8 Transmitters and receptors
Electrical Synapses
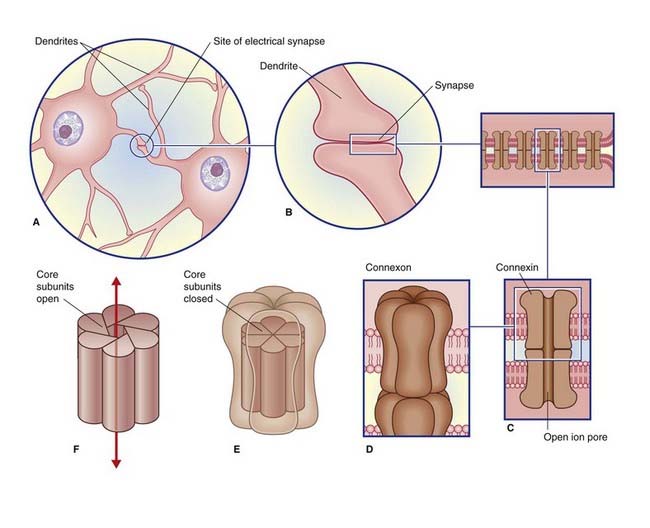
Electrical synapses are scarce in the mammalian nervous system. As seen in Figure 8.1, they consist of gap junctions (nexuses) between dendrites or somas of contiguous neurons, where there is cytoplasmic continuity through 1.5-nm channels. No transmitter is involved, and there is no synaptic delay.
Chemical Synapses
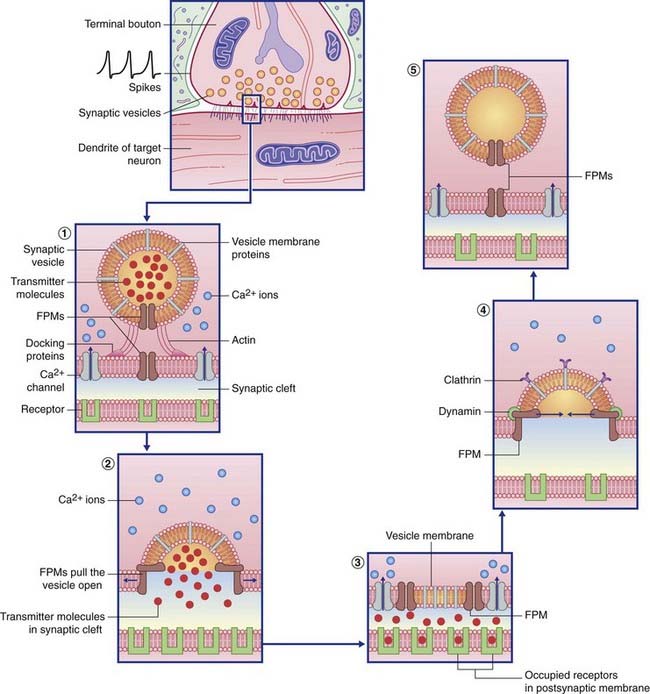
Transmitter liberation (Table 8.1 and Figure 8.2)
Table 8.1 Some named proteins involved in transmitter transport and vesicle recycling
| Named protein | Function |
|---|---|
| Actin | Brings vesicle into contact with presynaptic membrane |
| Synaptophysin | Creates the membrane fusion pore |
| Calmodulin | Expels vesicle content into synaptic cleft |
| Clathrin | Withdraws vesicle membrane from synaptic cleft |
| Dynamin | Pinches the neck of the developing vesicle to complete its separation |
| Ligand | Receptor protein that binds with the transmitter molecule |
Target cell receptor binding
Ionotropic receptors
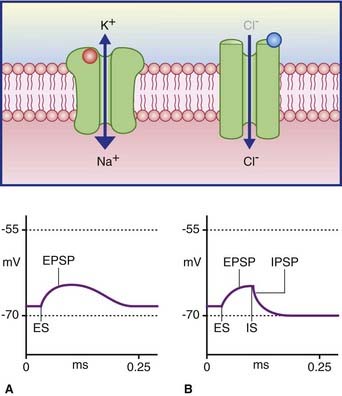
Ionotropic receptors are characterized by the presence of an ion channel within each receptor macromolecule (Figure 8.2). The transmitter binds with its specific receptor facing the synaptic cleft, causing it to change its conformation so as to open or close its ion pore. Ionotropic receptor channels are said to be transmitter-gated, or ligand-gated (from the Latin ligandum, ‘binding’), signifying their capacity to bind a transmitter molecule or a drug substitute.
In Figure 8.3A, the excitatory channel has been opened by the transmitter, causing a major influx of sodium and a minor efflux of potassium; the excitatory postsynaptic potential (EPSP) has depolarized the cell membrane almost to firing point. In Figure 8.3B, the membrane can be hyperpolarized only to −70 mV, the chloride equilibrium potential. A greater level of depolarization requires a second messenger system (see below).
Metabotropic receptors
Three second messenger systems are well recognized.
Cyclic AMP system
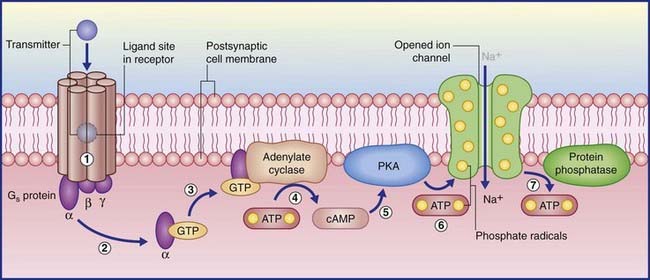
(The abbreviation cyclic AMP represents cyclic adenosine monophosphate.) In the examples shown in Figure 8.4, transmitter–receptor binding releases the alpha subunit of a Gs protein, leaving it free to link with GTP, which in turn facilitates adenosine cyclase to convert ATP to cyclic AMP. Protein kinase A in the membrane is stimulated by cyclic AMP to transfer phosphate ions from ATP to an ion channel, causing its pore to admit sodium ions, initiating depolarization of the target neuron. When the Gs protein is switched off, the membrane-attached enzyme protein phosphatase catalyzes extraction of the phosphate ions, resulting in pore closure.
Phosphoinositol system
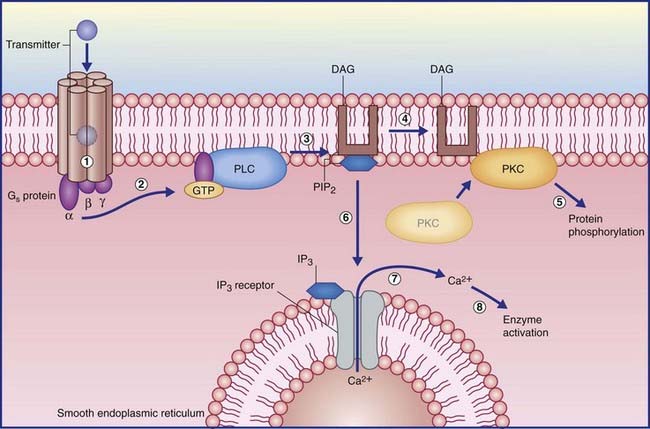
In the example shown in Figure 8.5, activation of another kind of Gs protein alpha subunit causes the effector enzyme phospholipase C to split a membrane phospholipid (PIP2) into a pair of second messengers: diacylglycerol (DAG) and inositol phosphate (IP3). DAG activates protein kinase C, which initiates protein phosphorylation. IP3 diffuses into the cytosol, where it opens calcium-gated channels, mainly in nearby membranes of smooth endoplasmic reticulum. The Ca2+ ions activate certain calcium-dependent enzymes downstream, and may open calcium-gated K+ (outward) and Cl− (inward) channels with excitatory effect.
Transmitters and Modulators
Several criteria should be fulfilled for a substance to be accepted as a neurotransmitter.
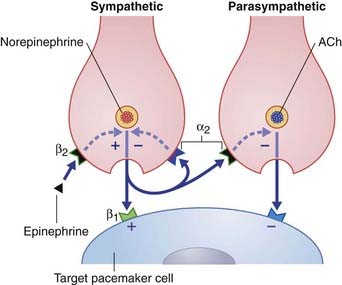
The term neuromodulator (L. modulare, to regulate) has been subject to several interpretations. The most satisfactory appears to derive from the terms amplitude modulation and frequency modulation in electrical engineering, signifying superimposition of one wave or signal onto another. Figure 8.6 represents a sympathetic and a parasympathetic nerve ending, close to a pacemaker cell (modified cardiac myocyte). This neighborly arrangement of nerve endings is common in the heart, and allows the respective transmitters to modulate each other’s activity. The sympathetic nerve ending liberates norepinephrine (noradrenaline), which has a stimulatory effect. The three modulators shown exert their effects via second messenger systems.
Fate of neurotransmitters
The principal transmitters and modulators are shown in Table 8.2. Respective receptor types are in Table 8.3.
Table 8.2 Main types of transmitters and modulators, with examples of eacha The neuropeptides are modulators
| Type | Example(s) |
|---|---|
| Amino acids | Glutamate |
| γ-Aminobutyric acid (GABA) | |
| Glycine | |
| Biogenic amines | Acetylcholine |
| Monoamines | |
| Catecholamines (dopamine, norepinephrine [noradrenaline], epinephrine [adrenaline]) | |
| Serotonin | |
| Histamine | |
| Neuropeptides | Vasoactive intestinal polypeptide |
| Substance P | |
| Enkephalin | |
| Endorphins | |
| Many others | |
| Adenosine | — |
| Gaseous | Nitric oxide |
a The five monoamines contain a single amine group. Catecholamines also contain a catechol nucleus.
Table 8.3 Receptor types activated by different neurotransmitters
| Ionotropic receptors | Metabotropic receptors |
|---|---|
| Glutamate (AMPA/K) | Glutamate (mGluR) |
| GABAA | GABAA |
| Acetylcholine (nicotinic) | Acetylcholine (muscarinic) |
| Glycine |