar nuclei (centromedian and parafascicular) and the mediodorsal nuclei of the thalamus. These projections are largely associated with limbic activities (Fig. 11.5). Output projections of the GPi reach the thalamic fasciculus via two different pathways. The first pathway, called the ansa lenticularis, loops ventrally and passes beneath the internal capsule before swinging dorsally to join the thalamic fasciculus and reach the thalamus. The second pathway, called the lenticular fasciculus, passes straight through the internal capsule to join the ansa lenticularis to form the thalamic fasciculus and enter the thalamus (Chusid 1982). The point at which the two pathways combine to form the thalamic fasciculus is sometimes referred to as the H fields of Forel. The H1 field of Forel refers to the thalamic fasciculus, the H2 field of Forel refers to the lenticular fasciculus, and the H or prerubral field of Forel refers to the area where the ansa lenticularis joins the thalamic fasciculus (Fig. 11.6). Finally, the GPi neurons also project to the complex reticular neurons in the pons and medulla known as the pontomedullary reticular formation (PMRF). These projections are involved in the modulation of the reticulospinal tracts (Afifi 1994).
The neurons in the SNr also project to the ventral anterior and ventrolateral nuclei of the thalamus. These projections are associated with motor control of the head and neck. The SNr neurons also project to the superior colliculus where they modulate actions of the tectospinal pathways. Finally, the SNr neurons project to the PMRF, where they also modulate the output of the reticulospinal tract neurons (Fig. 11.5).
The neuron in the substantia nigra pars compacta release dopamine as their neuromodulators. These neurons project to the neostriatum where they have complex modulatory effects on the output neurons of the neostriatum. The net effect of the SNc release of dopamine in the neostriatum is an excitation of the output neurons of the direct pathway and an inhibition of the output neurons of the indirect pathway (Parent & Cicchetti 1998).
Functional modulatory outputs of the direct and indirect pathways may result in movement and cognitive dysfunctions
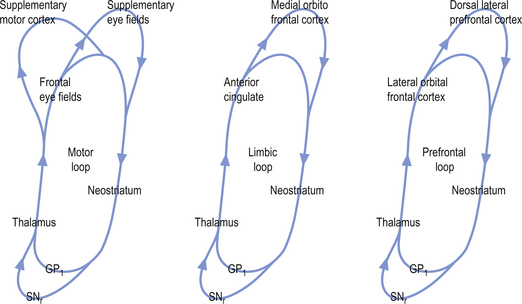
The activation pathways of the cortico-neostriatal-thalamo-cortical system are thought to operate through parallel segregated circuits that maintain their segregation throughout the neostriatal-thalamo-cortical projections. Several loops including a motor loop, a limbic loop, and a frontal cortical loop function to modulate motor, limbic, and frontal cortical activities, respectively (Fig. 11.7).
Cortical activation of the direct basal ganglionic pathway results in a net excitation of the thalamus via disinhibition of the basal ganglionic projections and subsequently excitation of the cortical areas that receive thalamic projections. Cortical activation of the indirect basal ganglionic pathway results in inhibition of the thalamus and subsequently inhibition of the cortical areas receiving thalamic projections (see Fig. 11.4). The large ACh-releasing neurons in the neostriatum tend to preferentially form excitatory synapses on the output neurons of the indirect pathway; thus, excitation of these neurons would result in an increased activation of the indirect pathway or an inhibition of movement and thought processes.
Summary of outputs from basal ganglionic structures
| Neostriatum (caudate, putamen) | All inhibitory |
| Globus pallidus pars internus | All inhibitory |
| Globus pallidus pars externus | All inhibitory |
| Subthalamic nucleus | All excitatory |
| Substantia nigra pars reticulata | All inhibitory |
| Substantia nigra pars compacta | Both excitatory and inhibitory |
Under normal conditions the inhibition and excitation of the thalamus from the basal ganglia occurs at the appropriate time and in the appropriate amounts to support the activities of the cortex. However, in certain circumstances dysfunction of the basal ganglionic circuits can result in a number of conditions that affect movement and thought processes. These include idiopathic Parkinson’s disease (PD), Huntington’s disease (HD), Sydenham’s chorea (SC), Tourette’s syndrome (TS), ballismus, dystonias, obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorders (ADHD), schizophrenia, depression, substance abuse disorders, and temporal lobe epilepsy (Marsden 1984; Javoy-Agid et al. 1984; Swerdlow & Koob 1987; Reiner et al. 1988; Modell et al. 1990; Baxter et al. 1992; Swerdlow 1996; Castellanos 1997; Van Paesschen et al. 1997; Leckman et al. 1998).