Figure 65.1. Flow chart of the different conditions that follow a cerebral insult. Classically vegetative state follows a coma; after 1 month the term “persistent vegetative state” is used; after 3 months (non-traumatic insult) or 1 year (traumatic insult) some authors use the term “permanent vegetative state” which implies no chance of recovery. Adapted from [Laureys et al., Lancet Neurology 2005].
Clinical practice shows that recognizing unambiguous signs of conscious perception of the environment and of the self in such patients with disorders of consciousness can be very challenging. This difficulty is reflected in the frequent misdiagnoses of VS, MCS and locked-in syndrome (LIS) [1-7]. Bedside evaluation of residual brain function in severely brain-damaged patients is difficult because motor responses may be very limited or inconsistent. In addition, consciousness is not an all-or-none phenomenon [8] and its clinical assessment relies on inferences made from observed responses to external stimuli at the time of the examination [7].
After briefly defining consciousness as it can be assessed at the patient’s bedside and reviewing the major clinical entities of altered states of consciousness following severe brain damage, we will here review evaluation scales available for the assessment of patients suffering from disorder of consciousness.
65.1.1 Consciousness, Awareness and Arousal
Consciousness is a multifaceted concept that has two major components: awareness of environment and of self (i.e., content of consciousness) and wakefulness (i.e., level of consciousness). One needs to be awake in order to be aware (REM-sleep being a notorious exception). Figure 65.2 shows that in normal physiological states (green); level and content are positively correlated (with the exception of the oneiric activity during REM-sleep). Patients in pathological or pharmacological coma (that is, general anesthesia) are unconscious because they cannot be awakened (red). The vegetative state (blue) is a unique persistent dissociated state of consciousness (i.e., patients being seemingly awake but lacking any behavioral evidence of “voluntary” or “willed” behavior).
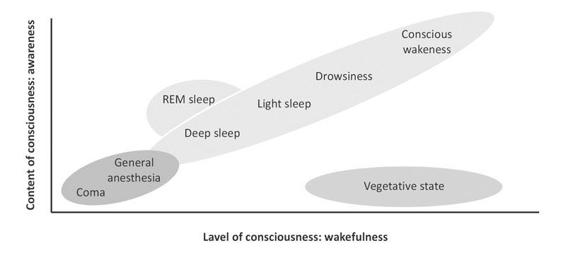
Figure 65.2. Oversimplified illustration of consciousness’ two major components: the level of consciousness (i.e., wakefulness or arousal) and the content of consciousness (i.e., awareness or experience). Adapted from [Laureys, Trends in Cognitive Sciences 2005].
Arousal is supported by several brainstem neuronal populations that directly project to both thalamic and cortical neurons [9]. Therefore depression of either brainstem or both cerebral hemispheres may cause reduced wakefulness. Brainstem reflexes are a key to the assessment of the functional integrity of the brainstem (Figure 65.3). However, profound impairment of brainstem reflexes can sometimes coexist with intact function of the reticular activating system if the tegmentum of the rostral pons and mesencephalon are preserved. Awareness is thought to be dependent upon the functional integrity of the cerebral cortex and its reciprocal subcortical connections; each of its many aspects resides to some extent in anatomically defined regions of the brain [10-12]. Unfortunately, for the time being, consciousness cannot be measured objectively by any machine. Its estimation requires the interpretation of several clinical signs. Many scoring systems have been developed for a standardized assessment of consciousness in severely brain damaged patients (For review see [13]).
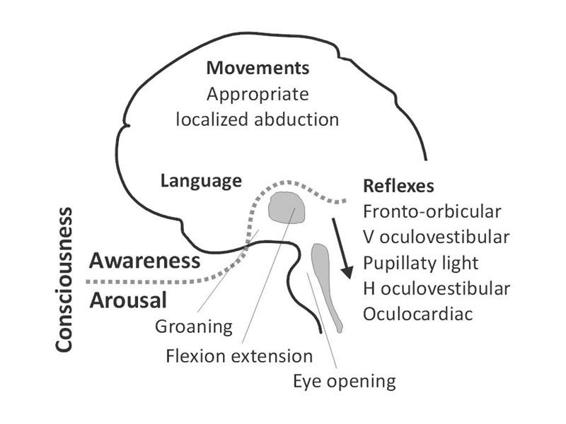
Figure 65.3. A simplified scheme of consciousness and its two major components: arousal and awareness. Note: the gray area represents the reticular activating system encompassing the brainstem and thalamus; the arrow near the brainstem denotes the progressive disappearance of brainstem reflexes during rostral-caudal deterioration (i.e., evolution from coma to brain death). Adapted from [13].
H = horizontal; V = vertical.
65.1.2 Clinical Definitions
Survivors of severe traumatic or hypoxic-ischemic brain damage classically go through different clinical entities before partially or fully recovering consciousness. Coma is defined as “unarousable unresponsiveness”. Irreversible coma may in some conditions equal brain death. After some days to weeks, comatose patients who recover will eventually open their eyes. When this return of “wakefulness” is accompanied by reflexive motor activity only, devoid of any voluntary interaction with the environment, the condition is called a vegetative state. The vegetative state may be a transition to further recovery, or not. Signs of voluntary motor activity should be actively searched for in vegetative state patients, as they herald the minimally conscious state (MCS). Functional communication indicates the next boundary – emergence from MCS – in the course of recovery. We will here briefly define brain death, coma, VS, MCS and LIS.
Brain Death
The concept of brain death as defining the death of the individual is largely accepted [14]. Most countries have published recommendations for the diagnosis of brain death but the diagnostic criteria differ from country to country [15]. Some rely on the death of the brainstem only [16] others require death of the whole brain including the brain stem [17]. However, the clinical assessments for brain death are very uniform and based on the loss of all brainstem reflexes and the demonstration of continuing cessation of respiration in a persistently comatose patient [procedures of a standardized apnea test can be found in 18]. There should be an evident cause of coma and confounding factors (including hypothermia, drugs, electrolyte, and endocrine disturbances) should be excluded. A repeat evaluation in 6h is advised, but the time period is considered arbitrary [19]. Confirmatory neurophysiological tests such as EEG, ERP, angiography, Doppler sonography, or scintigraphy or are only required when specific components of the clinical testing cannot be reliably evaluated and are recommended by a number of national professional societies to confirm the clinical diagnosis of brain death [20].
Coma
Coma is characterized by the absence of arousal and thus also of consciousness. It is a state of unarousable unresponsiveness in which the patient lies with the eyes closed and has no awareness of self and surroundings. The patient lacks the spontaneous periods of wakefulness and eye-opening induced by stimulation that can be observed in the VS [21]. To be clearly distinguished from syncope, concussion, or other states of transient unconsciousness, coma must persist for at least one hour. In general, comatose patients who survive begin to awaken and recover gradually within 2 to 4 weeks. This recovery may go no further than VS or MCS, or these may be stages (brief or prolonged) on the way to more complete recovery of consciousness.
Vegetative state
Patients in a VS are awake but are unaware of self or of the environment [22, 23]. Jennett and Plum cited the Oxford English Dictionary to clarify their choice of the term “vegetative”: to vegetate is to “live merely a physical life devoid of intellectual activity or social intercourse” and vegetative describes “an organic body capable of growth and development but devoid of sensation and thought”. “Persistent VS” has been arbitrarily defined as a vegetative state still present one month after acute traumatic or non-traumatic brain damage but does not imply irreversibility [24]. “Permanent VS” denotes irreversibility. The Multi-Society Task Force on PVS concluded that three months following a non-traumatic brain damage and 12 months after traumatic injury, the condition of VS patients may be regarded as ‘permanent’ and therefore irreversible. However, these guidelines are best applied to patients who have suffered diffuse traumatic brain injuries and post anoxic events; other non-traumatic etiologies may be less well predicted (see for example [25,26]) and require further considerations of etiology and mechanism in evaluating prognosis. Even after these long and arbitrary delays, some exceptional patients may show some limited recovery. This is more likely in patients with non-traumatic coma without cardiac arrest, who survive in VS for more than 3 months. The diagnosis of VS should be questioned when there is any degree of sustained visual pursuit, consistent and reproducible visual fixation, or response to threatening gestures [24] but these responses are observed in some patients who remain in VS for years. It is essential to establish repetitively the formal absence of any sign of conscious perception or deliberate action before making the diagnosis.
It is very important to stress the difference between persistent and permanent vegetative state which are, unfortunately, too often abbreviated identically as PVS, causing unnecessary confusion [27]. When the term “persistent vegetative state” was first described [22], it was emphasized that persistent didn’t mean permanent and it is now recommended to omit “persistent” and to describe a patient as having been vegetative for a certain time. When there is no recovery after a specified period (depending on etiology three to twelve months) the state can be declared permanent and only then do the ethical and legal issues around withdrawal of treatment arise [28,29]. The vegetative state can also be observed in the end-stages of some chronic neurodegenerative diseases, such as Alzheimer’s, and in anencephalic infants.
65.2 Clinical Evaluation of the Neurocritical Care Patient: The Coma Scales
The adequate evaluation of consciousness in patients suffering from severe brain damage is of a major importance for their optimal management over from the emergency department throughout their acute hospital stay and their rehabilitation. On this evaluation will indeed depend, not only the therapeutic surgical or medical decisions, but also often cares limitation. Before the development and the diffusion of the scales of coma and in particular of the scale of Glasgow [30], the evaluation of the patient presenting with altered consciousness was based on a rather vague nomenclature. The description of the depth of coma was made by means of terms often vague such as: drowsy, comatose, somnolent, obtunded, obnubilated, obstreperous or combative.
During the World War II and later, during the Korean and Vietnam campaigns, it appeared important to physician to set up methods making it possible to evaluate patients after a traumatic brain injury so as to facilitate the transmission of information on the clinical state of wounded between the doctors of ground and the doctors ensuring the continuation of the treatment [31]. From these efforts were born the first classifications for altered consciousnesses states [32]. The neurosurgical literature on head injuries sustained in the Vietnam conflict classified their initial state in three grades, variously defined [33,34]. These classification suffered from their lack of precision and from the hence ambiguity of the terms employed. This type of classification should be abandoned for the assessment of altered consciousnesses.
65.2.1 The Glasgow Coma Scale
The publication of the Glasgow coma scale (GCS) in 1974 by Teasdale and Jennet [30] brought a major change in the clinical assessment of patients presenting with post-traumatic altered consciousness. These authors took special care to the construction of their scale to overcome the ambiguities that arose when information about comatose patients was presented and groups of patients compared. Among the objectives of its originators, there was the desire to establish an evaluation tool based on simple, clear, unambiguous items that could be easily translated in various languages. Moreover, the scale had to be utilizable in a reliable way not only by any doctor but also by nurses and paramedics. Considering that the deterioration of consciousness varies according to a continuum and thus does not lend to a categorization, these authors preferred to create an instrument based on the distinct clinical evaluation from three aspects of the behavioral response to the stimulation of the patient with altered consciousness (Figure 65.4): eye opening (E, 4 levels), verbal activity (V, 5 levels) and motor response (M, 5 then 6 levels [35]).
Eye Opening
Eye opening in response to pain should be tested by stimulation at the level of the limbs, because the grimacing associated with supra-orbital or jaw-angle pressure may cause eye closure. The eye opening either spontaneously or on stimulation defines the transition from coma to vegetative state.
Verbal Activity
The presence of verbal responses indicates the restoration of a high degree of interaction with the environment (i.e., awareness). An oriented conversation implies awareness of the self and environment. Confused speech is recorded when the patient is capable of producing language, for instance phrases and sentences, but is unable to answer the questions about orientation. When the patient presents intelligible articulation but exclaims only isolated words in a random way (often swear words, obtained by physical stimulation rather than by a verbal approach), this is scored as ‘‘inappropriate speech’’. Incomprehensible sounds refer to moaning and groaning without any recognizable words. This rudimentary vocalization does not necessitate awareness and is thought to depend upon sub cortical functioning as it can be observed in anencephalic children and vegetative patients.
Motor Response
The motor response first assesses whether the patient obeys simple commands, given in verbal, gestural or written form. A non-specific sound stimulus may induce a reflex contraction of the patient’s fingers or alternatively such a reflex response can result from the physical presence of the examiner’s fingers against the palm of the patient (i.e., grasping reflex). Before accepting that the patient is truly obeying commands, it is advised to test that the patient will also release and squeeze again to repeated commands. If there is no response on command a painful stimulus is applied. First, pressure is applied to the fingernail bed. If flexion is observed, stimulation is then applied to other sites (applying pressure to the supra-orbital ridge, pinching the trapezium or rubbing the sternum) to differentiate between localization (i.e., attempt to remove a noxious stimulus by crossing the midline), withdrawal flexion (i.e., flexion of the elbow associated with abduction of the shoulder) or stereotyped flexion (i.e., stereotyped flexion of the elbow with adduction of the shoulder that can be achieved when stimulated at other sites). Extensor posturing is more easily distinguished and is usually associated with adduction, internal rotation of the shoulder and pronation of the forearm. Abnormal flexion and extension motor responses often coexist [36].
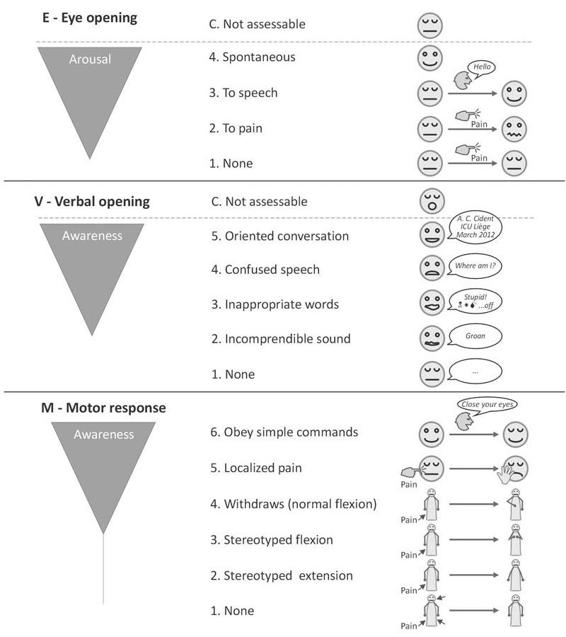
Figure 65.4. Pictographic representation of the Glasgow coma scale. Adapted from [13].
In a paper published the year following the original publication, numbers were ascribed to each level of response so that the responsiveness could be easily expressed, for example as E4, V3, M5, making communication more easy [37]. The use of these scores allowed, by summation the three component of the scale, to obtain a single total score [35]. This led to a score that ranged from 3 for deepest coma to 15 points for fully alert and oriented. The total score allows assessing the link between the gravity of altered consciousnesses and patients’ outcome. However, in spite of its interest, the use of the total score induces a loss of information during the individual evaluation [38,39]. Moreover, the sum of the components causes a mathematical problem since weighting is in favor of motor response (6 points) as compared to eye opening (4 points) and verbal activity (5 points). The total score is consequently more influenced by the motor response than by the other two components [40]. It therefore appears essential, on the clinical level, to communicate the Glasgow coma scale by giving its three components (E, V, M) rather than their sum alone [41]. Although the Glasgows coma scale was initially built for post-traumatic brain injury evaluation, it was later also validated for the assessment of patients suffering from nontraumatic consciousness disorders [42,43].
The Glasgow coma scale was also studied in General Intensive Care [44] and, in addition, it is integrated in several general scores of gravity such as APACHE II [45], SAPS II [46] and SAPS 3 [47] where it has an important weight in the hospital risk of death prediction. The Glasgow coma scale remains to date the most internationally used coma scale with, since its publication, more than 5900 citations (Medline research from July 1974 to June 2008) (Figure 65.5). Apart from the Apgar score [48] with nearly 6500 quotations over the same period of time, there is probably no patients other severity of illness score which is quoted as much in the literature nor which has such an extended clinical application.
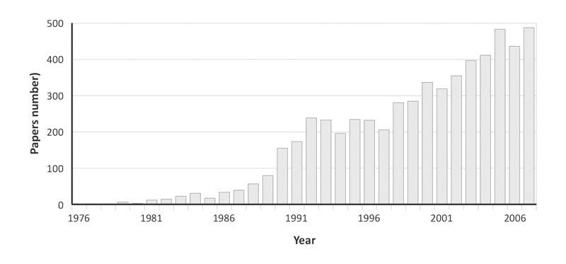
Figure 65.5. Number of publication making reference to the Glasgow coma scale (Medline research from July 1974 to June 2008).
In spite of its undeniable qualities, the scale of Glasgow suffers from a certain number of imperfections. Its use causes problem in mechanically ventilated patients which a very common situation for patients with severe consciousness disorders. Indeed, in this circumstance, it is not possible to evaluate the verbal component of the scale and consequently the provided information is somewhat truncated in a substantial proportion of patients. Murray et al. indeed showed, in a study including 1000 head injured patients, that 40% of these patients could not be assessed by the Glasgow coma scale on the prior their hospital admission; this rate of unevaluable patients raised up to 50% when patients were assessed in the intensive care [49]. Another shortcoming of the Glasgow coma scale is that eye opening is insufficient to properly assess activity in the brainstem arousal system. This limitation induces a considerable loss of information for the prognosis assessment of brain injured patients. The Glasgow-Liege scale (Figure 65.6) is an adaptation of the scale of Glasgow; it combines the Glasgow coma scale with five brainstem reflexes [50]. This allows an approach of the brain as a whole and consequently provides more elements to evoke the diagnosis of brain death. Finally the Glasgow coma scale lacks reliability when assessing patients progressively recovering from their coma and entering a vegetative or minimally conscious state [51]. It also fails to detect more subtle the state of consciousness such as Locked-in Syndrome (LIS). For these patients, more sensitive scales are the coma recovery scale-revised, sensory modality assessment and rehabilitation technique, or Wessex head injury matrix [52-54]. These scales, however, are not adapted for use in acute settings.
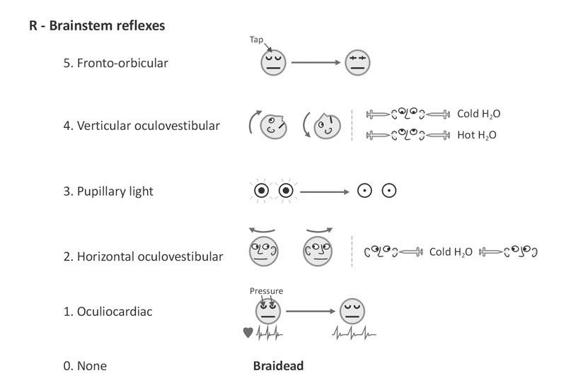
Figure 65.6. Pictographic representation of the Glasgow-Liège scale (Born et al. 1982). Note: when oculocephalic reflexes (doll’s eyes) cannot be tested or are absent, the (vertical and horizontal) oculovestibular reflexes (ice water testing) should be evaluated. Adapted from [13].
65.2.2 Other Scales for Acute Consciousness Disorders Assessment
Since the publication of the Glasgow coma scale, several other scales have been proposed. In 1981, Salcman et al. published the Scale of coma of Maryland [55], as compared to the Glasgow coma scale this scale had the advantage to include indicators for the brainstem assessment; it however never convinced the medical community. The Edinburgh-2 coma scale [56] proposed by Sugiura et al. (1983) is a one-dimensional scale (Table 65.1) varying from zero to nine in a way inversely proportional to deterioration of the state conscience. This scale is simpler than the scale of Glasgow yet it never took a real rise.
Stimulation (maximal) | Response (best) | Score |
Two sets of questions: | Answers correctly to both | 0 |
1. Month? | Answers correctly to either | 1 |
2. Age? | Incorrect for both | 2 |
Two sets of commands: | Obeys correctly to both | 3 |
1. Close and open hands | Obeys correctly to either | 4 |
2. Close and open eyes | Neither correct | 5 |
Strong pain | Localizing | 6 |
Flexion | 7 | |
Extension | 8 | |
None | 9 |
Table 65.1. Edinburgh-2 coma scale [56].
Among the imperfections of this scale, one could mention the fact that it cannot be validly applied to individuals that are unable to give a verbal answer – such as intubated patients – and the fact that, in this single dimension scale is based on various neurological characteristics take place. The Clinical Neurologic Assessment scale (CNA), published in 1989 by Crosby et al. [57], is a 21 items instrument evaluating not only the verbal answer and the capacity to answer simple orders but also neurological aspects such as the muscular tone, the position of the body, movements, chewing or yawn. Although it offers subtle assessment possibilities than the Glasgow coma scale, this tool remained unexploited. In 1991, Benzer et al. published the Innsbruck coma scale [58]. In its principle, this scale is rather similar to the Glasgow coma scale; with various components scored separately then aggregated into a total score varying from 0 to 23 points. The Innsbruck coma scale however differs from the Glasgow coma scale by its number of components – eight instead of three – and by their nature: if eye opening and motor response are similar components, there is not in this scale an assessment of verbal activity but instead items assessing reaction to acoustic stimuli, body posture, pupils size and response to light, position and movement of the eyeballs and oral automatisms. Although this scale has potential advantages as compared to the Glasgow coma scale, it is cited in very few publications and its clinical use is limited. A Swedish scale, the Reaction Level Scale (RLS85), was proposed by Starmark et al. [59]. This tool is a simple ordinal scale varying from 1 to 8 combining verbal and motor responses (Table 65.2). This scale correlates with the scale of Glasgow, moreover it has a better inter-observers agreement than the latter [60]. However the use of this instrument remains primarily limited to Sweden.
Clinical descriptor | Responsiveness | Score |
Alert | No delay in response, responsive without simulation | 1 |
Drowsy or confused | Responsive to light stimulation | 2 |
Very drowsy or confused | Responsive to strong stimulation | 3 |
Unconscious | Localizes but does not ward of pain | 4 |
Unconscious | Withdrawing movements on pain stimulation | 5 |
Unconscious | Stereotyped flexion movements on pain stimulation | 6 |
Unconscious | Stereotyped extension movements on pain stimulation | 7 |
Unconscious | No response on pain stimulation | 8 |
Table 65.2. The Reaction Level Scale (RLS85).
65.2.3 The Full Outline off UnResponsiveness Score (FOUR score)
The Full Outline off UnResponsiveness score (FOUR) published by Wijdicks et al. in 2005 [61] is certainly a very interesting alternative on a Glasgow scale represents. This acronym reflects the number of components tested (eye response, motor response, brainstem reflexes and respiratory function) and the maximum score assigned to each of these (E4, M4, B4 and R4) (Table 65.3). The sum of the points attributed to each component can thus vary from 0 to 16. With all FOUR categories graded zero, the scale alerts to consider brain death or standard apnoea (oxygen-diffusion) testing. The main characteristic of the FOUR score are described below.
E | Eye response |
4 | Eye tracking (at least 3 times), or eyelids blinking to command (at least 2 of 3). Open eyes and assess tracking (horizontally and vertically) if necessary |
3 | Eyelids open but not tracking |
2 | Eyelids closed but open to loud voice |
1 | Eyelids closed but open to pain* |
0 | Eyelids remain closed with pain* |
M | Motor response |
4 | Thumbs-up, fist, or peace sign (at least one of these) |
3 | Localizing to pain* |
2 | Flexion response (normal or stereotyped) to pain* |
1 | Extension response to pain* |
0 | No response to pain or generalized myoclonus status |
B | Brainstem reflexes |
4 | Pupil and corneal reflexes present° |
3 | One pupil wide and fixed |
2 | Pupil OR corneal reflexes absent |
1 | Pupil AND corneal reflexes absent |
0 | Absent pupil AND corneal, AND cough reflex** |
R | Respiration |
4 | Not intubated, regular breathing pattern |
3 | Not intubated, Cheyne-Stokes breathing pattern |
2 | Not intubated, irregular breathing |
1 | Breathes above ventilator rate^ |
0 | Breathes at ventilator rate OR apnea§ |
Table 65.3. The Full Outline UnResponsiveness (FOUR) score Adapted from [Wijdicks, 2005]. Instructions for the assessment of the individual categories of the FOUR score: Grade the best possible response.
* Temporo-mandibular joint or supra-orbital nerve nociceptive stimulation;
° Corneal reflexes are tested by instilling two to three drops sterile saline on the cornea from a distance of 10-15 cm (this minimizes corneal trauma from repeated examinations);
** The cough reflex to tracheal suctioning is tested only when both pupil and corneal reflexes are absent;
^ No adjustments are made to the ventilator while the patient is graded, but grading is done preferably with PaCO2 within normal limits;
§ A standard apnea (oxygen-diffusion) test may be needed when patient breathes at ventilator rate (R0).
Eye Response
The FOUR score specifically evaluates ocular movements or the eye blinking on command, this implies to open the eyes manually if the patient does not open them spontaneously. This assessment facilitates the early diagnosis of locked-in syndrome which represents a considerable improvement since recent studies show that, at the initial phase, the clinicians miss this diagnosis in up to 50% of the cases [62].The FOUR score also evaluates eye tracking which is known as being this is the first sign heralding the transition from a vegetative to a minimally conscious state [63]. This distinction is not only a matter of semantics since patients in minimal state of consciousness have a more favorable outcome than patients in vegetative state [51]. The others items of the FOUR’s eye score are similar to those of the scale of Glasgow.
Motor Response
With regard to the motor response, the most innovative item of the FOUR score is the hand position test, in which patients are asked to make thumbs-up, fist, or peace signs. This is a smart alternative to the V-score of the Glasgow coma scale and remains testable in intubated patients. The rest of the M-score is taken from the Glasgow coma scale, with the exception that no difference is made between abnormal stereotyped flexion and normal flexion to pain (similar to the early version of the Glasgow coma scale [30]). This difference may be difficult for inexperienced observers to appreciate but might lead to lower prognostic power of the FOUR scale. Generalized myoclonic status epilepticus, which is a sign of poor prognosis in anoxic coma, is scored the same as absent motor response to pain.
Brainstem Reflexes
Amending the Glasgow coma scale’s lack of brainstem-reflexes assessment, FOUR tests pupil, cornea, and cough reflexes and separately scores respiration. For untrained users, evaluation of the brainstem component is probably the most complex because it proposes different combinations of the presence or absence of each of its three reflexes. Unilateral fixed midriasis, alerting uncal herniation, has a separate score. To avoid corneal trauma by repeated testing, it is cleverly proposed to instil some drops of saline on the cornea.
Respiration
The last category of FOUR scores respiration as spontaneous regular, irregular, Cheyne-Stokes, ventilator-assessed patient-generated breaths, or absent. Whether pulmonary disease and respiratory settings will bias the assessment and how reliably inexperienced users can separate Cheyne-Stokes from irregular respiration are unknown.
In the past 30 years, many coma scales have been proposed as an alternative to the Glasgow coma scale, but none with success. The FOUR score has not been widely validated yet. To date only one study validated the score and assessed the inter-rater agreement [64]. The validity of this new scale needs to be corroborated when used in a general ICU setting by examiners other than neuroscience professionals. By virtue of its simplicity, and despite its drawbacks, the Glasgow coma scale became the most universally used and validated consciousness scale worldwide. Albeit convinced of the valuable improvement of the FOUR score, the Glasgow coma scale will not be dethroned effortlessly.
References
1. Andrews K, Murphy L, Munday R, et al. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ 1996; 313: 13-6
2. Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology 1993; 43: 1465-67
3. Ostrum AE. The ‘locked-in’ syndrome–comments from a survivor. Brain Inj 1994; 8: 95-8
4. Tresch DD, Sims FH, Duthie EH, et al. Clinical characteristics of patients in the persistent vegetative state. Arch Intern Med 1991; 151: 930-2
5. Vigand S. Only the eyes say yes (original title: Putain de silence). Arcade Publishing, 2000
6. Bauby JD. The diving bell and the butterfly (original title: Le scaphandre et le papillon). Ed. E.R. Laffont, 1997
7. Bernat JL. The boundaries of the persistent vegetative state. J Clin Ethics 1992; 3: 176-80
8. Wade DT, Johnston C. The permanent vegetative state: practical guidance on diagnosis and management. BMJ 1999; 319: 841-4
9. Steriade M, Jones EG, McCormick D. Thalamus. Amsterdam-New York: Elsevier, 1997
10. Dehaene S, Changeux JP, Naccache L, et al. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci 2006; 10: 204-11
11. Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 2001; 79: 1-37
12. Zeman A. Consciousness. Brain 2001; 124: 1263-89
13. Laureys S, Majerus S, Moonen G. Assessing consciousness in critically ill patients. In: Vincent JL (ed.). Yearbook of Intensive Care and Emergency Medicine. Heidelberg: Springer-Verlag, 2002; pp. 715-27
14. Laureys S. Science and society: death, unconsciousness and the brain. Nat Rev Neurosci 2005; 6: 899-909
15. Haupt WF, Rudolf J. European brain death codes: a comparison of national guidelines. J Neurol 1999; 246: 432-7
16. Medical Royal Colleges and their Faculties in the United Kingdom. Diagnosis of brain death. BMJ 1976; 2: 1187-8
17. Medical Consultants on the Diagnosis of Death, Guidelines for the determination of death. Report of the medical consultants on the diagnosis of death to the President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research. JAMA 1981; 246: 2184-6
18. Wijdicks EF. The diagnosis of brain death. N Engl J Med 2001; 344: 1215-21
19. The Quality Standards Subcommittee of the American Academy of Neurology, Practice parameters for determining brain death in adults (summary statement). Neurology 1995; 45: 1012-4
20. Wijdicks EF. Brain death worldwide: accepted fact but no global consensus in diagnostic criteria. Neurology 2002; 58: 20-5
21. Plum F, Posner JB. The diagnosis of stupor and coma. 3rd ed. Philadelphia: Davis, F.A, 1983
22. Jennett B, Plum F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet 1972; 1: 734-7
23. Jennett B. The vegetative state. Medical facts, ethical and legal dilemmas. Cambridge: Cambridge University Press, 2002
24. The Multi-Society Task Force on PVS, Medical aspects of the persistent vegetative state (1). N Engl J Med 1994; 330: 1499-508
25. Menon DK, Owen AM, Williams EJ, et al. Cortical processing in persistent vegetative state. Lancet 1998; 352: 200
26. Wilson BA, Gracey F, Bainbridge K. Cognitive recovery from “persistent vegetative state”: psychological and personal perspectives. Brain Inj 2001; 15: 1083-92
27. Laureys S, Faymonville ME, Berre J. Permanent vegetative state and persistent vegetative state are not interchangeable terms. BMJ 2000
28. Jennett B. The assessment and rehabilitation of vegetative and minimally conscious patients: Definitions, diagnosis, prevalence and ethics. Neuropsychol Rehabil 2005; 15: 163-165
29. American Congress of Rehabilitation Medicine, Recommendations for use of uniform nomenclature pertinent to patients with severe alterations of consciousness. Arch Phys Med Rehabil 1995; 76: 205-9
30. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2: 81-4
31. Medical Reaseach Council Brain Injuries Committee. A Glossary of Psychological Terms Commonly Used in Cases of Head Injury, M.W. Memorandum, Editor, HSMO: London, 1941
32. Jepson R, Whitty CWM. The neurological state and post-operative course of penetrating head wounds. In War Surgery Supplement No.1. Wounds of the head. Br J Surg 1947: 243-50
33. Rish BL, Caveness WF, Dillon JD, et al. Analysis of brain abscess after penetrating craniocerebral injuries in Vietnam. Neurosurgery 1981; 9: 535-41
34. Salazar AM, Jabbari B, Vance SC, et al. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology 1985; 35: 1406-14
35. Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976; 34: 45-55
36. Bricolo A, Turazzi S, Alexandre A, et al. Decerebrate rigidity in acute head injury. J Neurosurg 1977; 47: 680-9
37. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975; 1: 480-4
38. Teasdale G, Jennett B, Murray L, et al. Glasgow coma scale: to sum or not to sum. Lancet 1983; 2: 678
39. Teoh LS, Gowardman JR, Larsen PD, et al. Glasgow Coma Scale: variation in mortality among permutations of specific total scores. Intensive Care Medicine 2000; 26: 157-61
40. Bhatty GB, Kapoor N. The Glasgow Coma Scale: a mathematical critique. Acta Neurochir (Wien) 1993; 120: 132-5
41. Bozza Marrubini M. Classifications of coma. Intensive Care Med 1984; 10: 217-26
42. Bates D, Caronna JJ, Cartlidge NE, et al. A prospective study of nontraumatic coma: methods and results in 310 patients. Ann Neurol 1977; 2: 211-20
43. Thacker AK, Singh BN, Sarkari NB, et al. Non-traumatic coma–profile and prognosis. J Assoc Physicians India 1997; 45: 267-70
44. Teres D, Brown RB, Lemeshow S. Predicting mortality of intensive care unit patients. The importance of coma. Crit Care Med 1982; 10: 86-95
45. Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818-29
46. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993; 270: 2957-63
47. Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3-From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005; 31: 1345-55
48. Apgar V. A proposal for a new method of evaluation of the newborn infant. Curr Res Anesth Analg 1953; 32: 260-7
49. Murray GD, Teasdale GM, Braakman R, et al. The European Brain Injury Consortium survey of head injuries. Acta Neurochir (Wien) 1999; 141: 223-36
50. Born JD. [Practical assessment of brain dysfunction in severe head trauma (author’s transl)]. Neurochirurgie 1982; 28: 1-7
51. Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002; 58: 349-353
52. Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004; 85: 2020-9
53. Gill-Thwaites H. The Sensory Modality Assessment Rehabilitation Technique–a tool for assessment and treatment of patients with severe brain injury in a vegetative state. Brain Inj 1997; 11: 723-34
54. Shiel A, Horn SA, Wilson BA, et al. The Wessex Head Injury Matrix (WHIM) main scale: a preliminary report on a scale to assess and monitor patient recovery after severe head injury. Clin Rehabil 2000; 14: 408-16
55. Salcman M, Schepp RS, Ducker TB. Calculated recovery rates in severe head trauma. Neurosurgery 1981; 8: 301-8
56. Sugiura K, Muraoka K, Chishiki T, et al. The Edinburgh-2 coma scale: a new scale for assessing impaired consciousness. Neurosurgery 1983; 12: 411-5
57. Crosby L, Parsons LC. Clinical neurologic assessment tool: development and testing of an instrument to index neurologic status. Heart Lung 1989; 18: 121-9
58. Benzer A, Mitterschiffthaler G, Marosi M, et al. Prediction of non-survival after trauma: Innsbruck Coma Scale. Lancet 1991; 338: 977-8
59. Starmark JE, Stalhammar D, Holmgren E. The Reaction Level Scale (RLS85). Manual and guidelines. Acta Neurochir (Wien) 1988; 91: 12-20
60. Segatore M, Way C. The Glasgow Coma Scale: time for change. Heart Lung 1992; 21: 548-57
61. Wijdicks EF, Bamlet WR, Maramattom BV, et al. Validation of a new coma scale: The FOUR score. Ann Neurol 2005; 58: 585-93
62. Laureys S, Pellas F, Van Eeckhout P, et al. The locked-in syndrome: what is it like to be conscious but paralyzed and voiceless? Prog Brain Res 2005; 150: 495-511
63. Majerus S, Gill-Thwaites H, Andrews K, et al. Behavioral evaluation of consciousness in severe brain damage. Prog Brain Res 2005; 150: 397-413
64. Wolf CA, Wijdicks EF, Bamlet WR, et al. Further Validation of the FOUR Score Coma Scale by Intensive Care Nurses. Mayo Clinic Proceedings 2007; 82: 435-8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






