Topiramate
Edward Faught
Tracy A. Glauser
Introduction
Topiramate (TPM) is a broad-spectrum antiepileptic drug with multiple mechanisms of action. It was originally synthesized as part of a search for fructose-related compounds with hypoglycemic activity.63 It was not developed for that purpose, but was discovered to have antiepileptic activity in animal models in the 1980s. Phase II clinical trials began in 1988 and it was approved for prescription use in the United States in 1996. It is available in over 80 countries.
Chemistry
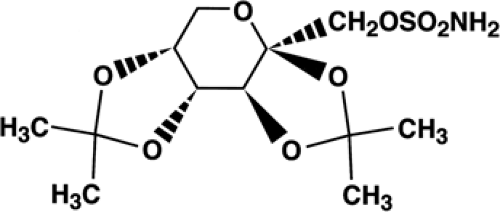
Topiramate(2,3:4,5-bis-O-(1-methylethylidene)-β-D-fructop- yranose sulfamate) is a monosaccharide derived from D-fructose, and is not structurally related to other antiepilepsy drugs (Fig. 1). It exists as a white crystalline powder, has a molecular weight of 339.37 and empirical formula of C12H21NO8S, and is soluble in water and organic solvents at physiologic pH.54
Pharmacology
Activity in Experimental Models of Seizures/Epilepsy
TPM is effective in the rodent maximal electroshock model, inhibiting tonic hind-limb extension;101 this indicates an action against seizure spread. It raises seizure thresholds lowered by pentylenetetrazole and bicuculline, but this effect is less robust.100 TPM is effective in the amygdalar kindling model71,114 and in genetic models including DBA/2J mice, spontaneously epileptic rats,72 genetic absence epilepsy rats, and Wistar rats with audiogenic convulsions.87
Mechanisms of Action
TPM has at least five putative mechanisms for its antiseizure effects, but their relative importance is unknown. The first action described was sodium channel modulation,19 which occurs at therapeutic concentrations and correlates with inhibition of sustained repetitive firing in a voltage- and use-dependent manner.64 It also has a modulatory action against glutamate-mediated excitatory neurotransmission, acting primarily on the kainate receptor type.99 It antagonizes the action of kainate receptors of the GluR5 subtype in cultured rat amygdalar neurons, apparently postsynaptically.42 Furthermore, unlike carbamazepine, it protects against seizures in mice induced by the convulsant substance ATPA, a GluR5 kainate receptor agonist.49 Although TPM also antagonizes the action of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) subtype of glutamate receptor, this effect is less striking than that seen with carbamazepine. TPM has no effect on the N-methyl-D-aspartate (NMDA)-mediated glutamate receptor type, unlike felbamate.19 Modulation of calcium currents may occur via the glutamate receptor effects,83 but direct effects on L-type voltage-sensitive calcium currents have also been described.120
In addition to these antiexcitatory mechanisms, TPM may enhance inhibition by promoting γ-aminobutyric acid (GABA) activity. TPM, like benzodiazepines, increases the frequency of opening of GABAA-mediated chloride channels, but does not bind to the benzodiazepine receptor site on the postsynaptic membrane.116,117 It appears to increase GABA content in human brain as measured by magnetic resonance spectroscopy in vivo,55,82 but how this may occur is uncertain. TPM may further enhance inhibition by increasing potassium channel conductance, as observed in rat neurons.47 Finally, TPM is a carbonic anhydrase inhibitor, but this effect is much weaker than that of acetazolamide and may contribute little to its efficacy.21
Clinical Pharmacokinetics
Absorption
Across all age groups, TPM has linear pharmacokinetics. In adults, after administration of single, oral doses ranging from 100 to 1,200 mg, TPM was rapidly absorbed from the gastrointestinal tract.23 Estimated bioavailability is approximately 80%.75,113 TPM’s absorption is not significantly affected by food.23
Plasma Protein Binding and Distribution
Topiramate is not highly bound to plasma proteins (9% to 17%).81 The volume of distribution in healthy adult volunteers was 0.6 to 0.8 L/kg, consistent with a distribution into total body water.26 Ninety percent of the maximal plasma concentration (Cmax) was achieved within 2 hours (range 1.4 to 4.3 hours) after oral administration.23 Mean values for Cmax and area under the concentration-time curve (AUC), reflections of drug absorption and clearance, increased linearly with respect to dose in adults.48 Steady state is achieved in about 4 days when renal function is normal.113
Metabolism and Elimination
In monotherapy, TPM is not extensively metabolized.48 Six trace metabolites of TPM, formed by hydroxylation,
hydrolysis, and glucuronidation, have been identified;118 none exhibits significant antiepileptic activity.48,118 In adults, the elimination half-life (t1/2) values for TPM when used as monotherapy range from 19 to 25 hours.95
hydrolysis, and glucuronidation, have been identified;118 none exhibits significant antiepileptic activity.48,118 In adults, the elimination half-life (t1/2) values for TPM when used as monotherapy range from 19 to 25 hours.95
Renal excretion is a major route of TPM elimination.118 In adults, estimates are that at least 51% of the dose is excreted by the kidney within the dose range of 200 to 1,200 mg.23 The renal clearance of TPM is much lower than its glomerular filtration rate,23 suggesting that it may undergo tubular reabsorption. A twofold increase in AUC and t1/2 occurs with renal failure (creatinine clearance <30 mL/min/1.73 m2),35 implying that patients with renal impairment may require reduced dosages. During hemodialysis, TPM is cleared from plasma roughly nine times faster than in patients with normal renal function, implying that patients may need additional doses after hemodialysis.34 Although moderate/severe liver impairment increased TPM’s half-life and lowered its oral clearance, there was not a clinically significant increase in plasma TPM concentration following a single 100-mg oral dose in adults.24
TPM pharmacokinetics in children and adolescents was determined from a single-center, open-label outpatient trial of 18 patients with epilepsy.89 Patients aged 4 to 17 years received oral adjunctive TPM therapy up to a target dose of 9 mg/kg/day. Plasma clearance was not affected by TPM dose. Compared to adults, clearance values were 54% greater in children when TPM was administered in the presence of enzyme-inducing drugs and 44% greater in the absence of enzyme-inducing drugs.89
Two studies have examined TPM pharmacokinetics in young children. In a study of five children (2 to 2.5 years old), mean plasma clearance was slightly higher than that reported for children and adolescents and higher in infants on concomitant enzyme-inducing antiepileptic drugs (AEDs) than in those on non–enzyme-inducing concomitant AEDs.38 A more recent study of 22 children (6 months to 4 years) found TPM oral clearance significantly higher in infants and young children on concomitant enzyme-inducing AEDs (85.4 ± 34.0 mL/hr/kg) compared with those taking valproic acid (VPA; 49.6 ± 13.6 mL/hr/kg) or non–enzyme-inducing AEDs (46.5 ± 12.8 mL/hr/kg).68
Plasma Concentrations
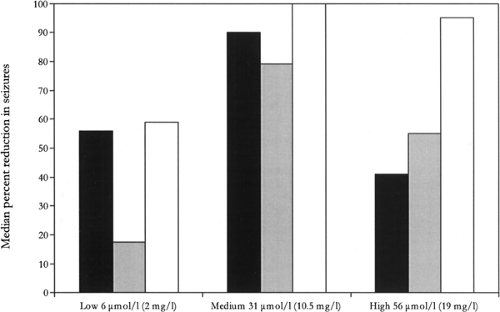
TPM plasma concentrations can be obtained through most large clinical laboratories. A randomized, concentration-controlled trial of topiramate in adults with treatment-resistant partial epilepsy demonstrated that optimal treatment response is most likely to occur at plasma concentrations ranging of about 10.5 μg/mL (Fig. 2). Another study found that for older children (6 to 12 years) on TPM monotherapy, serum concentrations ranged from 1.5 to 20.4 μg/mL, while children aged
5 and younger had higher TPM serum concentrations (3.2 to 28.8 μg/mL).98
5 and younger had higher TPM serum concentrations (3.2 to 28.8 μg/mL).98
Efficacy in Adults and Children
Partial-onset Seizures in Adults: Adjunctive Therapy
Results from eight multicenter, randomized, placebo-controlled trials of TPM as adjunctive therapy for refractory partial-onset seizures in adults have documented efficacy at all dosages of 200 mg/day or more.10,29,41,43,84,102,106,119 In early trials, the minimum effective daily dose was found to be 200 mg/day,29 with 400 mg/day being the most effective dose in intent-to-treat analyses.8,29 Doses from 600 to 1,000 mg/day conferred no additional benefit but increased the rate of adverse effects.8,29,84 Later clinical trials featured a slower titration rate41,43 or lower target dose.43,119 Good results from these later studies have led to recommendations for an adjunctive-therapy initial target dose for adults of 200 to 400 mg/day, achieved by weekly 25- to 50-mg/day upward dose titration. Nearly all patients in these studies had TPM added to enzyme-inducing drugs; in their absence, lower doses of TPM may result in similar serum levels.
Partial-onset Seizures in Children: Adjunctive Therapy
A multicenter, double-blind, randomized, placebo-controlled trial demonstrated the efficacy of adjunctive TPM in children between 1 and 16 years with refractory partial-onset seizures with or without secondary generalization.27 An 8-week baseline was followed by a 16-week double-blind treatment phase (8-week titration period followed by an 8-week stabilization period) with a weight-based target dose of 6 mg/kg/day. Forty-one TPM-treated and 45 placebo-treated children aged 2 to 16 years completed treatment. For partial-onset seizures, the median percent reduction from baseline in the average monthly seizure rate in the TPM-treated group was 33% compared to 11% in the placebo group (p = 0.034).27
Partial and Generalized-onset Seizures: Monotherapy
Four monotherapy trials have been conducted.32,33,34,35 In the first, adult outpatients with refractory partial-onset seizures were converted gradually from one or two standard drugs to either 100 mg/day or 1,000 mg/day TPM.94 Stopping rules for patient safety were used, such as a doubling of the baseline seizure frequency. Success, defined as conversion without invoking a stopping rule, was more common with the high dose, and 13% of the high-dose patients but no low-dose patients became seizure free.
Two randomized, controlled multicenter trials with a similar design explored lower TPM dosages in recently diagnosed epilepsy in children and adults.5,33 As in most new-onset trials, patients with both partial-onset seizures and generalized-onset tonic–clonic seizures were included. In the first trial, 252 patients 3 years of age or older with epilepsy for <3 years took either a low dose (25 mg/day if weight <50 kg, 50 mg/day if weight >50 kg) or a high dose (200 mg if weight <50 kg, 500 mg if weight >50 kg).33 Patients were either on no medication at baseline (56%) or on one drug, which was withdrawn within 6 weeks. Although the primary end-point, time to second seizure, was not statistically significant between these doses, when the time to first seizure was added as a covariate, the high dose proved superior (p = 0.01). Fifty-four percent of high-dose and 39% of low-dose patients were seizure free during the 6-month trial (p = 0.02). In the second multinational study, patients 6 years of age or older with untreated epilepsy for <3 months took either 50 mg/day or 400 mg/day.5 The primary end-point, time to first seizure, favored the high dose. Seizure-free rates at 12 months were 76% and 59% for the high and low doses, respectively (p = 0.001).5
Lower doses, 100 and 200 mg/day, were used in a fourth study of new-onset seizures.85 For each patient, physicians were given their choice of either valproate 1,200 mg/day or carbamazepine 600 mg/day as the active control agent. Patients were then randomized to TPM 100 mg/day, TPM 200 mg/day, or the control drug. Seizure control was statistically equal among all patient groups. As expected, physicians usually chose valproate for suspected generalized-onset seizures and carbamazepine for suspected partial-onset seizures, but in both groups the TPM efficacy was equal to that of the control agent. Because 100 mg/day TPM proved just as effective as 200 mg/day, the authors suggested 100 mg/day as an initial target dose for patients with new-onset seizures. In the subset of pediatric partial-onset seizure patients, the time to first seizure was similar for patients in the TPM, carbamazepine, or valproate arms, as were the proportions of seizure-free patients during the last 6 months of treatment.115
Generalized Tonic–Clonic Seizures of Nonfocal Origin
The efficacy of TPM for children and adults with uncontrolled generalized tonic–clonic (GTC) seizures of nonfocal origin was examined in a double-blind, placebo-controlled, multicenter study.14 Patients had to be at least 4 years old, have had at least three GTC seizures during an 8-week baseline phase, have EEG findings consistent with generalized epilepsy, and be taking no more than two standard AEDs. Patients were randomized to either TPM or placebo adjunctive treatment, titrated to target dosages of 6 mg/kg/day over 8 weeks, and maintained at that dose for 12 weeks.
Eighty patients (3 to 59 years) were randomized to either TPM or placebo adjunctive therapy. The median baseline average monthly rate of GTC seizures was 5.0 in the TPM group and 4.5 in the placebo group. The mean TPM dosage during the stabilization period was 5.0 mg/kg/day. At the end of the 20-week double-blind phase, the median percent reduction from baseline in the average monthly GTC seizure rate was 57% for the TPM group compared with 9% for the placebo group (p = 0.019). A larger percentage of the TPM-treated patients experienced a ≥50% reduction in GTC seizures compared with the placebo-treated controls (56% vs. 20%; p = 0.001).
Lennox-Gastaut Syndrome
A multicenter, double-blind, placebo-controlled trial established TPM’s efficacy as adjunctive therapy in patients with Lennox-Gastaut syndrome.93 Patients had to have active drop attacks (either tonic or atonic seizures), a history of or active atypical absence seizures, and a prior EEG showing a slow spike-and-wave pattern, and be taking one to two concomitant AEDs. After a 4-week baseline, they were randomized to either TPM or placebo adjunctive therapy. TPM dosage was increased at weekly intervals over 3 weeks to 6 mg/kg/day and then maintained at that dose for 8 weeks.
Ninety-eight patients, aged 2 to 42 years (mean age 11), were randomized. The median monthly drop attack frequency during the baseline period was 90 in the TPM group and 98 in the placebo group; the median monthly frequency of all seizure types was 267 in the TPM group and 244 in the placebo group. The median average TPM dosage during the stabilization period was 5.8 mg/kg/day.93
Patients in the TPM group had a greater median percent reduction from baseline in drop attacks (15%) compared to the placebo group (–5%; p = 0.041). TPM-treated patients were nearly twice as likely to show an improvement in seizure severity as compared with controls (52% vs. 28%; p = 0.040) based on parental global evaluations.93
Infantile Spasms (West Syndrome)
At least eight open-label trials have examined efficacy in infantile spasms.3,36,40,46,68,108,111,112 A pilot study of 11 patients with infantile spasms documented by 24-hour video-EEG utilized an initial dose of 25 mg TPM, followed by a “rapid-dose” titration schedule of 25-mg increments every 2 to 3 days over a 4-week period until either a maximal tolerated dose was reached, spasms were controlled, or a maximal dose of 24 mg/kg/day was achieved. Five patients (45%) became spasm free with absence of infantile spasms and hypsarrhythmia proven by repeat 24-hour video-EEG.36 Seven of the 11 patients (64%) were able to achieve TPM monotherapy, while the others were able to reduce their intake of concomitant AEDs.36
In a retrospective study of TPM in patients with intractable epilepsy, four of seven infants with West syndrome became seizure free with TPM therapy.108 Another study reported the efficacy of TPM in 13 infants with West syndrome as part of a multicenter study of 224 unselected patients with a variety of epilepsy types. Seven of the 13 (54%) West syndrome infants had a >50% reduction of their seizures and two (15%) became seizure free. Nine of the patients used TPM as initial monotherapy with doses up to 16 mg/kg/day.46 An open-label, multicenter chart review study of 28 infants treated with TPM included eight infants with West syndrome. Almost all (seven of eight, 88%) of the patients with infantile spasms improved with TPM therapy and three patients were treated with TPM monotherapy.112 An open, prospective, pragmatic study of TPM in 59 infants with epilepsy included 19 patients with West syndrome. Four of the six patients with cryptogenic West syndrome became seizure free after a median follow-up of 14 months. Among 13 patients with symptomatic West syndrome, four patients were classified as responders but only 1 of 13 (8%) became seizure free.40 In contrast, one study of 18 patients with spasms (14 infantile, four late onset) found six responders but none seizure free.68 A prospective open-label trial of TPM in 47 children (6 to 60 months old) with refractory epilepsy included nine patients with infantile spasms. Two of the nine patients became seizure free and another two experienced a >50% reduction in seizure frequency.3 A retrospective review of TPM use in 13 children younger than 2 years old reported four infants with infantile spasms; following initiation of TPM therapy two became spasm free, one had a >75% reduction in spasms, and the other had no response to TPM.111
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree