Maintaining an optimal cerebral environment and correctly treating intracranial hypertension are fundamental in avoiding added injury to our patients and achieving the continual decrease in mortality and poor results.
27.1 Introduction
Traumatic brain injury (TBI) continues to be the leading cause of death and disability in people under 45 years of age. Furthermore, incidence is on the increase; at present this disease is considered pandemic, with particular repercussions for less developed countries. However, this pessimistic finding contrasts with a clear, objectively positive observation in recent years: although high-energy injuries involving the severest acceleration/deceleration mechanisms have increased, mortality rates and poor results have decreased significantly. In 1991 the information gathered by the American Traumatic Coma Data Bank (TCDB) indicated that mortality rates were situated at 36%, with an overall percentage of poor outcome (death, vegetative state or severe disability) reaching nearly 60% of patients with severe TBI [1]. Recent analysis indicates that this figure has been reduced very significantly to 40% in centers with extensive experience in the management of brain injured patients [2].
The main explanation for this decrease should be sought in the significant changes in treatment applied to these patients. However, drugs and therapeutic maneuvers used on patients with TBI (mannitol, hyperventilation, barbiturates, etc.) are unchanged in the last 20 years and all attempts to introduce new neuroprotective drugs (glutamate inhibitors, calcium channel blockers, or oxygen-free radical inhibitors, among others) have failed. The causes of this decline in poor outcome may be explained by a combination of factors, including the following: 1) improved understanding of the pathophysiology of TBI, 2) the multidisciplinary management applied to this condition, 3) the creation of units specialized in the management of these types of neurocritical patients, and 4) the development and following of clinical practice guidelines (CPG) that have advocated the use of standardized and systematic treatment protocols for the treatment of intracranial hypertension (ICH) [3,4]. Nevertheless, the treatment of TBI is still prone to excessive variability in the numerous hospitals that receive and treat such patients. This variability, while justified in some cases, can be a reflection of reluctance on the part of the various medical groups implicated to accept that the systematic treatment of these cases brings about a clear benefit, not only for patients but also for the social environment in which they live.
In the last decade, our center has observed a progressive reduction in mortality and an increase in good outcome in patients with severe TBI. We are convinced that this change is due to the fact that our center was able to carry out the 4 measures described above, especially the systematic, stepwise protocol for the management of these patients optimized on the basis of a multimodal neuromonitoring protocol. This chapter summarizes the series of general measures and the systematic approach used by our hospital for TBI patients admitted to the Neurotraumatology Intensive Care Unit (ICU).
In this chapter, the general measures and specific treatment for ICH given to in-hospital patients with severe traumatic brain injury are outlined. We assume that the foundations of “evidence-based medicine” and other aspects, including multimodal neuromonitoring or the management of specific aspects, such as fluid therapy or nutrition, have been covered extensively in other chapters of this work. In this chapter we focus on general measures and first level measures in the treatment of ICH based mainly on recommendations of the Brain Trauma Foundation (BTF) without ruling out additional sources of evidence, such as the Cochrane Collaboration.
We aim at providing information that is above all practical and concise by supplementing the text with a series of useful tables and algorithms. In addition to theoretical information, we have included an illustrative case report in which we describe the maneuvers used and identify the controversial points or inadequate aspects of patient management.
27.2 Neurotraumatic Patient Treatment in the Hospital
In this section we refer to the treatment of patients once they have arrived at the final hospital. Treatment at the receiving hospital should begin in the emergency department. The therapeutic conduct and the type and number of complementary testing to be carried out must be individualized in all cases. As a general rule, however, once the airway and hemodynamic status is stable, patients should be evaluated according to the Glasgow Coma Scale (GCS), noting whether there are differences at the initial assessment made after resuscitation, during transport of the patient, or at the first reference center. In addition to the GCS score, breathing pattern and the size and shape of the pupils, pupil reactivity to light and the presence of corneal reflexes should all be noted during the neurological examination. In paralyzed patients undergoing mechanical ventilation, prehospital evaluation using the GCS will guide the therapeutic management. In cases where this assessment is unreliable and the CT brain scan shows no obvious injuries, it is advisable to reverse paralysis and re-examine the patient before proceeding to monitoring and aggressive, and possibly unjustified, treatment.
Any associated systemic injuries (pulmonary, genitourinary, abdominal, etc.) should be evaluated and treated in order of priority. At the same time, external craniofacial injuries are evaluated (otorrhagia, head contusions, etc.) and the possible existence of cervical injuries are determined before transferring the patient to the neuroradiology unit for tomography scanning. It is important that the transfer to the CT area is under optimal conditions, which includes ensuring flow of the airway, adequate ventilation and proper functioning of the fluid delivery pathways. Too often, these patients suffer significant deterioration in their neurological status during the periods spent in the hospital corridors waiting for various complementary examinations.
Tomography scans should be performed in a quick and technically correct manner because this first exploration allows existing brain injury to be assessed and classify the patient according to the different groups of pathologies described by Marshall et al. [5]. Based on the volume of the lesions, the status of cistern compression and the degree of deviation from the midline, this classification consists of 4 types of diffuse lesions and the presence of intra- or extra-axial focal lesions requiring surgical evacuation. The Marshall classification also provides initial prognostic information because each of the injury categories is associated with an increased risk of ICH and a poor outcome (Figure 27.1) [6].
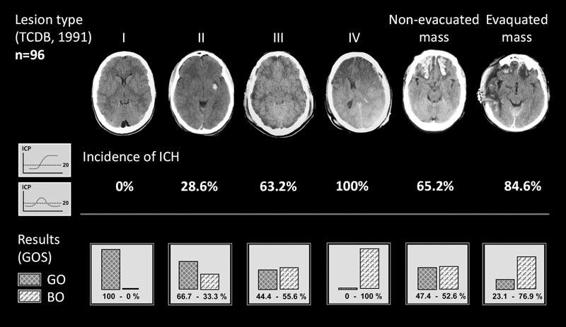
Figure 27.1. Traumatic brain injury according to the classification system of the American Traumatic Coma Data Bank. (I) Diffuse type I injury (comatose patient with normal CT). (II) Diffuse type II injury (permeable cisterns, midline centered with small lesions). (III) Diffuse type III injury (bilateral brain swelling). (IV) Diffuse type IV injury (midline shift >5 mm with no focal lesions to account for this distortion in the brain). Non-evacuated mass is any intra- or extracerebral lesions with a volume >25 ml that were not evacuated. Evacuated mass is any evacuated focal lesion. The image shows the different percentages of intracranial hypertension incidence and associated poor outcome in the 96 patients included in the study [6].
GO = Good Outcome; BO = Bad Outcome.
Early surgical treatment of space-occupying lesions is essential for the correct treatment of patients with severe TBI. At our center, all space-occupying accesible lesions with a volume exceeding 25 ml are evacuated surgically. In our opinion, the use of the initial levels of intracranial pressure (ICP) in making surgical decisions, while controversial, is helpful in doubtful cases. In general, we can say that regardless of their level of consciousness, patients with focal lesions whose ICP levels are moderately elevated will present late neurologic deterioration, the result of uncontrolled ICP elevations in a large percentage of cases [7,8]. We feel this point justifies an aggressive therapeutic approach aimed at preventing these situations. The wait-and-see approach in these types of patients, especially those presenting concussions, often has catastrophic results. Although the predominant type of lesion may vary depending on the damaging mechanism, it is generally estimated that 25-45% of patients with severe TBI, 3-12% of those with moderate TBI, and 1 in 500 mild TBI patients present a focal lesion requiring intervention.
27.3 Multimodal Monitoring in Patients With Severe Traumatic Brain Injury
Once the CT scan is performed and any space-occupying lesions are evacuated, patients are transferred to the ICU, where the therapeutic approach should be aimed at creating a means favorable to the recovery from primary lesions and the early treatment and prevention of secondary injuries. The difficulty in carrying out appropriate clinical monitoring in these patients requires the use of a multimodal monitoring protocol that includes neurological and systemic parameters. It is well established that a score less than or equal to 8 on the GCS reflects a risk of ICH. In studies performed by the Traumatic Coma Data Bank during the 1990s, it was shown that between 50-75% of these patients had ICH at some point during the evolution of the injury [9]. At present, we know that this incidence can be adjusted according to the type of brain injury[6] and that it decreases dramatically with an aggressive surgical approach to focal lesions.
The high risk of ICH in severe TBI explains why continuous monitoring of ICP has been implemented almost routinely in the management of these patients, especially since the 1995 publication of the CPG for TBI management developed by the BTF. These guidelines have been updated and endorsed by most international scientific societies. The latest version, published in 2007, reaffirmed with level II evidence that ICP monitoring should be applied in all severe TBI patients with an abnormal CT scan upon admission [10]. The implementation of an ICP sensor is also indicated in TBI patients with an initial CT scan that is normal if a GCS score of less than 8 can be confirmed. This is based on the fact that 1 of every 3 of these patients show new brain lesions in the subsequent CT scan [11]. In recent years, however, we have found that the value of ICP as a parameter alone is insufficient for decision making in complex cases. In addition to the ICP sensor in the latest edition of the BTF guidelines, it is also recommended that additional monitoring systems be included to provide overall or specific information regarding cerebral oxygen availability, such as oxygen saturation in the jugular bulb (SjO2) or catheters for direct measurement of oxygen pressure at the tissue level (PtiO2) [12].
In our center, all patients with TBI who present a diffuse lesion, or those in whom a focal lesion was evacuated, undergo implantation of an intracranial pressure (ICP), intraventricular, or intraparenchymal sensor. When using an intraparenchymal sensor, the more damaged brain hemisphere is selected [13]. In addition to the ICP sensor, TBI patients are implanted with a retrograde catheter in the predominant jugular vein and/or a catheter for tissue oximetry in the less injured hemisphere. In selected cases, 1 or 2 cerebral microdialysis catheters are also placed in the patient. These new monitoring techniques allow continuous information on various aspects of blood flow and cerebral metabolism to be obtained. At the systemic level, multimonitoring of patients is also recommended. This basically involves the control of diuresis, heart rate, blood pressure (with the transducer leveled at the foramen of Monro), and central venous pressure, in addition to measuring the pressure in the pulmonary artery in cases of hemodynamic instability.
27.4 General Measures for Treating Brain Injured Patients
While in the ICU, severe TBI patients undergo monitoring of both ICP and cerebral and systemic hemodynamics. This is what guides the course of treatment for these patients. Although many of these cases involve associated systemic injuries, we generally refer to the patient with “pure” severe TBI or with associated lesions that do not significantly modify treatment. To simplify, we could define the key therapeutic targets in patients with intracranial hypertension as reducing ICP and maintaining a mean arterial pressure (MAP) sufficient to maintain adecuate cerebral perfusion pressure (CPP). In severe TBI patients, a series of general measures, regardless of the initial value of the ICP, should be taken early on to achieve a correct hemodynamic stability and an adequate supply of nutrients, and to avoid any factors that increase ICP. The latter may include maladaptive breathing, improper positioning of the patient (Figure 27.2), hypoxia, hypercapnia, high fever, seizures, hypo- or hypertension, and hyponatremia.
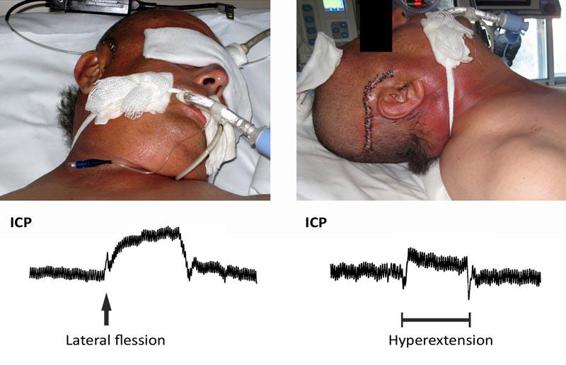
Figure 27.2. Image showing the effect of cranial position on intracranial pressure (ICP). Both hyperextension (right) and lateral flexion (left) in moderate amounts cause evident increases in ICP.
Maintaining normovolemia and making a correct selection of serums to administer play an essential role in the treatment of these patients. Electrolyte management includes maintaining an adequate circulating blood volume (which helps maintain the correct CPP and availability of O2), a volume in the brain interstitium that is somewhat lowered, and a discrete serum hyperosmolality. It is recommended that isotonic solutions are administered in TBI patients. A NaCl solution at 0.9% is considered the crystalloid of choice. A 5% albumin or hydroxy-ethyl starch of low molecular weight are the more widely accepted colloids. The administration of glucose solutions is not advised unless there is a risk of hypoglycemia because this condition may exacerbate ischemic injury. In addition, glucose causes an osmotic drag of water in the transport, which may contribute to the formation of cerebral edema.
Ideally, the objectives of this set of comprehensive measures are aimed at: 1) evacuating lesions with a volume >25 ml occupying intra- or extracerebral space, 2) maintaining normothermia, with a core temperature (rectal, esophageal or bladder) <37 °C, 3) maintaining an ICP below 20 mmHg, 4) maintaining levels of MAP >90 mmHg that allow CPP values ≥ 60 mmHg [14], 5) maintaining a total content of hemoglobin and a level of PaO2 to ensure proper transport of oxygen to the brain (recommended: hemoglobin >10 g/dl and PaO2 of 100-110 mmHg), 6) maintaining values of SaO2 >95% associated, if possible, with normocapnia and 7) maintaining values for SjO2 above 60% [15].
General measures also include proper analgesia and sedation of the patient. Analgesics and sedatives should be used in combination to enhance their effects and thereby reduce the individual doses. Given the depressant effect of these drugs on the respiratory system, mechanical ventilation should be used. To address their cardiovascular impact, doses may be adjusted according to the patient’s hemodynamic status. At present, midazolam is considered the sedative of choice due to its short half-life in comparison to other benzodiazepines. Other sedatives, such as propofol, may cause arterial hypotension, an important drawback. Morphine hydrochloride and fentanyl are the more adequate analgesics because a conventional dose not increase cerebral blood flow (CBF) or ICP.
27.5 Specific Treatment of Intracranial Hypertension
In cases where ICP >20 mmHg and the abovementioned maneuvers have been carried out correctly while ensuring that there are no new space-occupying lesions requiring surgical evacuation, a stepwise, additive treatment for ICH should be initiated. From the time of the publication of the first version of CPG (1995) by the BTF, our center designed a treatment algorithm largely adapted to the recommendations of these guidelines. After almost 15 years, this algorithm remains essentially unchanged because the latest versions of these guides have not produced significant changes in most of the therapeutic measures used. However, this algorithm included, and includes, differential aspects, such as a less frequent use of barbiturates (this is discussed extensively in the chapter on second level measures in the treatment of ICH), additional maneuvers, such as hypertonic saline, as well as specific considerations for certain injuries obtained from other sources of evidence.
For patients with persistent intracranial hypertension, first level measures for the treatment of ICH, as outlined in the first 2 editions of the BTF CPG, will be initiated in addition to reviewing previous general measures. These measures include muscle relaxation, the evacuation of cerebrospinal fluid (CSF), the administration of hyperosmolar solutions, and hyperventilation. Almost all are based on type I or II levels of evidence (Table 27.1).
Measurement | Recommendation | Level of evidence |
Muscle relaxants | There are no specific recommendations | – |
Evacuation of CSF | There are no specific recommendations | – |
Mannitol | Without monitoring ICP, mannitol may only be given if signs of transtentorial herniation or progressive neurological deterioration are observed | III |
Mannitol is effective in controlling ICH when used at doses of 0.25-1 g/kg. When used, decreases in systolic arterial pressure (SAP) to below 90 mmHg should be avoided | II | |
Hypertonic saline solution | There are no specific recommendations | – |
Hyperventilation (HV) | HV should not be used prophylactically (PaCO2 <25 mmHg) | II |
HV should be used intermittently for ICH | III | |
HV should be avoided during the first 24 hours of injury, when CBF is reduced | III | |
If using HV, monitoring the availability of brain oxygen using SjO2 or PtiO2 is recommended | III |
Table 27.1. Table of evidence of the Brain Trauma Foundation (2007 edition) for the treatment of intracranial hypertension in patients with severe traumatic brain injury.
CBF = cerebral blood flow; CSF = cerebrospinal fluid; ICH = intracranial hypertension; ICP = intracranial pressure; PaCO2 = arterial pressure of carbon dioxide; PtiO2 = brain tissue oxygen pressure; SAP = systolic arterial pressure; SjO2 = jugular bulb oxygen saturation.
In the sequence of application for treating ICH, the administration of muscle relaxants follows the evacuation of CSF, if possible. This therapeutic maneuver is only possible in cases in which ICP monitoring is done using an intraventricular catheter. Hyperventilation and/or the administration of hyperosmolar solutions are the subsequent therapeutic stages. When all these measures are insufficient, the BTF recommends the administration of barbiturates at doses sufficient to induce a burst-suppression pattern [16]. However, based on the controversies generated by the dissemination of the results of the Cochrane Collaboration on the real effectiveness of this drug [17], our center does not routinely consider using this therapeutic measure.
27.6 Muscle Relaxation
Despite the controversy that still exists in the literature on the use of muscle relaxants in the treatment of patients with severe TBI, it is generally accepted that the first specific maneuver to treat ICH should be muscle relaxation [18-21]. The periodic tracheal aspirations performed on these patients may incur significant elevations in ICP despite the additional administration of sedatives and analgesics. When the patient is given muscle relaxants continuously, these changes in ICP are reduced significantly. As an additional advantage, muscle relaxants reduce elevations in ICP that may occur during habitual movements of the patients (daily hygiene, postural changes, clapping, etc.), and facilitate mechanical ventilation. Despite the fact that the BTF do not include a specific chapter on muscle relaxants in its CPG, in its previous version and in the chapter on the early management of patients, the panel recognized that muscle relaxants facilitate the ventilation and treatment of ICH, although they do not address its use on specific patients [22]. For children, there are no specific recommendations for these drugs [23].
While the use of muscle relaxants during the prehospital phase or hypothermia treatment is unquestionable [24], a continuous infusion of muscle relaxants during hospital treatment has been associated with longer stays in the ICU and a greater dependence on the ventilator, both of which increase rates of septic complication in the patient [19,25]. Other problems associated with the prolonged administration of relaxants are the impossibility of making an appropriate neurological assessment, drug accumulation and the appearance, in some cases, of secondary myopathy [26]. The latter is enhanced by the co-administration of aminoglycosides and corticosteroids [26]. Despite these drawbacks, recent studies have not associated the use of muscle relaxants with a worse patient outcome [24].
Current recommendations on the use of muscle relaxants indicate that they should always be administered simultaneously with sedatives and analgesics. The dose should be the lowest possible amount, with monitoring of its effect using the train of four (TOF – peripheral nerve stimulator), and should be stopped at an early stage [22]. Adequate relaxation should cause a contraction by electrical stimulation. The administration of relaxants should be optimized according to the responses obtained in the TOF [15] (Table 27.2). Of the drugs emerging on the market, vecuronium at a dose of 0.01 to 0.05 mg/kg/hour is recommended because it allows for more hemodynamic stability [15]. Other alternatives continue to be pancuronium or cisatracurium. In the absence of contraindications, preventative measures for deep vein thrombosis and bedsores should be taken [22].
Response* | Cause | Recommendation |
TOF=0 | Excessive muscle relaxation | Dose reduction or temporary suspension of relaxant administration |
TOF=1 | Appropriate level of muscular block | None |
TOF=2-4 | Inadequate level or absence of muscular block | Increase dosage of the drug |
Table 27.2. Monitoring the effectiveness of muscle relaxants using the ”Train of Four” (TOF).
TOF = 0: no muscle response by electrical stimulus; TOF = 1: muscular contraction by electrical stimulus; TOF = 2-4: two to four muscular contractions by electrical stimulus.
27.7 Evacuation of Cerebrospinal Fluid
When ICP increases, there is a physiological tendency toward the displacement of CSF from the cranial cavity to the spinal canal and an increase in elimination, mainly via the venous compartment. This phenomenon is one of the main mechanisms for compensating for increased volumes in a “closed” system like the cranial cavity and explains why patient ICP initially remains stable despite this increase (the “flat” area of the pressure-volume curve). In contrast, CSF formation is relatively constant and independent of ICP values. A slight but noticeable decrease in CSF production is observed only at very high levels of ICP. From a therapeutic standpoint, we can enhance the elimination of CSF by diverting it to outside of the cranio-spinal system using a ventricular or lumbar catheter. The implantation of a ventricular catheter is a routine maneuver in neurocritical patients, but the placement of a lumbar drain should be reserved as an alternative measure in the treatment of ICH, only applicable to certain patients and never in the initial phase of trauma.
Intracranial pressure monitoring via ventricular catheter permits not only the evacuation of CSF but also access to the CSF for sampling and/or drug administration. It is recommended that the catheter is open intermittently if needed, limiting the evacuation to 2-5 ml without exceeding 20 ml/h [15]. The ventricular catheter reservoir should be located about 20 cm from the external auditory canal (EAC), an anatomical reference for locating the foramen of Monro. In this position, CSF drains against a pressure of 20 cm of H2O (15 mmHg). The need to evacuate CSF volumes >20 ml/h should be regarded as a treatment failure and requires an additional therapeutic measure, such as hyperosmolar solutions and/or moderate hyperventilation. Taking into account that the treatment of ICH is additive and not a substitutive, CSF drainage in these patients should not be dispensed with a priori, unless the ventricular system collapses. Ventricular drainage should not be left open permanently because it distorts the ICP readings [27] (Figure 27.3) and subjects the patient to unnecessary risks. The only exception to this rule is in patients who present a dilated ventricular system secondary to trauma. In these cases, post-traumatic hydrocephalus plays a key role in ICH and requires a continuous opening in the system. In this subgroup of patients, it is advisable to measure ICP with a device separate from the ventricular catheter.
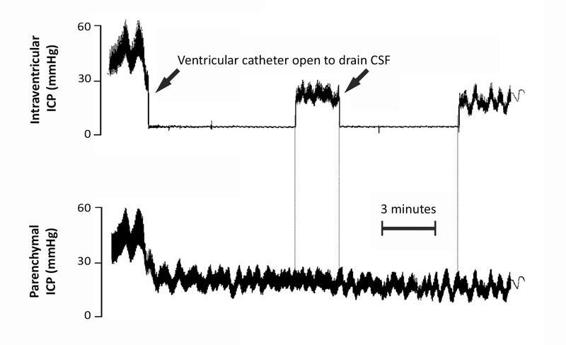
Figure 27.3. Recording of simultaneous intracranial pressure (ICP) monitoring in parenchymal and intraventricular compartments. Observe how when the ventricular catheter remains closed the recordings match. However, when the ventricular catheter is opened to drain CSF, the intraventricular ICP recording is lost.
A) Insertion of the catheter | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



