5
The Evaluation of Pediatric Sleep Disorders
Sleep is a major component of a child’s life. About half of early childhood is spent sleeping. Rapid changes occur in the development and maturation of the neurologic structures responsible for sleep in the early years of life, resulting in a fairly predictable progression of sleep patterns. However, disruption of these processes can lead to developmental, behavioral, and cognitive consequences, which can be either helped or hindered by a caregiver’s responses and actions. In addition, a child’s sleep difficulties can have effects on other family members, including parents and siblings and can impair the child’s daytime functioning in school and social and emotional development. It is important, therefore, to understand what is normal and abnormal in childhood sleep, and when sleep disorders occur how to appropriately assess and treat them in an attempt to significantly improve not only the child’s life but also those of their families (1).
Common sleep concerns for children include not enough sleep, disrupted sleep, and, less commonly, too much sleep. These problems can present as difficulties with sleep initiation and maintenance, sleep at inappropriate times, and abnormal activity or behaviors during sleep. All of these can lead to daytime behavioral problems, including inattention, irritability, and learning difficulties.
Evaluation of pediatric sleep disorders consists of obtaining a detailed pediatric sleep history, performing a sleep-focused physical examination, and in select cases, obtaining and interpreting a pediatric polysomnogram (PSG). Each of these components, as well as case studies of common pediatric sleep disorders, are discussed in the following text.
HISTORY
In addition to a standard pediatric medical history, a sleep history should also be reviewed. Obtaining a history of the presenting sleep problem in children requires interviewing the caretakers, who are typically the parents. This often adds a layer of complexity that is typically not seen in adult patients, who usually give their own history. Often the pediatric patient is not concerned about sleep, but rather it is the parents who are voicing the complaint.
A helpful mnemonic device for gathering a history of sleep symptoms is BEARS (Bedtime, Excessive daytime sleepiness, Awakenings, Regularity, Snoring) (2). Starting with dinnertime is a useful way to start asking a sleep history, including inquiring if any caffeinated beverages are consumed, the time that dinner is usually eaten, and how is it structured—ie, does the family sit down together at the table? Following dinner, what activities does the child do? When does the child start the bedtime ritual, and what does the child do? Many children have a bath as a part of the bedtime activities, but for some, this may be very stimulating and cause them to have difficulties settling down. Also, sometimes children can get a “second wind” or schlafbereitschaft. This is a brief burst of energy that usually lasts around 20 minutes and occurs just before sleep readiness. Often parents allow their children to watch TV before going to sleep, and many children even have TVs in their bedrooms. Evening TV shows are frequently stimulating and can increase sleep latency. A much better strategy is to encourage parents to spend time reading bedtime stories together with their children. When does the child typically fall asleep and how long does that take from the time lights are turned off? If they do not fall asleep immediately, what do they do during that time? What conditions are needed for the child to fall asleep, and are they able to initiate sleep on their own or do they need the presence of the parent or someone (or something) else?
Questions should also be asked of the sleep environment. Is the child in a crib, toddler bed, a bunk bed, or a regular bed? Does the child share a bed or bedroom, and if so with whom? Does he or she sleep with pets? How dark and how quiet is the bedroom? Most children sleep better in a darkened environment, but some anxious children may do better with a dim night light. What is the temperature of the room? An ideal bedroom should be a bit on the cool side but not uncomfortably cold. How close, or far, is the child’s bedroom from the parents’ room? This can indicate how easy or difficult it may be for the parents to hear what happens in the child’s room at night.
Do the children sleep through the night? If not, when and how often do they wake up? What do they do during the waking? Do they go back to sleep on their own, or do the parents have to put them back to sleep? Do they snore and if so, how loud is it? Do they gasp, choke, or stop breathing? Do they wet the bed? Are they quiet or restless? Do they sleep in unusual positions? When do they wake up? Do they wake up on their own, or do parents have to wake them up? How easy or difficult is it to wake the child up? How long does it take for the child to become wide awake? Is the child refreshed upon awakening or still tired? How many hours do the children sleep at night? Do they nap during the day and if so, at what times and for how long? While young children typically nap during the day, pediatric sleepiness scales have been published and can help provide an objective measure of daytime sleepiness (3).
Finally, a detailed sleep diary should be kept. While this may need to be completed by the parents and some of it may have to be their best estimates, it can still provide significant information, especially over an extended period of time, typically about 2 weeks. The sleep diary should be in a graph form, with times noted for in bed, asleep, awake, and out of bed. Any unusual events, such as sleepwalking, should be noted.
PHYSICAL EXAMINATION
While many, if not most, children with sleep problems have normal physical and neurological examinations, there are several important findings that should be assessed. Vital signs and measurements (height, weight, head and neck circumference, heart rate, blood pressure, and respiratory rate) should be obtained and plotted on standardized growth charts. The body mass index (BMI; weight in kilograms [kg]/square of height in meters) should be calculated and also plotted, as both obesity and relative emaciation can associate with obstructive sleep apnea (OSA) in certain childhood syndromes. A detailed oropharyngeal evaluation should be performed, with attention to the tonsil size, palate elevation, posterior airway size, tongue size and thickness, and dentition. The Mallampati scales are often used to describe the posterior airway size (4). The nasopharynx should also be examined, with attention to nasal obstruction and septal deviation. Any significant craniofacial features should be noted, such as jaw size, midface hypoplasia, or “adenoidal facies” (5). In addition to the neck circumference, any neck masses or thyroid enlargement should also be assessed. Cardiopulmonary evaluation should note any respiratory abnormalities such as wheezing, rales, or rhonchi, and a persistently split S2 can indicate pulmonary hypertension (6). Breathing patterns, including mouth breathing and nasal speech should be noted if present. The neurological examination should also include a brief developmental assessment as well as any behavior concerns, such as tiredness, hyperkinesis, or attention problems.
POLYSOMNOGRAPHY
PSG describes a procedure of objective, simultaneous recording of many different physiologic parameters (electroencephalogram [EEG], electrooculogram [EOG], and electromyogram [EMG]) during sleep (7). In 1875, Caton performed the first EEG animal studies, and in 1929 Berger published recordings from humans (8–11). The first continuously recorded all-night EEG sleep studies by Loomis et al. in 1937 were followed by the discovery of rapid eye movement (REM) sleep by Aserinsky and Kleitman in 1953, which showed the utility of the EOG (12,13). In 1967, Jovet’s associating REM sleep with hypotonia justified the present use of EMG for PSG (14). In 1968 the combination of EEG, EOG, and EMG allowed for formal PSG and the first published standardized technique for scoring sleep stages by Rechtschaffen and Kales (15). Presently, PSG analysis allows the differentiation of three specific non–rapid eye movement (NREM) sleep stages; stage N1 (NREM 1), stage N2 (NREM 2), stage N3 (NREM 3), and stage R, REM sleep (16).
For children younger than 2 months of age, the PSG is scored using the accepted criteria of Anders, Parmalee, and Emdee (17). Four EEG patterns are described during full-term neonatal sleep: (a) low voltage irregular (LVI); low voltage (14–35 µV), fast theta (5–8 Hz) with significant slow (1–5 Hz) activity, (b) high voltage slow (HVS); continuous, rhythmic 50–150 µV, slow activity, (c) trace alternant (TA); 3- to 8-second bursts of HVS, separated by similar periods of attenuated mixed frequency activity, with occasional rapid low-voltage and 2 to 4 Hz sharp wave activity, and (d) mixed (M); LVI and HVS mixed, with little periodicity.
In children, 2 to 3 months post-term, standard child/adult nomenclature for stages W (wake), N1, N2, N3, and R can often be used, as V-waves (defining stage N1 sleep) often appear within 2 to 3 months, sleep spindles and K-complexes (defining stage N2 sleep) appear within 2 to 3 and 4 to 6 months, respectively, and slow wave activity (SWA; defining stage N3 sleep) appears within 2 to 5 months. The occipital EEG, dominant posterior rhythm (DPR) during restful wakefulness upon eye closure, attenuates with eyes open and when falling asleep. The DPR at 3 to 4 months is 3.5 to 4.5 Hz; at 5 to 6 months, 4 to 6 Hz; and by 3 years, 7.5 to 9.5 Hz (normal adult values, 8–13 Hz, are defined as alpha rhythm).
In addition to V-waves and loss of DPR, stage N1 sleep is also defined by a general low-voltage, mixed frequency pattern on EEG, with slow roving eye movements on EOG and occasionally hypnagogic hypersynchrony. In stage R sleep there is a general low-voltage mixed frequency pattern that appears around 3 Hz at 7 weeks post-term, 4 to 5 Hz at 5 months, 4 to 6 Hz at 9 months, 5 to 7 Hz at 1 to 5 years, and from 5 to 10 years, 8 to 13 Hz, with a variable heart and respiratory rate, relatively frequent phasic muscle twitches, grimaces, and vocalizations. In a sleeping child, 2 to 6 months old, if there are no sleep spindles, K-complexes, SWA, or characteristics of stage R sleep, the sleep stage can be given the general designation N (NREM).
A PSG study should last a minimum of 6 hours, but optimally 8 or more hours for children, who typically sleep longer than adults. They should be conducted during the child’s normal sleep times, ie, beginning in the early evening and lasting through to the next morning. Proper patient and parent preparation is crucial and will alleviate anxiety about the procedure and improve compliance. A tour of the laboratory can significantly ease tension for both the child and the parent. The sleep laboratory environment should be child-friendly, but also simulate a home environment as much as possible and should be light and sound attenuated. Patient setup should be done in a separate room from the sleep room to avoid unpleasant conditioning effects. The technician working with pediatric patients should be comfortable with children and parents, and realize that it may take more time, effort, and patience to gain a child’s trust and cooperation. Rushing or forcing children to do something they do not want to do is rarely successful and usually counterproductive. Often, using distraction (watching a video or TV show) during electrode application can be helpful, along with providing a small incentive, such as a small toy, for completion of the setup.
SPECIFIC SLEEP DISORDERS
The third edition of the International Classification of Sleep Disorders (ICSD), published in 2014, describes 7 major categories of sleep disorders with 2 appendices, describing approximately 75 different diagnoses (18). This chapter addresses major diagnoses for which clinical neurophysiological monitoring techniques are useful (see Table 5.1).
TABLE 5.1 Topic Outline From the International Classification of Sleep Disorders, Third Edition
1. Sleep-related breathing disorders i. Obstructive sleep apnea disorders a. Obstructive sleep apnea, pediatric |
2. Central disorders of hypersomnolence i. Narcolepsy |
3. Parasomnias i. NREM-related parasomnias a. Confusional arousals b. Sleepwalking c. Sleep terrors ii. REM-related parasomnias a. REM sleep behavior disorder a. Recurrent isolated sleep paralysis |
4. Sleep-related movement disorders i. Sleep-related bruxism ii. Sleep-related rhythmic movement disorder |
5. Sleep-related medical and neurological disorders i. Sleep-related epilepsy |
Source: Adapted from American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien IL: American Academy of Sleep Medicine, 2014.
SLEEP-RELATED BREATHING DISORDERS
Obstructive Sleep Apnea
In 1978, utilizing the PSG, Guilleminault et al. coined the term apnea index (the average number of apneas and hypopneas per hour of sleep) to precisely define the presence and severity of OSA (19). In 2007 the American Academy of Sleep Medicine (AASM) published the first pediatric standard definition for hypopnea, allowing for the routine reporting of the apnea-hypopnea index (AHI; the average number of apneas plus hypopneas per hour of sleep) in children (20). Today, the AHI is “the key measure used for case identification, for quantifying disease severity, and for defining disease prevalence in normal and clinical populations” (21).
PSG monitoring for OSA mandates the use of transcutaneous or end-tidal PCO2 (partial pressure of carbon dioxide) monitoring for children less than 13 years (16). There are specific recommended standards for scoring obstructive respiratory events in children less than 18 years of age although depending on the child’s relative physical level of maturity, the sleep scoring expert can choose to use adult standards for patients greater than 13 years (16). An obstructive apnea in a child is scored when there is a 90% reduction of amplitude on the thermal airflow channel for a duration of two missed breaths when compared to baseline but continued respiratory effort. An obstructive hypopnea is defined as greater than 30% reduction in the nasal pressure transducer channel from baseline, for a duration of greater than two missed breaths, with greater than 3% oxygen desaturation or the event is associated with an arousal.
CENTRAL DISORDERS OF HYPERSOMNOLENCE
Narcolepsy
Narcolepsy is defined in the ICSD as either type 1 or type 2 (with and without cataplexy, respectively), and is associated with periods of irrepressible sleep or sleepiness for at least 3 months (18). In type 1 there is one or both of the following:
1. Cataplexy and a mean sleep latency (MSL) of less than or equal to 8 minutes and two or more sleep-onset REM periods (SOREMPs) on a mean sleep latency test (MSLT; 4–5 daytime 20-min nap attempts separated by approximately 2-hour intervals, performed the day following overnight PSG). A SOREMP within 15 minutes of sleep onset on the preceding PSG may replace one SOREMP on the MSLT.
2. Cerebrospinal fluid (CSF) hypocretin-1 concentration less than or equal to 110 pg/mL (or < 1/3 of normal mean values).
Type 2 follows these criteria except there is no cataplexy and the CSF hypocretin-1 has either not been measured or is greater than 110 pg/mL or greater than one-third of the mean normal.
The onset of type 1 narcolepsy is usually after 5 years, peaking at 15 years. Typically, sleepiness is followed within a year by cataplexy, with hypnagogic hallucinations, sleep paralysis, and insomnia potentially gradually developing over years. In young children, sleepiness can present as excessively long night sleep or as a resumption of previously discontinued daytime naps.
Almost all patients with cataplexy are positive for the human leukocyte antigen (HLA) subtype DQB1*0602, compared with 12% to 38% of the general population. Although anticipation of reward is a common precipitant, cataplexy may present atypically in young children where it can be severe at disease onset and may occur with weakness of face, eyelids, mouth, and tongue protrusion (“cataplectic facies”) not clearly associated with emotion (22).
Hyperactive behavior, poor school performance, inattentiveness, lack of energy, and hallucinations can lead to psychiatric misdiagnosis of depression and schizophrenia. In addition, depending upon the child’s verbal capabilities, sleep paralysis and hypnagogic hallucinations can be difficult to confirm. Finally, precocious puberty, obesity, REM sleep behavior disorder (RBD; and REM without atonia) can also manifest at symptom onset.
There are no normative MSLT values for children less than 6 years. As such, CSF hypocretin-1 measurements may prove invaluable as 90% to 95% of type 1 patients have undetectable or low CSF hypocretin-1 levels. In children, the minimum 7 hours of sleep recommended for adults on the preceding PSG should be longer.
There is evidence that in type 1 narcolepsy during prolonged “global” cataplectic attacks (with quadriparesis and areflexia), the PSG can show a “REM sleep pattern” with EOG bursts of rapid eye movements, atonic EMG electrical silencing, and EEG patterns with low-voltage mixed frequency activity and sporadic sawtooth waves (Figure 5.1) (23–27). In patients with frequent attacks, provoking and documenting the clinical exam and PSG findings during a cataplectic attack can prove useful.
FIGURE 5.1 PSG rapid eye movement (REM) patterns generated by a 17-year-old boy with narcolepsy type 1 clearly contrast with his normal waking study (top left) and show general atonia on EMG, with REM on EOG (top right and bottom left and right). While preparing for a routine diagnostic overnight PSG the patient complained of paralysis and stated in a very hypophonic, rather dysarthric manner “I think I’m having a hypnagogic hallucination,” while demonstrating a classic REM-PSG pattern with a marked paucity of muscle tone on chin EMG, with EEG sawtooth waveforms, and rapid eye movements on the EOG (bottom left). This patient’s REM patterns were indistinguishable when visual comparisons were made between studies recorded during events that represented cataplexy (top right), sleep paralysis with a hypnagogic hallucination (bottom left), and normal sleep (bottom right).
Abbreviations: EOG, electrooculogram; EMG, electroencephalogram; LOC, left outer canthus; PSG, polysomnography; ROC, right outer canthus.
Source: Adapted from Ref. (24). Dyken ME, Yamada T, Lin-Dyken DC, et al. Diagnosing narcolepsy through the simultaneous clinical and electrophysiologic analysis of cataplexy. Arch Neurol. 1996;53:456–460; with permission.
PARASOMNIAS
The ICSD describes parasomnias as undesired physical phenomena, associated with sleep, often with central nervous system (CNS) activation, evidenced as an elevation of autonomic and skeletal muscle activity, and occasionally an experiential element (18).
In the ICSD, parasomnias have been divided into four major categories: the NREM-related parasomnias, the REM-related parasomnias, other parasomnias, and isolated symptoms and normal variants (Table 5.1).
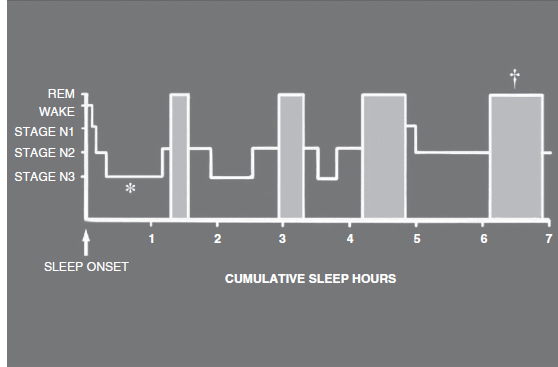
As suggested by the ICSD classification scheme, many parasomnias associate specifically with stages N3 and R sleep (28,29). Normally, there is a preponderance of stage N3 sleep in the first third of the night, and stage R sleep in the last third of the sleeping period (Figure 5.2) (28). This makes the sleep history helpful, because when a parasomnia consistently occurs soon after sleep onset, or just before waking, the differential diagnosis narrows.
Although a single PSG may not capture a suspected parasomnia, PSG correlates often suggest the diagnosis. When a parasomnia is captured, attention should focus on the PSG data and the clinical presentation immediately prior to, during, and following the event. The differential diagnosis frequently includes seizures and a variety of sleep-related movement disorders not otherwise classified as parasomnias. The documentation of a specific sleep stage, an ictal (seizure) EEG pattern, and behaviors characteristic of a parasomnia or seizure with split-screen, video-PSG is mandated for accurate diagnosis.
FIGURE 5.2 This histogram shows the general progression of sleep stages throughout a relatively normal night of sleep in a young adult, with the majority of consolidated stage N3 sleep occurring early in the night (as depicted by the asterisk) and the majority of consolidated/relatively prolonged stage R sleep occurring in the early morning hours (as depicted by the dagger) relatively close to the expected waking time.
Source: Modified from the educational slide set “Sleep Disorders;” Scope Publications, The Upjohn Company, 1983.
NREM-Related Parasomnias
Confusional Arousals
Confusional arousals in children classically occur early in the night during the first or second period of N3 sleep (27,28). They can last minutes to hours and can occur during daytime naps (30). Although confusional arousals are common and benign in children, they may predispose to adolescent sleepwalking (31). Violent behavior can occur if there is an attempt to awaken them. Two variants of the adolescent/adult type disorder include morning sleep inertia (sleep drunkenness) occurring primarily from light NREM sleep and abnormal sexual, at times assaultive, behavior (sexsomnia) (32,33).
The prevalence of confusional arousals from age 3 to 13 years has been reported at 17.3% (30). Predisposing factors include stress, anxiety, sleep deprivation, untreated OSA, and bipolar and depressive disorders (30). A genetic predisposition may be exacerbated by stressors that include sleep deprivation and drug use (34).
Sleepwalking
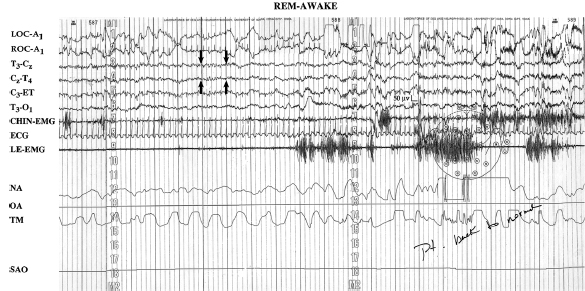
The PSG tracing in Figure 5.3 shows sleepwalking from stage N3 sleep (see technician’s note, “get up walking”). Sleepwalking classically occurs at the end of the first or second period of stage N3 sleep (29,31). The differential diagnosis includes seizures and other sleep-related movement disorders not considered parasomnias. Adolescent sleepwalking may be preceded by early childhood confusional arousals (31). As a single PSG may not capture sleepwalking, the history and PSG capture of confusional arousals support a sleepwalking diagnosis.
Sleepwalking can be precipitated by stress and major depressive disorders (MDDs). Reynolds et al. reported that almost 90% of the patients with MDD show some sleep-related EEG disturbance, most commonly, alterations in N3 sleep (35). Espa et al., in a controlled study of 11 subjects with sleepwalking or sleep terrors, showed increased slow-wave sleep “intensity” with increased total time and percentage of total sleep time and fragmentation of stage N3 sleep (36). Increased stage N3 sleep intensity was hypothesized to inhibit full arousal from N3 sleep, predisposing to sleepwalking and sleep terrors.
Sleep Terrors
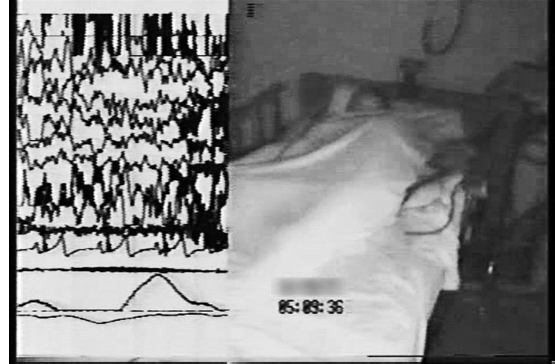
The PSG tracing in Figure 5.4 captures a classical stage N3 sleep terror from a 16-year-old girl with recurrent sleep spells with combative behavior (during one she fell down the stairs and chipped a few teeth) (37). During the PSG spell she tore off electrodes, and the technician’s restraining efforts exacerbated combative physical activity and screaming, for which the patient was later amnestic.
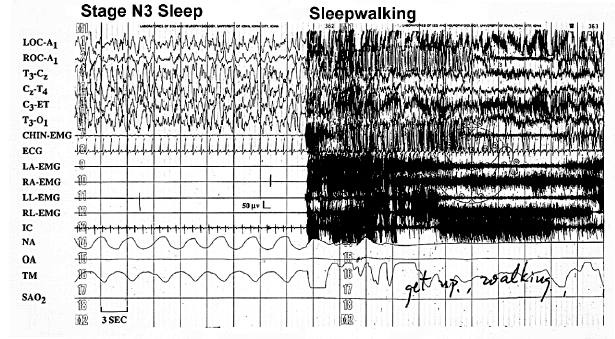
FIGURE 5.3 This PSG tracing captures a classical sleepwalking event from stage N3 sleep (see the technician’s note, “get up walking”).
Abbreviations: A1, left ear; C, central; ECG, electrocardiogram; EMG, electromyogram; ET, ears tied; IC, intercostal EMG; LA, left arm; LL, left leg; LOC, left outer canthus; NA, nasal airflow; O, occipital; OA, oral airflow; RA, right arm; RL, right leg; ROC, right outer canthus; SAO2, oxygen saturation; T; temporal, TM, thoracic movement.
Source: Modified from Ref. (28). Dyken ME, Yamada T, Lin-Dyken DC. Polysomnographic assessment of spells in sleep: nocturnal seizures versus parasomnias. Semin Neurol. 2001;21:377–390; with permission.
FIGURE 5.4 A 16-year-old girl with recurrent spells of nocturnal combative behaviors for which she was amnestic. This figure shows the split screen video analysis of the PSG, which captured a classical sleep terror that began in stage N3 sleep.
Source: From Ref. (37). Dyken ME, Lin-Dyken DC, Boyle J. Vignette 8; Sleep terrors, In: Chokroverty S, Thomas RJ, eds. Atlas of Sleep Medicine: Second Edition. Philadelphia: Elsevier Saunders; 2014:386–387; with permission.
Sleep terrors differ from confusional arousals in that they are often associated with significant autonomic hyperactivity and signs of fear, frequently with bloodcurdling screams. Sleep terrors (pavor nocturnus in children) are abrupt episodes from sleep, associated with motor agitation, vocalization, and extreme fear, often with screaming and crying (29,31). Autonomic hyperactivity is often evidenced by diaphoresis, mydriasis, tachypnea, and tachycardia (31). Although patients appear alert, attempts at interaction are usually unsuccessful and may prolong the spell (29,31).
The onset of sleep terrors ranges between 4 and 12 years of age, with a prevalence in up to 6.5% (31). Although they usually resolve in adolescence, the prevalence may reach 2.6% in the adult population up to 65 years (31). Precipitants include medications, stress, illness, insufficient sleep, and altered sleep schedule or environment. Sleep terrors are usually not associated with psychopathology in children (31,38). Although patients are generally amnestic for spells of sleep terror, some can recall feeling fear or threat as an episode resolves. This can lead to clinophobia (fear of going to bed) and sleep-onset insomnia (psychophysiological; conditioned insomnia), a stress that could exacerbate further sleep terrors (39).
Treatment centers on patient, family, and caregiver education. Detailed description should be given regarding gently directing the patient back to bed, and avoiding attempts to awaken them as this generally exacerbates fear, confusion, and the potential for injury (39,40). The family should avoid discussing the events with the patient in the mornings after the events occur as it could engender psychosocial stress (40). Scheduled awakenings should be considered if episodes occur at a consistent time during the night (39,41). This involves gently awakening the patient 15 to 30 minutes prior to the usual onset of the episode (39,41). Medications are rarely used. If the events become dangerous or highly disruptive, imipramine or low-dose clonazepam (starting dose, 0.5 mg) at bedtime has been reported to be potentially beneficial (39).
REM-Related Parasomnias
REM Sleep Behavior Disorder
RBD occurs in REM sleep in association with violent, directed behavior, frequently leading to injury, followed by spontaneous report of detailed dreams corresponding with observed movements (isomorphism) (28,42,43). Although, primarily affecting elderly males, it has been reported in children and adolescents (44).
In adults, RBD may be the initial manifestation of a synucleinopathy, a group of neurodegenerative disorders that includes Parkinson’s disease (PD), and in children RBD has been linked to juvenile PD (43,45). In children, RBD has also been associated with various neurological disorders with putative brainstem lesions, including infiltrating tumors of the pons and cerebellar astrocytomas (46,47).
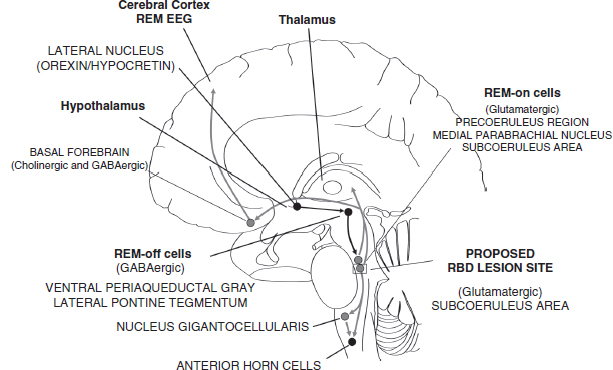
In normal REM sleep, large-amplitude movements do not occur because uninhibited REM-on cells in the brainstem with caudally directed neuronal tracts lead to atonia (48,49). In RBD, a lesion may affect the brainstem structure in humans, analogous to the subcoeruleus area (SCA) in the cat (Figure 5.5) (49,50). From animal studies, it has been hypothesized that degeneration of the SCA disrupts descending tracts that normally cause atonia/paresis, thus allowing violent behaviors during REM (“dreaming/paralyzed”) sleep.
PSG criteria for RBD demands the presence of REM without atonia, as characteristic behaviors are captured in only 8% of adults (Figure 5.5) (42,43,51). RBD is also associated with periodic limb movements in sleep (75% of adult patients) (43). Clonazepam can control RBD in up to 90% of adults, and in an account of five cases of childhood RBD, 0.25 mg led to complete resolution of nighttime disturbances (52–54). It has been speculated that clonazepam may act preferentially through serotonergic-like inhibition of excitatory motor systems (54).
Recurrent Isolated Sleep Paralysis
Although classically associated with narcolepsy, at least one episode of sleep paralysis occurs in 40% of the general population (18,55). Isolated events may last minutes, while spells that tend to recur can last hours (56). Sleep paralysis can be precipitated by stress, sleep deprivation, and apnea (57). Sleep paralysis can associate with hallucinations that include the presence of an intruder (an incubus), or an out-of-body experience (58). In severe cases, therapy has included tricyclic antidepressants and selective serotonin reuptake inhibitors (56).
FIGURE 5.5 This parasagittal section of the brain and brainstem shows the suspected pathology explaining RBD, based upon the recently proposed “flip-flop” model of sleep state transitions by Saper et al. (48). In normal REM sleep, glutamatergic REM-on cells in what would be considered the SCA in cats and the sublaterodorsal nucleus in rats (an area presently not well defined in humans), directly and indirectly (through the nucleus gigantocellularis, one of the ventromedial groups of the reticular nuclei in the medulla oblongata) cause hyperpolarization of anterior horn cells in the spinal cord, resulting in atonia. From animal studies, it has been hypothesized that in RBD, degeneration of the SCA disrupts descending tracts that would normally lead to atonia/paresis, thus allowing violent behaviors during REM (dreaming/paralyzed) sleep. Black circles and lines indicate nuclei and neuronal tracts normally inhibited during REM sleep; gray circles and lines indicated nuclei and neuronal tracts normally activated during REM sleep; rectangle indicates proposed lesion site in RBD.
Abbreviations: GABA, gamma-aminobutyric acid; RBD, rapid eye movement sleep behavior disorder; SCA, subcoeruleus area.
Source: From Ref. (49). Dyken ME, Afifi AK, Lin-Dyken DC. Sleep-related problems in neurologic diseases. Chest. 2012;141:528–544; with permission.
SLEEP-RELATED MOVEMENT DISORDERS
Restless Legs Syndrome and Periodic Limb Movements in Sleep
Restless legs syndrome (RLS) is classically described as a sleep-related movement disorder although it is a sensorimotor problem defined by the waking symptoms (defined by the acronym URGE) that includes the urge to move the legs, that is worsened by rest, relieved by going (movement of the limb), and is worst in the evening (18,31). It is only associated with sleep-related movements that are known as periodic limb movement in sleep (PLMs) in 80% to 90% of the cases (18,31). Disruption of sleep onset and maintenance can lead to insomnia, sleepiness, memory and motivational problems, and potentially to anxiety and depression.
Pediatric prevalence rates for RLS are 2% to 4% (moderate to severe in approximately 0.5% to 1%) (18). Boys and girls are affected equally until the late teens where the prevalence is approximately two times greater in women (18). Pediatric RLS is highly familial, with up to 92% of the cases reporting affected family members.
The typical age of onset for the periodic limb movement disorder (PLMD; where PLMs significantly affects sleep) is unknown, but it has been reported to occur as early as infancy. There is potential concern that PLMs-associated overactivity of the sympathetic nervous system may lead to a higher risk of vascular disease (59). About 70% of the children with RLS demonstrate greater than 5 PLMs per hour on PSG study (18,31).
Therapy has been based largely on a dopamine deficiency for the hypothesis regarding the etiology of RLS and PLMs (60). There is also a high prevalence of iron deficiency with these problems, which may be explained by the fact that iron is a cofactor for tyrosine hydroxylase in dopamine production (61). In such cases iron replacement is recommended, otherwise dopaminergic therapy is generally considered the treatment of choice (61,62).
Sleep-Related Bruxism
Sleep bruxism is stereotypic teeth-grinding movements during sleep, often seen in normal children, with a prevalence up to 17% (31). It is divided into a primary form (most often seen in healthy children) and a secondary form (often seen in children with disabilities) (63). Up to 50% of the patients have a family history of bruxism. Bruxism is associated with two types of jaw contractions: tonic/isolated sustained contractions and rhythmic masticatory muscle activity, which appears as a series of repetitive jaw clenchings (31). Bruxism can lead to dental damage, temporal-mandibular injuries, facial pain, headaches, and insomnia, although the natural course is usually benign (64,65).
Sleep-Related Rhythmic Movement Disorder
Rhythmic movements of the body during drowsy wakefulness and sleep are common in normal infants and children, occurring in 59% of the infants at 9 months, in 33% of the children at 18 months, and in 5% by 5 years (31). The formal diagnosis of sleep-related rhythmic movement disorder (RMD) can only be considered if the movements interfere with sleep, impair daytime function, or result in injury. Most patients with sleep-related rhythmic movements are normal, but in older children there may be a higher association with intellectual disabilities, and there have been reports of soft-tissue injuries, with significant injury being rare (66).
In RMD, the subtypes of movements include body rocking, headbanging, and head rolling (less commonly body rolling, leg banging, or leg rolling), often in association with rhythmic humming or inarticulate oral sounds (31). The movement frequency is between 0.5 and 2.0 movements per second and the duration of repetitive movements is generally less than 15 minutes (18).
One study of six children with RMD found that 3 weeks of controlled sleep restriction with the concomitant use of hypnotics during the first week of therapy led to an almost complete resolution of pathological movements (67). The authors of that paper believed their results suggested that the RMD is a voluntary, self-soothing behavior.
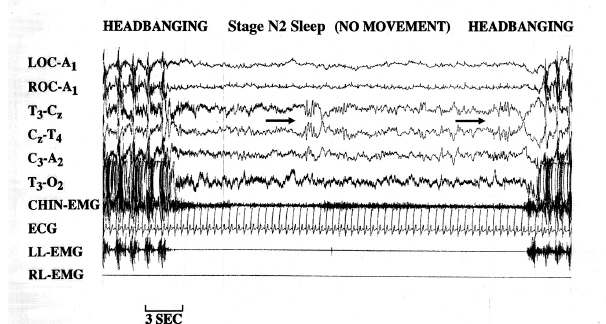
In 1997, a video-PSG study of children 1 to 12 years of age, referred for the evaluation of violent nocturnal behavior, characterized the movement, responsiveness, and sleep stage during each spell (66). Thirty-seven periods of headbanging, body rocking, and leg banging occurred during sleep; 26 were associated with stage N2 sleep, one appeared in stage N3 sleep, one occurred in stage REM sleep, whereas only nine events were recognized in N1 sleep (see Figure 5.6).
FIGURE 5.6 Immediately after and before respective episodes of headbanging, a young girl with rhythmic movement disorder stopped moving for a period of time, which allowed the recognition of stage N2 (NREM) sleep with characteristic K-complexes (as depicted by the 2 black arrows) with negative sharp waves followed by positive components (total duration lasting ³ 0.5 seconds).
Abbreviations: A1, left ear; A2, right ear; C, central; ECG, electrocardiogram; EMG, electromyogram; LL, left leg; LOC, left outer canthus; O, occipital; RL, right leg; ROC, right outer canthus; T, temporal.
Source: From Ref. (66). Dyken ME, Lin-Dyken DC, Yamada T. Diagnosing rhythmic movement disorder with video-polysomnography. Pediatr Neurol. 1997;16:37–41; with permission.
SLEEP-RELATED MEDICAL AND NEUROLOGICAL DISORDERS
Sleep-Related Epilepsy
Nocturnal frontal lobe epilepsy (NFLE) is a sleep-related epilepsy with a mean age of onset of 14 +/− 10 years, characterized by seizures that occur predominately during sleep, with 34% of the patients reporting occasional seizures (similar to their sleep seizures) during wakefulness (68). The neurological examination is normal in up to 92% of all cases and although there are no major clinical differences between the sporadic and the autosomal dominantly inherited form of NFLE, diurnal behavioral problems including impulsivity, aggression, and hyperactivity have been reported in some forms of NFLE in relation to mutations of the subunits of the nicotinic acetylcholine receptor (69).
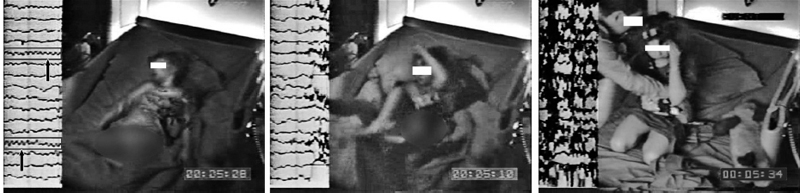
FIGURE 5.7 Sleep-related epilepsy. Of the 40 stereotypical spells captured during a 10-year-old girl’s sleep video-EEG, only one showed rhythmic theta activity from the right frontal region (as shown by the arrows in the EEG channels enclosed by the rectangular boxes in the first picture frame) prior to a clinical spell, which was characterized by a sudden frightened awakening and yelling for “Daddy” (see the second picture frame), followed by crouching on bended hands and knees and subsequently (see the third picture frame) clutching her father.
Source: From Ref. (70). Dyken ME, Lin-Dyken DC. Seizure, parasomnia or behavioral disorder? In: Culebras A, ed. Case Studies in Sleep Neurology; Common and Uncommon Presentations. New York, NY: Cambridge University Press; 2010;193–199; with permission.
NFLE is associated with brief hypermotor seizures, with marked autonomic activation and stereotypical arousals with emotive vocalizations and bending and rocking of the body that often suggest a parasomnia or waking behavior (28,68,69). The firm diagnosis is also complicated by the fact that the EEG is often normal and may only show epileptiform discharges during an actual nocturnal event (see Figure 5.7) (70). In one study carbamazepine abolished all seizures in 20%, with a significant reduction in seizure activity by at least 50% in another 48% of the patients treated (68).
CONCLUSIONS
Sleep comprises approximately half of the time spent in early childhood. Maturation of the neurological structures responsible for sleep normally leads to a fairly predictable progression of sleep patterns; nevertheless disruption of this process can lead to negative consequences. Sleep disorders in children can present as difficulties with sleep initiation and maintenance, inappropriate sleep, and undesired behaviors. The appropriate evaluation of pediatric sleep disorders requires a detailed pediatric sleep history, a focused physical examination, and in some cases PSG to assure accurate diagnosis and proper therapeutic intervention.
REFERENCES
1. Sheldon SH, Ferber R, Kryger M. Preface. In Sheldon SH, Ferber R, Kryger MH, eds. Principles and Practice of Pediatric Sleep Medicine. Philadelphia, PA: Elsevier Saunders; 2005:vii.
2. Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. Philadelphia, PA: Lippincott, Williams & Wilkins;2003.
3. Drake C, Nickel C, Burduvali E, et al. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26(4):455–458.
4. Kumar HV, Schroeder JW, Gang Z, Sheldon SH. Mallampati score and pediatric obstructive sleep apnea. J Clin Sleep Med. 2014;10(9):985–990.
5. Ballard CF. Adenoidal facies and mouth-breathing: a clinical analysis. Med Press, 1952;228(15): 347–351.
6. Chan W, Woldeyohannes M, Colman R, et al. Haemodynamic and structural correlates of the first and second heart sounds in pulmonary arterial hypertension: an acoustic cardiography cohort study. BMJ Open. 2013;3(4):1–10.
7. Sheldon SH. Polysomnography in infants and children. In: Sheldon SH, Ferber R, Kryger MH, eds. Principles and Practice of Pediatric Sleep Medicine. Philadelphia, PS: W.B. Saunders;200 5:49–70.
8. Caton R. The electric currents of the brain. Br Med J. 1875;2:278.
9. Berger H. Uber das Elektrenkephalogramm des Menschen. Arch of Psychiat. 1929;87:527–570.
10. Brazier MAB. A History of the Electrical Activity of the Brain: The First Half-Century. London: Pitman Medical Publishing Co;1961.
11. Yamada T, Meng E. Chapter 1, Introduction: history and perspective of clinical neurophysiologic diagnostic tests. In: Yamada T, Meng E, eds. Practical Guide for Clinical Neurophysiologic Testing– EEG. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins;2010:1–4.
12. Loomis AL, Harvey N, Hobart GA. Cerebral states during sleep, as studied by human brain potentials. J Exp Psychol. 1937;21:127–144.
13. Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274.
14. Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967;47:117–177.
15. Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages in human subjects. Washington, DC: US Government Printing Office;1968. (NIH Publication No. 204)
16. Berry RB, Brooks R, Gamaldo CE, et al. American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, Version 2.0. Darien, IL: American Academy of Sleep Medicine;2012. www.aasmnet.org
17. Anders T, Emde R, Parmalee A, eds. A Manual of Standardized Terminology, Techniques and Criteria for Scoring of States of Sleep and Wakefulness in Newborn Infants. Los Angeles: UCLA Brain Information Service, NINDS Neurological Information Network;1971.
18. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine;2014.
19. Guilleminault C, van den Hoed J, Mitler M. Clinical overview of the sleep apnea syndromes. In: Guilleminault C, Dement WC, eds. Sleep apnea syndromes. New York, NY: Alan R Liss, Inc;1978:1–12.I
20. Iber C, Ancoli-Israel S, Chesson A, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st ed. Westchester, IL: American Academy of Sleep Medicine;2007.
21. Ruehland WR, Rochford PD, O’Donoghue FJ, et al. The new AASM criteria for scoring hypopneas: Impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157.
22. Prasad M, Setty G, Ponnusamy A, et al. Cataplectic facies: clinical marker in the diagnosis of childhood narcolepsy-report of two cases. Pediatr Neurol. 2014;50(5):515–517.
23. Dyken ME, Yamada TY, Lin-Dyken DC, et al. Narcolepsy: unequivocal diagnosis after split-screen, video-polysomnographic analysis of a prolonged cataplectic attack. Neurology. 1994;44:760–761.
24. Dyken ME, Yamada T, Lin-Dyken DC, et al. Diagnosing narcolepsy through the simultaneous clinical and electrophysiologic analysis of cataplexy. Arch Neurol. 1996;53:456–460.
25. Krahn LE, Boeve BF, Olson EJ, et al. A standardized test for cataplexy. Sleep Med. 2000;1(2):125–130.
26. Saper CB, Fuller PM, Pedersen NP, et al. Sleep state switching. Neuron. 2010;68(6):1023–1042.
27. Dyken ME, Afifi AK, Lin-Dyken DC. Sleep-related problems in neurologic diseases. Chest. 2012;141(2):528–544.
28. Dyken ME, Yamada T, Lin-Dyken DC. Polysomnographic assessment of spells in sleep: nocturnal seizures versus parasomnias. Semin Neurol. 2001;21:377–390.
29. Dyken ME, Lin-Dyken DC. Parasomnias. In: Yamada T, Meng E, eds. Practical Guide for clinical neurophysiologic testing: EP, LTM, IOM, PSG, and NCS. Philadelphia, PA:Wolters Kluwer/Lippincott Williams & Wilkens;2011:260–277.
30. Berry RB. Fundamentals of Sleep Medicine. Elsevier Health, Kindle Edition. 2011:27849–27861.
31. American Academy of Sleep Medicine. International Classification of Sleep Disorders:Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine;2005.
32. Roth B, Nevismalova S, Rechtschaffen A. Hypersomnia with “sleep drunkenness.” Arch Gen Psychiatry. 1972;26:456–462.
33. Guilleminault C, Moscovitch A, Yuen K, Poyares D. Atypical sexual behavior during sleep. Psychosom Med. 2002;64:328–336.
34. Hublin C, Kaprio J, Partinen M, et al. Parasomnias: co-occurrence and genetics. Psychiatr Genet. 2001;11:65–70.
35. Reynolds C, Kupfer D, Sleep research in affective illness: state of the art circa 1987. Sleep. 1987;10:199–215.
36. Espa F, Ondze B, Deglise P, et al. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;11:929–939.
37. Dyken ME, Lin-Dyken DC, Boyle J. Vignette 8; Sleep terrors, In: Chokroverty S, Thomas RJ, eds. Atlas of Sleep Medicine. 2nd ed. Philadelphia, PA: Elsevier Saunders;2014:386–387.
38. Schenck C, Mahowald M. On the reported association of psychopathology with sleep terrors in adults. Sleep. 2000;23:448–449.
39. Provini F, Tinuper P, Bisulli F, Lugaresi E. Arousal disorders. Sleep Med. 2011;12 Suppl 2:S22–S26.
40. Meltzer L, Mindell J. Sleep and sleep disorders in children and adolescents. Sleep Med. 2008;3(2):269–279.
41. Lask B. Sleep disorders. “Waking treatment” best for night terrors. BMJ. 1993;306(6890):1477.
42. Schenck CH, Bundlie SR, Mahowald MW. Human REM sleep chronic behavior disorders: a new category of parasomnia. Sleep Res. 1985;14:208.
44. Stores G. Rapid eye movement sleep behaviour disorder in children and adolescents. Dev Med Child Neurol. 2008:50:728–732.
45. Rye DB, Johnston LH, Watts RL, Bliwise DL. Juvenile Parkinson’s disease with REM sleep behavior disorder, sleepiness, and daytime REM onset. Neurology. 1999;53:1868–1870.
46. Barros-Ferreira M, Chodkiewicz JP, Lairy GC, Salzarulo P. Disorganized relations of tonic and phasic events in REM sleep in a case of brain-stem tumour. Electroenceph Clin Neurophysiol. 1975;38:202–207.
47. Schenck CH, Bundlie SR, Smith SA, et al. REM behavior disorder in a 10 year old girl and aperiodic REM and NREM sleep movements in an 8 year old brother. Sleep Res. 1986;15:162.
48. Saper CB, Fuller PM, Pedersen NP, et al. Sleep state switching. Neuron. 2010;68(6):1023–1042.
49. Dyken ME, Afifi AK, Lin-Dyken DC. Sleep-related problems in neurologic diseases. Chest. 2012;141:528–544.
50. Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130(pt 11):2770–2788.
51. Dyken ME, Lin-Dyken DC, Seaba P, et al. Violent sleep-related behavior leading to subdural hemorrhage. Arch Neurol. 1995;52:318–321.
52. Sheldon SH, Jacobson J. REM-sleep motor disorder in children. J Child Neurol. 1998;13:257–260.
53. Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. Cleve Clin J Med. 1990;57(Suppl):9–23.
54. Mahowald MW, Schenck CH. REM sleep Parasomnias. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders;2005: 897–916.
55. Dyken ME, Wenger EJ, Yamada T. REM alpha rhythm: diagnostic for narcolepsy? J Clin Neurophysiol. 2006;23:254–257.
56. Terrillon JC, Marques-Bonham, S. Does recurrent isolated sleep paralysis involve more than cognitive neurosciences? J Sci Explor. 2001;15:97–123.
57. Dyken ME, Lin-Dyken DC, Jerath N. Isolated sleep paralysis: an REM-“Sleep” polysomnographic phenomenon as documented with simultaneous clinical and electrophysiological assessment. In; Chokroverty S, Thomas RJ eds. Atlas of Sleep Medicine; 2nd ed. Philadelphia, PA; Elsevier Saunders;2014:375–377.
58. Cheyne JA, Rueffer SD, Newby-Clark IR. Hypnagogic and hypnopompic hallucinations during sleep paralysis: neurological and cultural construction of the night-mare. Conscious Cogn. 1999;8(3):319–337.
59. Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease and stroke. Sleep. 2009;32(5):589–597.
60. Lee SJ, Kim JS, Song IU, et al. Poststroke restless legs syndrome and lesion location: anatomical considerations. Mov Disord. 2009;24(1):77–84.
61. Im KB, Strader S, Dyken ME. Management of sleep disorders in stroke. Curr Treat Options Neurol. 2010;12(5):379–395.
62. Littner MR, Kushida C, Anderson WM, et al. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:557–559.
63. Ohayon M, Li K, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest. 2001;119:53–61.
64. Rugh J, Harlan J. Nocturnal bruxism and temporomandibular disorders. Adv Neurol. 1988;49:329–341.
65. Ware J, Rugh J. Destructive bruxism: sleep stage relationship. Sleep. 1988;11:172–181.
66. Dyken ME, Lin-Dyken DC, Yamada T. Diagnosing rhythmic movement disorder with video-polysomnography. Pediatr Neurol. 1997;16:37–41.
67. Etzioni T, Katz N, Hering E, et al. Controlled sleep restriction for rhythmic movement disorder. J Pediatr. 2005;3:393–395.
69. Ryvlin P, Rheims S, Risse G. Nocturnal frontal lobe epilepsy. Epilepsia. 2006;47:83–86.
70. Dyken ME, Lin-Dyken DC. Seizure, parasomnia or behavioral disorder? In; Culebras A., ed. Case Studies in Sleep Neurology; Common and Uncommon Presentations. New York, NY: Cambridge University Press:2010;193–199.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree