Figure 63.1. Topiramate (2,3:4,5-di-O-isopropylidene-β-d– fructopyranose sulfamate).
MECHANISMS OF ACTION
TPM has a unique combination of activities at various receptor sites and ion channels, which may account for its broad-spectrum profile in epilepsy and other neurologic disorders. It blocks the kainate/AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) subtype of the glutamate receptor (2–5), with no direct effect on NMDA (N-methyl- d-aspartate) receptor activity; blocks voltage-activated sodium channels to limit sustained repetitive firing (6–10); enhances α-aminobutyric acid (GABA)–mediated chloride flux at GABAA receptors (11,12); reduces the amplitude of high-voltage–activated calcium currents (13,14); and activates potassium conductance (15,16). It has been hypothesized that effects of TPM on voltage-activated sodium channels, high-voltage–activated calcium channels, GABAA receptors, and AMPA/kainate receptors reflect a common modulator involving protein phosphorylation (17). TPM is also a weak inhibitor of carbonic anhydrase isoenzymes (CA II and CA IV), which may modulate pH-dependent activation of voltage- and receptor-gated ion channels (18); its inhibitory effect is less than acetazolamide.
ANIMAL MODELS
The anticonvulsant properties of TPM have been demonstrated in several animal models of epilepsy. TPM exhibited potent and long-lasting anticonvulsant activity when evaluated using the MES test in rodents with a median effective dose of 47.6 mg/kg in the mouse and 15.8 mg/kg in the rat (19). TPM inhibited chronic motor seizures and absence-like seizures when administered intraperitoneally (17). TPM blocked sound-induced clonic and tonic–clonic seizures (20). TPM effectively inhibited tonic, clonic, and wild running seizures in a postischemia model of epilepsy in rats, and its potency was similar to that of phenytoin for all three seizure types (21). TPM also produced a dose-related inhibition of amygdala-kindled seizures in rats (22). Experimental studies have shown that TPM reduced seizure-induced hippocampal neuronal injury (23) and prevented spontaneous seizures following status epilepticus (24). In an experimental model of neonatal hypoxia/ischemia, TPM suppressed acute seizures and reduced subsequent susceptibility to neuronal injury and seizures induced by a second insult (kainate) (25).
PHARMACOKINETICS
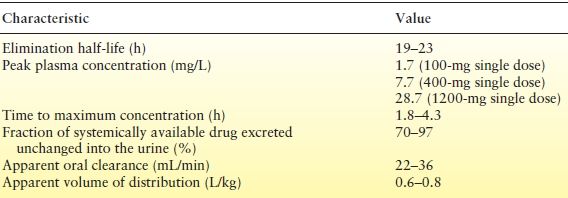
Renal elimination, low protein binding, and a long half-life make TPM relatively easy to manage from a pharmacokinetic perspective (Table 63.1) (26,27).
Table 63.1 Pharmacokinetic Characteristics of TPM
Absorption
TPM is rapidly absorbed with peak plasma concentrations occurring in 1 to 4 hours with TPM doses of 100 to 400 mg. Absorption is nearly complete with <80% of a 100-mg dose recovered in urine. Coadministration with food slightly delays absorption but does not decrease bioavailability (28). TPM exhibits linear kinetics; plasma concentrations increase in proportion to dose increases (29).
Distribution and Protein Binding
The apparent volume of distribution for TPM is 38.5 to 58 L (0.6 to 0.8 L/kg, weight normalized), consistent with distribution to total body water. Binding to plasma proteins is minimal (13% to 17%) and is not considered to be a major factor in dosing and drug interactions (29).
Metabolism and Excretion
In the absence of hepatic enzyme induction, approximately 20% of a TPM dose is metabolized. When TPM is coadministered with enzyme-inducing antiepileptic drugs (AEDs), up to 50% of the TPM dose may be metabolized. Hepatic metabolism appears to involve hydroxylation, hydrolysis, and glucuronidation; none of the metabolites constitutes >5% of an administered dose, and they are quickly cleared (29).
Elimination of TPM is primarily via renal excretion, with 50% to 80% being eliminated in the urine unchanged. The half-life of TPM in adults is 20 to 30 hours in the absence of enzyme induction, allowing steady-state plasma concentrations to be reached in 4 to 8 days. In the presence of enzyme induction, the TPM half-life in adults is 12 to 15 hours (29). In children 4 to 17 years of age, clearance is approximately 50% higher than in adults (30). Steady-state concentrations for the same mg/kg dose were correspondingly lower in children than in adults. Consistent with the higher clearance, the calculated half-life of TPM in children is approximately 15 hours without enzyme induction and 7.5 hours with enzyme induction. In young children (younger than 4 years old), clearance rates were the same or slightly higher than in older children (31). In elderly patients (65 to 85 years of age), clearance decreases only to the extent that renal function itself is reduced by age; age alone does not alter clearance in adults (32).
TPM clearance is reduced by 40% to 50% in patients with moderate (creatinine clearance 30 to 69 mL/min) or severe (creatinine clearance, <30 mL/min) renal impairment compared to subjects with normal renal function (creatinine clearance, >70 mL/min) (29). One-half of the usual TPM dose is recommended in patients with moderate or severe renal impairment. TPM plasma concentrations fell by an average of 50.1% during hemodialysis. The mean hemodialysis plasma clearance of TPM has been reported to be approximately nine times higher than that found in subjects not receiving hemodialysis (33). Modest decreases in TPM clearance have been reported when comparing age- and sex-matched healthy controls to individuals with moderate to severe hepatic impairment; mean clearance was decreased to 26% (31.8 vs. 23.5 mL/minute) and half-life increased to 36% (25 vs. 34 hours), with parallel increases in plasma concentrations (29).
THERAPEUTIC DRUG MONITORING
Drug monitoring is of relatively little importance in initial titration of TPM. It is most important for compliance and also if utilizing higher-dose therapy. Steady-state plasma concentrations of TPM are generally linear, with dose- proportional increases in plasma concentration (29). Mean plasma concentrations achieved during maintenance in randomized, controlled trials of TPM monotherapy were as follows: 50 mg/day, 1.6 and 1.9 μg/mL; 97 mg/day, 3.8 μg/mL; 189 mg/day, 6.4 μg/mL; 313 mg/day, 11.7 μg/mL; and 367 mg/day, 12.4 μg/mL (34). Studies of TPM as monotherapy have provided the opportunity to examine the relationship between TPM plasma levels and clinical response. In a study comparing 50 and 500 mg/day TPM as monotherapy, plasma concentrations >9.91 μg/mL were associated with better seizure control compared with plasma concentrations of 1.77 to 9.91 μg/mL and ≤1.76 μg/mL (35). However, because of the intraindividual variations in blood levels associated with seizure control and side effects, a traditional “therapeutic range” cannot be identified. As expected, plasma concentrations are higher when TPM is administered as monotherapy (6.4 to 12.4 μg/mL with approximately 200 to 400 mg/day) versus its use as add-on to enzyme-inducing AEDs (1.4 to 5.3 μg/mL with approximately 200 to 400 mg/day). Despite the substantially higher plasma concentration with monotherapy, the incidence of central nervous system (CNS)-related adverse events, particularly cognitive effects, was substantially lower with TPM monotherapy than with adjunctive therapy. This finding underscores the contribution of pharmacodynamic interactions to the occurrence of adverse events during TPM polytherapy and the limited benefit of therapeutic drug monitoring in TPM-treated patients.
The relationship between TPM dose and plasma level was examined in children in whom TPM was titrated to clinical response or side effects (36). Among 21 children aged 6 to 12 years, TPM plasma levels were predictably related to dose (1:1 ratio). With monotherapy, a mean dose of 9.7 mg/kg/day (range, 5.5 to 16.5 mg/kg/day) resulted in a mean plasma level of 9.8 μg/mL (range, 3.4 to 16.6 μg/mL). For 20 younger children (younger than 6 years of age), however, higher monotherapy doses were needed (mean, 22.5 mg/kg/day; range, 11 to 35 mg/kg/day) to achieve seizure control; mean plasma level was 14.8 μg/mL (range, 6.1 to 23.7 μg/mL). When TPM was administered with an enzyme-inducing drug, the TPM dosage in younger children (mean, 14.2 mg/kg/day) was double than that in older children (7.0 mg/kg/day) (36).
Patients with levels close to 25 μg/mL or more rarely obtained additional benefit at higher dosages and side effects increased. Therapeutic ranges are often quoted in the 2 to 25 μg/mL range. It is this author’s opinion that there are two ranges. Monotherapy patients who are relatively easy to control can often be controlled in the 2 to 6 μg/mL range, and those who are more intractable may need higher doses.
DRUG INTERACTIONS
Interaction studies were performed with the three leading AEDs (at time of initial release of TPM)—carbamazepine, phenytoin, and valproic acid and also later with lamotrigine. Similar study designs were utilized.
Topiramate and Carbamazepine
The steady-state pharmacokinetics of carbamazepine and TPM as adjunctive therapy and monotherapy were determined in 12 adults whose epilepsy was stabilized with carbamazepine 300 to 800 mg t.i.d. (37). No significant differences were observed in the pharmacokinetics of total or unbound carbamazepine or carbamazepine epoxide in the absence of TPM or with TPM 100 to 400 mg b.i.d. TPM AUC, Cmax, average concentration, and minimum concentration levels were approximately 40% lower in the presence of carbamazepine than with TPM monotherapy (27).
Topiramate and Phenytoin
The steady-state pharmacokinetics of phenytoin and TPM were determined in 12 adults with partial epilepsy who were stabilized on phenytoin (38). During concomitant phenytoin therapy, TPM pharmacokinetics were proportional for dosages ranging from 100 to 400 mg. During TPM adjunctive therapy with phenytoin, TPM concentrations were reduced by approximately 50%. The investigators hypothesized that the increase in TPM clearance during concomitant phenytoin therapy was due to enzyme induction by phenytoin. In half the patients, TPM had no measurable effect on the pharmacokinetics of phenytoin; in the other patients, however, particularly those taking phenytoin twice a day, phenytoin concentrations were approximately 25% higher. No patients required adjustment of phenytoin or discontinued the trial. In clinical practice, patients receiving dosages in the higher therapeutic ranges of phenytoin should be observed carefully, because they may be more likely to require a downward adjustment of phenytoin dosage (27).
Topiramate and Valproic Acid
The steady-state pharmacokinetics of valproic acid and TPM were determined in 12 patients whose partial epilepsy was treated with valproic acid (39). TPM plasma concentrations were approximately 14% lower during adjunctive therapy with valproic acid. Valproic acid concentrations decrease by 11% when TPM 400 mg b.i.d. was added. The clinical significance of these changes is probably minimal (27).
Topiramate and Lamotrigine
An open-label, sequential, single-group, dose-escalating, PK study was performed in 13 patients with epilepsy. No PK interactions were noted between TPM and lamotrigine at observed doses of 100 to 400 mg/day TPM (40).
Topiramate and Oral Contraceptives
Interaction studies evaluating the effect of TPM on combination oral contraceptives showed that TPM has no effect on the progestin (norethindrone 1.0 mg) component (41,42). At doses of ≤200 mg/day, TPM has no significant effect on estrogen (ethinyl estradiol 35 μg) concentrations (41,42). Initial studies showed the mean serum estradiol to be reduced by 18% at 200 mg/day, but repeat testing at the same 200 mg dosage showed only an 11% decrease. At higher doses (400 and 800 mg/day), TPM was associated with 21% and 30% reductions, respectively, in ethinyl estradiol concentrations, suggesting a modest induction of estrogen clearance (42). The level of induction is substantially less than that associated with potent enzyme-inducing agents such as carbamazepine (42% reduction in estrogen concentration) (41). The dose- related effect of TPM on estrogen clearance is consistent with the concentration-dependent induction of cytochrome P450 (CYP450) CYP3A4 activity measured in vitro (43). TPM induced CYP3A4 enzymes only at concentrations >50 μM, a concentration that is unlikely to be achieved with dosages up to 400 mg/day; enzyme induction was still less than that associated with known inducers (phenobarbital and rifampicin) used in this study.
Predominantly, renal elimination and low protein binding minimize the potential for drug interactions. Pharmacokinetic interactions between TPM and other AEDs are limited primarily to the effects of enzyme-inducing drugs on TPM. TPM plasma levels are approximately 50% lower when TPM is given with an enzyme-inducing AED (37–45) compared to TPM use alone or in combination with non–enzyme- inducing drugs (37–39,39–45). The addition of TPM does not significantly affect plasma concentrations of carbamazepine (37), valproate (39), phenobarbital/primidone (44), or lamotrigine (40). However, phenytoin plasma levels may be increased as much as 25% in some patients, particularly those in whom phenytoin metabolism may be at or near saturation (45). Studies of TPM in models designed to predict drug interactions related to the CYP450 enzyme system have shown inhibition of only the CYP2C19 isozyme, which may account for the potential interaction with phenytoin (46). Although pharmacokinetic interactions between TPM and other AEDs are limited, the lower incidence of adverse effects with TPM monotherapy (35,47,48) suggests that pharmacodynamic interactions may affect tolerability when TPM is added to existing therapy.
A slight decrease in digoxin clearance has been observed with the addition of TPM (49) but generally does not require dosage adjustments. Changes in metformin pharmacokinetics suggest that diabetic control should be monitored when TPM is added or withdrawn (50).
EFFICACY
Adjunctive Therapy
Partial-Onset Seizures
The effectiveness of TPM as adjunctive therapy across a wide range of doses (200 to 1000 mg/day) in adults with refractory partial-onset seizures has been well documented in randomized, double-blind, placebo-controlled trials (51–59). Similarity of trial design and patient populations allowed pooled analysis of data from six of these trials (51–56). Among 743 adults (median baseline frequency, 12 seizures per month), median seizure reduction was 44% with TPM treatment versus 2% with placebo (P ≤ 0.001); 43% of TPM-related patients (placebo, 12%; P ≤ 0.001) achieved at least 50% seizure reduction (60). During 11 to 19 weeks of double-blind treatment, 5% of patients in the TPM group were seizure free, while no patients in the placebo group were seizure free (P ≤ 0.001) (60). Initially, it was felt that dosages of 200 mg/day would be placebo-like, and therefore, 79% of the original patients were at dosages of 400 to 1000 mg/day. On initial review of the data, it appeared that there was a flattening of the efficacy curve at higher dosages. However, one must remember that this was intent to treat data. If a patient due to side effects did not make it to his assigned upper dosage (even if seizure free or significantly reduced in seizure frequency), the patient was considered as not succeeding at that dosage. Therefore, from an efficacy point of view, there was a dose–response curve. Although dosages as high as 1000 mg/day were evaluated, the most clinically useful adjunctive therapy dosages appear to be 200 to 400 mg/day. In a 12-week, double-blind trial to further evaluate the lower end of the presumed dosing range (59), 200 mg/day TPM was added to carbamazepine. Median seizure reduction in TPM-treated patients (N = 168) was 44% (vs. 20% with placebo, N = 91; P < 0.001); 45% of TPM-treated patients (placebo, 24%; P = 0.001) achieved at least 50% seizure reduction. After 2 weeks, median seizure reduction in patients receiving TPM 100 mg/day (N = 84) was 60% (placebo, 17%; P < 0.001), which suggests that 100 mg/day may be a target dose at which seizure control should be initially evaluated.
The initial overestimation of TPM dosage needs is evident from prospective, in-practice studies in which adults with refractory partial-onset seizures achieved good seizure control with 264 mg/day (48% of patients had 50% or more seizure reduction rate; 9% were seizure free) (61) and 323 mg/day (68% of patients had a 50% or more seizure reduction rate) (62). When titrating to response, patients with fewer baseline seizures (>4 per month) required lower TPM dosages (303 mg/day) than those with higher baseline seizure frequency (341 mg/day in patients with four or more seizures per month) (62). In a prospective study, 17% of refractory patients had at least 50% seizure reduction and 8% were seizure free with TPM dosages of 100 or less mg/day (63).
In treatment-resistant epilepsy patients treated at a tertiary epilepsy center, estimated long-term retention rates among 393 TPM-treated patients were 52% after 1 year, 42% at 2 years, 30% at 3 years, and 28% at 5 years (64,65). Although these rates were higher than those with another new-generation agent (lamotrigine), the low retention rate at 5 years reflects the limitations of medical therapy in patients with refractory epilepsy.
TPM was evaluated as adjunctive therapy in 86 children (2 to 16 years of age) with refractory partial-onset seizures (66). With a mean daily dose of 6 mg/kg (target dose, 5 to 9 mg/kg/day), median seizure reduction was 33% (placebo, 11%; P = 0.03). More TPM-treated children had at least 50% reduction in seizures (39% vs. 20% with placebo; P = 0.08); 5% of children receiving TPM had no seizures, while no placebo-treated children were seizure free.
All 83 children completing the double-blind phase entered the long-term, open-label extension in which the dosages of TPM and concomitant AEDs could be adjusted according to clinical response (67). Mean treatment duration was 15 months, with some children being treated as long as 2.5 years; the mean TPM dosage was 9 mg/kg/day (range, 4 to 22 mg/kg/day). Among children treated for at least 6 months, 64% had at least a 50% reduction in seizures; 14% were seizure free for a minimum of 6 months. During open-label in-practice studies in children with refractory partial-onset seizures (68–71), 4% to 20% of TPM-treated children were seizure free during treatment periods as long as 33 months.
Lennox–Gastaut Syndrome
TPM was evaluated as adjunctive therapy in 98 patients with Lennox–Gastaut syndrome confirmed by an electroencephalographic (EEG) pattern of slow spike-and-wave, multiple seizure types, including drop attacks, and a history of atypical absence episodes (72). At a maximum dose of 6 mg/kg/day, median reduction for drop attacks was 15% compared with a 5% increase with placebo; 28% of TPM-treated patients were responders (placebo 14%). A combined measure of drop attacks and tonic–clonic seizures showed a 26% reduction with TPM and a 5% increase with placebo (P = 0.015); respective responder rates were 33% and 8% (P = 0.002). These outcomes compared favorably with those reported for lamotrigine in this population (73). The placebo-adjusted responder rate for drop attacks was 14% for TPM and 15% for lamotrigine; respective rates for major motor seizures were 25% and 17% (72,73).
During the long-term, open-label extension in which the dosages of TPM and concomitant AEDs could be adjusted according to clinical response (74), 55% of the 82 children treated with TPM for more than 6 months had at least a 50% reduction in drop attacks during the last 6 months of treatment; 15% experienced no drop attacks. Two patients were free of all seizures. The mean duration of TPM treatment was 18 months, with treatment periods as long as 3.4 years. The mean TPM dosage was 10 mg/kg/day (range, 1 to 29 mg/kg/day). Among patients treated as long as 8 years, 21% to 40% of patients had at least 50% seizure reduction, with major motor seizures being the most responsive (75,76).
Generalized Tonic–Clonic Seizures of Nonfocal Origin
Two double-blind, placebo-controlled trials (77,78) evaluated TPM in the treatment of generalized, nonfocal tonic–clonic seizures (i.e., primary generalized tonic–clonic seizures). Inclusion criteria specified tonic–clonic seizures with or without other generalized seizure types and EEG or CCTV/EEG patterns consistent with generalized epilepsy (generalized, symmetric, synchronous spike–wave discharges, and normal background activity); patients with Lennox–Gastaut syndrome or partial-onset seizures were excluded. In the two trials, more than 70% of patients had primary generalized tonic–clonic seizures plus at least one other type of generalized seizure (i.e., absence, myoclonic, or tonic).
TPM was initiated as adjunctive therapy in adults and children (at least 4 years of age) with refractory generalized tonic–clonic seizures despite treatment with one or two AEDs. The target dose was 5 to 9 mg/kg/day, and the maximum daily dose was 400 mg. In one trial (77), baseline seizure frequency in the TPM-treated group (N = 39) was five generalized tonic–clonic seizures per month (placebo 4.5 generalized tonic–clonic seizures per month; N = 41). Median seizure reduction was 57% (placebo 9%; P < 0.02) for tonic–clonic seizures and 42% (placebo, 1%; P = 0.003) for all generalized seizures. Among TPM-treated patients, generalized tonic–clonic seizures and all generalized seizures were reduced at least 50% in 56% and 46%, respectively (respective placebo values: 20%, P = 0.001; 17%, P = 0.003). No generalized tonic–clonic seizures occurred during the 20-week study in 13% of TPM-treated patients (placebo 5%); 5% had no generalized seizures of any type (placebo 0% of patients).
Because the two trials were identically designed, data were pooled and analyzed. As had been observed in the single trial, TPM reduced the frequency of generalized tonic–clonic and all generalized seizures, with significantly more patients achieving 50% or greater reduction in generalized tonic–clonic (55% vs. 28% with placebo; P ≤ 0.001) and all generalized seizures (43% vs. 19% with placebo; P = 0.001). Although small sample sizes limited analysis, TPM was also more effective than placebo in reducing the frequency of tonic and myoclonic seizures and did not exacerbate absence seizures.
All 131 patients who completed the double-blind phase entered an open-label extension phase (79). During the last 6 months of treatment, 16% had no generalized tonic–clonic seizures and 7% were seizure free for at least 6 months. TPM was also effective against other generalized seizure types; during the last 6 months of treatment, 10% of patients with absence seizures, 33% of patients with myoclonic seizures, and 21% of patients with tonic seizures were seizure free for at least 6 months.
In a study evaluating EEG changes and seizure control in TPM-treated patients with primary generalized epilepsies (80), more than half of the patients showed reductions in epileptiform spike-wave activity, although TPM was less likely to suppress activity in patients with very high discharge frequencies at baseline. As with other broad-spectrum AEDs, seizure reduction (36% seizure free) did not correlate with EEG response, and no correlation was observed between clinical or EEG response and TPM blood levels.
Juvenile Myoclonic Epilepsy
In a pilot study, 15 patients who had previously failed valproic acid (one wanted off strictly due to weight gain and 5 wished to get off due to weight issues) were switched from valproic acid to TPM. Myoclonic seizures stopped in 60% of patients, 49% of generalized tonic–clonic seizure patients became seizure free of that type of seizure, and 25% of absence seizure patients stopped having this type of seizure (81).
A small subset of patients with juvenile myoclonic epilepsy (JME) was included in the controlled trials evaluating TPM in primary generalized tonic–clonic seizures (77,78). Among 11 patients with JME receiving TPM, primary generalized tonic–clonic seizures were reduced at least 50% in 73% (vs. 18% of patients receiving placebo, N = 11; P = 0.03) (82). In addition, the frequency of myoclonic seizures was reduced, and the number of weeks without absence seizures was increased in TPM-treated patients. In a randomized, open-label study in patients with JME (83), TPM and valproate were similarly effective (seizure-free rates following 12 weeks’ treatment: 47% and 33%, respectively). The treatment groups were similar in neurotoxicity scores; however, TPM was associated with less systemic toxicity than valproate.
West Syndrome
Eleven children with refractory West syndrome participated in a pilot study of TPM (84). At a maximum daily dose of 24 mg/kg, the frequency of infantile spasms was reduced by at least 50% in nine children, including five (45%) who were completely controlled. Ancillary seizures responded in four of six children. After 18 months of TPM (mean dosage, 29 mg/kg/day), eight children (73%) continued on medication, four (50%) children were free of spasms, and seven (88%) children had spasms reduced by at least one-half (85).
Childhood Absence Epilepsy
Five children 4 to 11 years of age with EEG-documented absence seizures and childhood absence epilepsy were treated with open-label TPM (maximum dose, 12 mg/kg/day) (86). Three children experienced a minimum reduction of 50% at daily dosages of 5 to 6 mg/kg; two children were seizure free. Frequency was unchanged in the remaining two children, even at the maximum dosage.
Severe Myoclonic Epilepsy in Infancy
During a prospective, multicenter, open-label study in 18 patients with severe myoclonic epilepsy in infancy and refractory seizures of different types, three patients became seizure free, six patients had >75% seizure reduction, and four patients had >50% seizure reduction with TPM treatment (87). Seizure frequency was unchanged in five patients; no patients experienced seizure worsening. Mean treatment duration was 12 months (range, 2 to 24 months); mean TPM dose was 5.4 mg/kg/day (range, 2.8 to 10 mg/kg/day).
Patients with Mental Retardation, Learning Disabilities, and/or Developmental Disabilities
Among 64 patients (16 to 65 years of age) with refractory epilepsy and learning disability treated with TPM in an open- label study, 16 patients became seizure free and 29 patients had at least a 50% seizure reduction (88). Many patients, including 63% of those who were seizure free and 66% of treatment responders, were receiving TPM dosages of ≤200 mg/day. In a study evaluating the effect of TPM in 20 adults (21 to 57 years of age) with intractable mixed seizures, mental retardation, and development disabilities, two patients became seizure free and 11 patients had at least a 50% seizure reduction with TPM treatment (89). In addition, the duration and/or severity of seizures were reduced in 44% of patients. The mean duration of treatment was 42 weeks (range, 20 to 54 weeks); the mean TPM dose was 189 mg/day (range, 50 to 350 mg/day).
Refractory Status Epilepticus
In six cases of refractory status epilepticus unresponsive to sequential trials of multiple agents, including one patient who had been in a prolonged pentobarbital coma, TPM (300 to 1600 mg/day) administered via nasogastric tube successfully terminated refractory status epilepticus (90). TPM was effective against both generalized convulsive and nonconvulsive status epilepticus. All patients were subsequently discharged from the hospital.
Monotherapy
The 1990s ushered in a new era—at least in the United States—for clinical studies in newly diagnosed, previously untreated epilepsy. The use of traditional AEDs (carbamazepine, phenytoin, valproate) as first-line monotherapy is largely based on landmark Veterans’ Administration Cooperative trials (91,92) and similar open-label trials in the United Kingdom (93,94). However, the U.S. Food and Drug Administration (FDA) began requiring randomized, double-blind trials demonstrating a statistically significant difference between treatments as evidence of efficacy, generating considerable debate as to how to safely and ethically accomplish this goal. One such approach is an active-control conversion-to-monotherapy design in which patients are randomized to study drug or a minimally effective active-control, and preexisting AED therapy is gradually withdrawn (95). Such a design parallels the technique clinicians use to switch patients to a second trial of AED monotherapy when the first agent has failed because of ineffective seizure control or intolerable side effects. Such a design was used as a proof-of-principle trial for TPM monotherapy (96).
Monotherapy trial design becomes particularly complex when evaluating new AEDs in patients with newly or recently diagnosed epilepsy. The use of a placebo control in untreated epilepsy patients remains controversial, and only one such trial has been conducted (97). Unlike their European counterparts, regulatory authorities in the United States are unwilling to accept monotherapy equivalence trials for AEDs already approved as adjunctive therapy (95). The argument is that a trial showing equivalence of two treatments could be interpreted as meaning that both treatments were equally ineffective or that the trial simply failed to detect existing differences (95,98). Given the responsiveness of patients with newly diagnosed epilepsy, some have doubted the possibility of demonstrating a treatment effect with active-control or dose-control trials. These trial types are also controversial in relation to ethical equipoise (99).
TPM has been evaluated as first-line monotherapy in adults and children with newly or recently diagnosed epilepsy in three multicenter, randomized, double-blind trials. Two trials were dose-controlled trials (35,48), and one trial used a novel trial design to simultaneously compare TPM with two standard AEDs (i.e., carbamazepine and valproate) (47).
In the first dose-controlled trial (35), 252 adults and children who had been diagnosed with epilepsy within 3 years of study entry and who had one to six partial-onset seizures during a 3-month retrospective baseline were randomized to 50 or 500 mg/day TPM (patients weighing ≤50 kg were randomized to 25 or 200 mg/day). Patients were untreated or had been treated for more than 1 month with one AED. The primary efficacy outcome was time to exit, which was time to second seizure in 96% of patients. Time to exit was longer in patients receiving TPM 200/500 mg/day (median 422 days vs. 293 days in patients receiving 25/50 mg/day), although the difference was not significant. When time to exit was analyzed using time to first seizure as a covariate, the difference between treatment groups was significant (P = 0.01). This finding reflected the higher seizure-free rate in patients receiving TPM 200/500 mg/day (54% vs. 39% with 25/50 mg/day; P = 0.02) as well as the longer interval before the first seizure (median 317 days vs. 108 days with 25/50 mg/day; P = 0.06). In this study, seizure-free rates with 50 mg/day (39%) and TPM 400 mg/day (54%) were at the lower and upper ends for the range of seizure-free rates (36% to 43%) reported with therapeutic dosages of other AEDs in double-blind studies (100,101). The mean dosage among patients randomized to TPM 500 mg/day was 366 mg/day. A significant difference between treatment groups was observed for patients with one or two seizures in the 3-month baseline, but not for patients with three or more seizures in the 3-month baseline. This finding suggested that higher seizure frequency may serve as an indicator of more treatment-resistant seizures in patients with untreated epilepsy and is consistent with other reports linking higher seizure frequency before initial treatment with refractory epilepsy (102).
Results from the first dose-controlled study (35) suggested that TPM 50 mg/day was an effective dose in some patients responsive to anticonvulsant therapy and could serve as an active control to treatment with TPM 400 mg/day. Moreover, patients with one or two seizures in a 3-month baseline may represent the population of patients with newly diagnosed epilepsy who are most likely to benefit from monotherapy and not require polytherapy because of drug-resistant epilepsy. In the second dose-controlled study (48), 470 adults and children (weighing at least 25 kg) were eligible if they had untreated epilepsy diagnosed within 3 months of study entry, or if epilepsy had relapsed while they were not receiving anticonvulsant therapy. Patients could have only one or two partial-onset or generalized tonic–clonic seizures during the 3-month retrospective baseline. The primary efficacy end point was time to first seizure; seizure-free rates at 6 months and 1 year were secondary efficacy measures. Kaplan–Meier survival analyses for time to first seizure showed a significantly greater treatment effect with the 400 mg/day group versus the 50 mg/day group (P = 0.0002). The probability of being seizure free was 83% with the 400 mg/day group and 71% with the 50 mg/day group (P = 0.005) after 6 months treatment and 76% and 59% (P = 0.001) after 12 months. A difference between dose groups emerged within the first week after randomization when patients were receiving 25 or 50 mg/day; the between-group difference was significant after 2 weeks when patients were receiving 25 or 100 mg/day. The mean dosage achieved for each of these groups was 46 mg/day (in the so-called 50 mg/day group) and 275 mg/day (in the so-called 400 mg group). The reason that the numbers were <50 and 400 mg/day was that for example for the higher dosage patients, they had to be increased to at least 150 mg/day but not necessarily to 400 mg/day. Approximately half of the patients were not fully titrated to 400 mg/day and approximately half were titrated up to 400 mg/day (investigator discretion). Similarly, one could stop at 25 mg/day for the low-dosage group and did not have to increase to 50 mg/day.
The effectiveness of TPM 100 mg/day as initial monotherapy in patients with newly diagnosed epilepsy was established further with a randomized, double-blind trial comparing TPM, carbamazepine, and valproate in adults and children (N = 613) with newly diagnosed epilepsy (47). No seizure types/epilepsy syndromes were excluded. During this trial, investigators selected carbamazepine (600 mg/day) or valproate (1250 mg/day) as the preferred therapy according to each patient’s clinical presentation. Patients were then assigned to the carbamazepine or valproate treatment branch. Within each branch, patients were randomized to double-blind treatment with the investigator’s choice of traditional AED (carbamazepine or valproate), TPM 100 mg/day or TPM 200 mg/day. Patients continued double-blind treatment until exiting the study or until 6 months after the last patient was randomized.
The initial efficacy analysis compared time to first seizure for the two TPM dosages (100 and 200 mg/day). If TPM 200 mg/day was significantly more effective than TPM 100 mg/day, then 200 mg/day was to be compared with carbamazepine and valproate. If 200 mg/day was not significantly more effective, the protocol required TPM dosage groups to be pooled within each branch and compared with traditional therapy. For the comparison between TPM and traditional therapy, the primary efficacy measure was time to exit; secondary efficacy end points were time to first seizure and proportion of patients seizure free during the last 6 months of double-blind treatment.
No difference was observed for the initial efficacy analysis comparing the two TPM dosages groups. Therefore, the combined TPM groups were compared with carbamazepine and valproate treatment. In both the carbamazepine and valproate branches, time to exit did not differ between the combined TPM treatment groups and traditional therapy. Because the branches were homogeneous, pooled data across branches were used to calculate 95% confidence intervals (CIs) for treatment differences. Although retention rates were higher among patients receiving TPM compared with those receiving carbamazepine or valproate, 95% CIs included zero, which indicated that between-group differences were not statistically significant. Similar results were observed for time to first seizure. The proportion of patients with no seizures during the last 6 months of double-blind treatment was 49% among patients receiving TPM 100 mg/day and 44% in each of the other three treatment groups (i.e., TPM 200 mg/day, carbamazepine, and valproate). The 95% CIs were narrow and included zero, indicating no difference among the four treatment groups.
Results from two trials showing that TPM 100 mg/day is effective in adults and children with newly diagnosed epilepsy support clinical findings suggesting that only low to moderate dosages of AEDs are required in patients with new-onset epilepsy that is responsive to treatment (103). Pharmacokinetic–pharmacodynamic (PK-PD) modeling and simulation bridging showed no difference in PK-PD of TPM between adult and pediatric patients. Monotherapy regimens were identified in children 2 < 10 years of age (104).
Intravenous Use
There is no intravenous formulation approved to date. The safety and pharmacokinetics following a single dose in patients with epilepsy or migraines taking oral TPM was however performed. A 25 mg single dose caused minimal infusion site or systemic adverse effects (105).
Other Clinical Uses
TPM is now indicated for adults for the prophylaxis for migraine headaches. Two randomized, double-blind, placebo- controlled trials evaluated the efficacy of TPM treatment (50, 100, and 200 mg/day) in 970 patients with migraine (106,107). The primary efficacy measure was change in mean monthly migraine frequency from baseline during double-blind treatment. Compared with placebo, significant reductions in monthly migraine frequency were reported with TPM dosages of 100 and 200 mg/day; migraine frequency was also reduced with 50 mg/day, although the difference from placebo was not statistically significant. The proportion of treatment responders with a 50% or more reduction in monthly migraine frequency was significantly greater in TPM-treated patients (36% to 54% vs. 23% with placebo) (104,105). TPM may also have favorable effects in patients with cluster headache; in a case series, cluster remission occurred in nine of 10 patients (108).
TPM may have a potential role in movement disorder treatment. In a double-blind, placebo-controlled, crossover trial in 62 patients with essential tremor, TPM was associated with significant improvements in tremor severity, motor task performance, and functional disability (109), findings that were consistent with those in an earlier pilot study (110). In a retrospective chart review, TPM seemed to be effective in reducing tics in children and adolescents with Tourette syndrome (111); 59% (19/32) of patients had at least 50% reduction in tic severity scores.
Several studies suggest that TPM may be effective in various impulse control disorders. In a randomized, double-blind, placebo-controlled trial in 150 patients with alcohol dependence, TPM-treated patients had significantly fewer drinks per day, drinks per drinking per day, and heavy drinking days and significantly more abstinent days compared with placebo (112). Plasma α-glutamyl transferase, an objective index of alcohol consumption, was also significantly lower in TPM-treated patients. Among 61 obese patients with binge-eating disorder who were participating in a randomized, double-blind, placebo-controlled trial, TPM treatment was associated with significantly greater reductions in binge frequency, binge per day frequency, body mass index (BMI), body weight, and obsessive–compulsive scores (113). Open-label treatment with TPM has been reported to improve behavior, mood, weight control, compulsive eating problems, and self-mutilating behavior (notably skin picking) associated with Prader–Willi syndrome (114,115).
The observation that TPM is associated with weight loss and expected improvement in metabolic parameters (e.g., lipids, blood pressure, glucose levels) (116) led to studies of TPM (64 to 384 mg/day) in obese patients (117). After 6 months, mean percent decrease in baseline body weight was significantly greater among TPM-treated patients (range, 4.8% to 6.3% depending on TPM dose; 2.6% with placebo). A similar pattern of weight loss was observed in patients with diabetes who participated in three double-blind, placebo-controlled trials evaluating the efficacy of TPM in painful diabetic neuropathy (118). Moreover, in these trials, diabetic control, measured as HbA1c levels, improved significantly compared with placebo, with reductions in HbA1c occurring independent of weight loss. These findings are supported by data from an animal model of diabetes, in which TPM demonstrated dose- dependent decreases in blood glucose and plasma triglycerides without significant body weight changes (119).
In view of the role of glutamate and AMPA in the pathobiology of neuronal injury, attention has been focused on TPM because of its activity as an AMPA antagonist. Potential neuroprotective and disease-modifying effects of TPM have been observed in models of seizure-related neuronal injury (23), focal cerebral ischemia (120), and glutamate excitotoxicity (121). Preliminary data in patients with diabetic neuropathy suggest that TPM may improve or restore nerve function through preservation/regeneration of C-fibers, with associated improvement in functional parameters (122). Studies using a cerebral microdialysis technique in patients with traumatic brain injury showed that TPM reduced glutamate levels compared with historical controls (123). In a double-blind, placebo-controlled trial, high-dose TPM (800 mg/day) did not provide beneficial effects in patients with amyotrophic lateral sclerosis and may have accelerated the loss of arm muscle strength. In this study, TPM treatment was associated with an increased risk of side effects (124). These findings are useful for advancing our understanding of potential therapeutic targets.
A recent translational medicine research report using a computational approach to discover new drug therapies for inflammatory bowel disease (IBD) suggested the possibility that TPM might be helpful. They utilized a trinitrobenzenesulfonic acid–induced rodent model of IBD (125).
Use in Pregnancy
“In 2011, TPM was reclassified from a Pregnancy Category C to a Pregnancy Category D drug.” Data in humans suggested a link between the drug’s use in the first trimester of pregnancy and oral cleft formation in newborns. “Pregnancy Category D drugs are those with positive evidence of human fetal risk based on human data but still may be used in pregnant women in certain situations when its benefits are thought to outweigh potential risks. Previously, data suggesting fetal harm with TPM were available only with animal studies” (126). In animal studies, fetal abnormalities were similar to those observed with other carbonic anhydrase inhibitors such as acetazolamide, whose use has not been linked to teratogenic effects in humans. “The recent FDA action stemmed from inspection of data provided by the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Upon review of these data, the agency found that newborns exposed to TPM monotherapy during the first trimester of pregnancy were more likely to develop an oral cleft compared to those who were not (RR = 21.3; 95% CI, 7.9–57.1). Similar conclusions were drawn from a review of the United Kingdom Epilepsy and Pregnancy Register (incidence of oral cleft: 3.2% vs. 0.2%, RR increase of approximately 16-fold). In their published safety communication, FDA agency officials stressed, ‘The benefits and the risks of TPM should be carefully weighed when prescribing this drug for women of childbearing age, particularly when TPM is considered for a condition not usually associated with permanent injury or death.” They continued, “Appropriate alternative treatment should be considered.” (126
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








