Antipsychotic treatment algorithm. Following several unsuccessful atypical antipsychotic monotherapy trials, a trial with a conventional antipsychotic or with clozapine is recommended. High-dose monotherapy may also be considered for such treatment-resistant patients. Antipsychotic polypharmacy is recommended only after antipsychotic monotherapy has failed. Note that throughout the treatment algorithm, monitoring of plasma drug levels of each antipsychotic is critical when determining the next course of action.
Evaluation of Violence and Treatment of Comorbidities Before Going Beyond the Guidelines
Patients with schizophrenia who exhibit violent behavior in inpatient settings should first be treated according to published guidelines for all patients with schizophrenia, including a series of monotherapies with atypical antipsychotics and a trial of clozapine (Figure 21.1) [1]. If a patient with schizophrenia continues to exhibit violent behavior, that violence should be categorized as psychotic, impulsive, or predatory; predatory behavior is not an appropriate target for antipsychotic treatment, but psychotic and impulsive violence can be [2,3]. Most violent acts in forensic and state hospital settings (where patients mostly suffer from psychotic disorders) are impulsive, with predatory violence and psychotic violence being less frequent [4–9]. Psychotic violence is hypothetically linked to excessive neuronal activity in the mesolimbic dopamine pathway, and can often, but not always, be successfully treated with standard antipsychotic monotherapies, including clozapine [10–13]. Impulsive violence is also common in psychotic patients in forensic and state hospital settings, even after positive symptoms of psychosis have been controlled with standard antipsychotic treatment [14–16]. Impulsive violence is hypothetically linked to an imbalance between “top-down” cortical inhibitory controls and “bottom-up” impulsive drives, and, empirically, high dosing and polypharmacy can reduce these behaviors in some patients who respond inadequately to standard treatments [2,11,12,17–19]. However, before considering high dosing or polypharmacy for schizophrenic patients with psychotic or impulsive violence who have failed to respond adequately to standard antipsychotic treatments, it is important to treat and stabilize any coexisting cognitive dysfunction or substance abuse issues [5,7,20–27].
Treatment of Violence and Aggression: Attaining Sufficient Dopamine D2 Receptor Occupancy
Neuroimaging studies have repeatedly shown that blockade of at least 60% of D2 receptors by antipsychotic treatment is necessary in order to reduce psychosis [10,11,28]. At greater than 80% occupancy of D2 receptors, the threshold for extrapyramidal symptoms (EPS) is reached in many patients. Thus, antipsychotics at standard doses aim to achieve between 60%–80% D2 receptor occupancy (Figure 21.2) [28–31]. Data indicate that obtaining sufficient D2 receptor occupancy and achieving the downstream therapeutic effects of D2 receptor blockade by an antipsychotic often take more than 6 weeks to manifest [32,33]. In fact, it may be necessary to treat schizophrenia with an antipsychotic for as long as 1–2 years before a significant improvement in psychotic symptoms is evident, although this may not be practical in forensic settings where violent behavior must be controlled [34–37]. Additionally, there are some data to suggest that nonresponse to an antipsychotic after 4 weeks of treatment predicts nonresponse at 12 weeks [38]. In this particular study by Stentebjerg-Olesen et al., patients who were early treatment responders had a significantly greater chance of being treatment-responsive at 12 weeks compared to early treatment nonresponders [38]. However, we do wish to point out that over one-third of patients who were considered early treatment nonresponders did ultimately respond to treatment by week 12 [38].
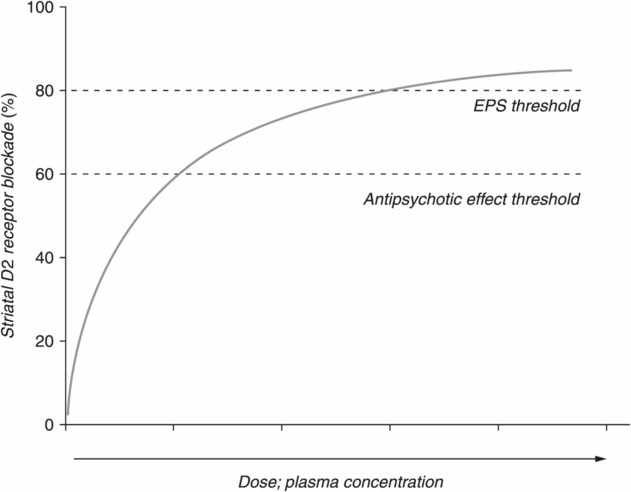
Dopamine D2 receptor occupancy. Antipsychotic blockade of at least 60% of D2 receptors in the striatum is necessary to ameliorate psychotic symptoms. However, when 80% or more of D2 receptors are blocked, extrapyramidal symptoms (EPS) are likely to occur. Standard doses of atypical antipsychotics are based on achieving 60% D2 receptor occupancy without exceeding the 80% EPS threshold. Note that the slope of the curve flattens out with increasing dose; that is, at higher doses, large increases in dose are needed to obtain substantial increases in D2 receptor occupancy.
Pharmacokinetic failure, treatment resistance, and violence
When a patient with schizophrenia who exhibits either psychotic or impulsive violence fails to respond to standard doses of antipsychotic monotherapy of adequate duration and with adherence to treatment, this can be due to either pharmacokinetic failure or pharmacodynamic failure [29]. Pharmacokinetic interactions describe the effects of a biological system on a medication and include rapid metabolization, cytochrome P450 polymorphisms, poor absorption (e.g., due to gastric bypass), and interactions with other medications/substances. In the case of pharmacokinetic failure, plasma drug levels do not reach adequate levels (and therefore D2 receptor occupancy is less than 60%) despite standard antipsychotic doses (Figure 21.3A). Often, pharmacokinetic failure presents as a lack of both therapeutic and adverse effects at standard antipsychotic doses. Therapeutic drug monitoring is essential for determining if a pharmacokinetic issue or treatment nonadherence underlies treatment nonresponse; in these cases, plasma drug levels will be lower than expected [18,39]. Solutions to pharmacokinetic failure include increasing the antipsychotic dose to achieve sufficient plasma levels, switching to a different antipsychotic monotherapy (such as one with a sublingual or intramuscular formulation), instituting antipsychotic polypharmacy, or simply taking the antipsychotic with food [17].
Pharmacokinetic and pharmacodynamic failures. The failure of a patient to respond to antipsychotic treatment may be due to either pharmacokinetic or pharmacodynamic failure.
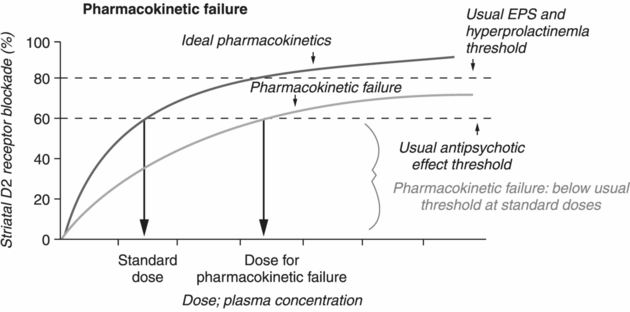
Pharmacokinetic failure occurs in cases in which the therapeutic threshold (~60% D2 occupancy) is not achieved despite dosing at standard therapeutic levels.
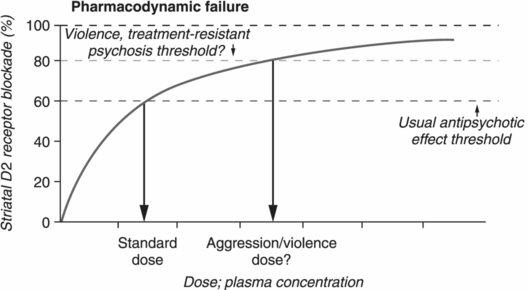
Pharmacodynamic failure occurs in cases in which occupancy of greater than 80% of D2 receptors may be required before therapeutic effects are achieved. Pharmacodynamic failure therefore alters antipsychotics’ threshold for therapeutic effects and may be quite prevalent in patients with psychotic or impulsive aggression.
Pharmacodynamic failure, treatment resistance, and violence
Pharmacodynamic interactions describe how antipsychotics impact biological systems once they occupy 60%–80% of D2 dopamine receptors. Pharmacodynamic failure occurs when there is a lack of therapeutic response despite attaining adequate plasma drug levels (Figure 21.3B) [29]. Why some patients do not respond to the usual degree of D2 receptor occupancy remains a quandary, but can include insensitive D2 receptors, or even supersensitive D2 receptors, where increasing doses of antipsychotics may be necessary in order to reduce psychotic symptoms [40–42]. Interestingly, several factors, including substance abuse, can increase dopamine supersensitivity [42]. These treatment-resistant patients may present with excessive psychotic symptoms and violence leading to institutionalization in forensic settings. For these individuals, it may be necessary to use treatment strategies (including high-dose antipsychotic monotherapy and antipsychotic polypharmacy) aimed at greater than 80% D2 receptor occupancy in order to relieve psychotic symptoms (Figure 21.1) [17,29].
Heroic treatment strategies such as high-dose monotherapy or antipsychotic polypharmacy may not be necessary for typical patients with schizophrenia included in clinical research studies and for which the evidence in the literature is generated. In fact, most clinical trial data do not show any superior benefit from using high-dose monotherapy or antipsychotic polypharmacy for such patients [36,37]. Those patients with pharmacodynamic or pharmacokinetic failures and who may require bold treatment measures are often treatment-resistant to standard doses of a single drug and present with violent or aggressive behaviors [18]. Unfortunately, these patients (who are the most likely candidates for high-dose antipsychotic monotherapy or antipsychotic polypharmacy) are excluded from clinical trials because they are too psychotic, too substance-abusing, too aggressive, or too treatment-resistant to meet inclusion criteria or give informed consent [29,43,44]. Thus, it is not surprising that many (but not all) of the published clinical trial data have failed to find any clear benefit of antipsychotic polypharmacy or high-dose monotherapy over standard therapeutic doses of a single antipsychotic. It may therefore be difficult for the prescribing clinician to know the best strategy to optimize care for treatment-resistant, violent, or aggressive patients given the paucity of studies that include the patients who require it. However, most studies that investigate the actual use of high antipsychotic dosing (including high dosing that results from combining two antipsychotics) find that those patients for whom high dosing is used are often the most treatment-resistant, aggressive, or otherwise difficult-to-treat cases, and that clinicians who utilize high-dosing strategies are often those with the most clinical experience [45–53].
These data suggest that currently available guidelines fall short for many patients in real-world clinical practice, especially in forensic and state hospital inpatient settings or for outpatients on compulsory treatment orders [45]. These same patients may exhibit psychotic or impulsive violence, and there is substantial practice-based evidence for the use of treatment measures including high-dose monotherapy and antipsychotic polypharmacy [36,37]. Most guidelines for the treatment of schizophrenia advocate several trials of antipsychotic monotherapy (using both first- and second-generation agents), followed by a trial of clozapine, and either do not advocate antipsychotic polypharmacy or reserve it for only the most difficult cases (Figure 21.1) [46,48]. A trial of clozapine is a critical, yet often bypassed, step, since there is an abundance of data that shows the superior efficacy of clozapine for treatment-resistant patients as well as for the amelioration of aggression [46,48,54,55]. Even so, as many as 40% of patients may experience only partial or no response to clozapine [56]. While adherence to these published guidelines is likely the best course of action for the majority of patients, what is the clinician to do when a patient is persistently psychotic and possibly aggressive following several standard-dose monotherapies and an unsuccessful trial of clozapine? In the following sections, we offer guidance and recommendations for using high-dose antipsychotic monotherapy and antipsychotic polypharmacy based on practice-based evidence involving patients who are chronically violent or aggressive and for whom standard guidelines typically fall short (Figure 21.1).
Antipsychotic Polypharmacy
Although data supporting the use of antipsychotic polypharmacy (the simultaneous use of two antipsychotics) are somewhat limited, this practice is very common in psychiatry; as many as 30% of patients receive antipsychotic polypharmacy [57,58]. In fact, despite several guidelines recommending that polypharmacy should only be used as a last resort (following failure of several monotherapies and a trial of clozapine), many clinicians attempt polypharmacy as the rule, rather than the exception [18,47]. Alarmingly, a recent study showed that as many as one-quarter of patients are not treated using prescribing pathways that are consistent with treatment guidelines, with up to 65% receiving antipsychotic polypharmacy as their first antipsychotic treatment [55]. Such prescribing practices appear to have led to some backlash, with calls and efforts to reduce antipsychotic polypharmacy, including several articles authored by ourselves [47,59–65]. We advocate here that published treatment guidelines should be adhered to, and will likely be effective for the majority of patients [1]. Recent studies have shown that as many as two-thirds of patients treated with antipsychotic polypharmacy can be successfully switched to monotherapy, supporting the notion that antipsychotic polypharmacy may not be necessary for the majority of patients [58,66]. The study by Essock et al. [58] in particular showed that not only did patients who were switched from polypharmacy to monotherapy have no worsening of symptoms or increased hospitalization, but many also had reversal of the metabolic effects that were presumably due to antipsychotic polypharmacy. However, it is important to note that polypharmacy was necessary for symptom management in one-third of all patients in the Essock et al. study. Also, although many studies have failed to show a benefit of antipsychotic polypharmacy over standard-dose monotherapy, more recent investigations do show some evidence for the benefit of combining antipsychotics [48,67,68]. Notably, in line with our previous assertion that time may itself be like a drug, there is some evidence to suggest that treatment with antipsychotic polypharmacy must be continued for at least 10 weeks before a significant therapeutic effect is seen [35,67]. Together, these data support the notion that a subpopulation of patients, likely including those who are treatment-resistant or violent, may require treatment measures such as antipsychotic polypharmacy [18]. Resorting to antipsychotic polypharmacy is probably not necessary for most patients and should be reserved for those patients for whom several antipsychotic monotherapy trials have failed and a trial with clozapine is unsuccessful or cannot be attempted.
Antipsychotic polypharmacy is often employed as a method for increasing dopamine D2 receptor occupancy, but also may be used to recruit additional properties of antipsychotics in order to treat non-positive symptoms such as depression and anxiety [47,67]. Atypical antipsychotics bind to a variety of receptors, some of which are hypothesized to have therapeutic benefit [10]. Indeed the recruitment of various serotonergic and noradrenergic receptors may help to normalize the aberrant neurotransmission associated with violence and aggression [69–71]. For example, increasing serotonergic neurotransmission in the prefrontal cortex (PFC) may, in theory, improve top-down cortical control of the limbic system and thereby improve impulsive aggression [10,11,15,69].
Unfortunately, each atypical antipsychotic also binds to receptors associated with increased risk of intolerable effects (e.g., sedation), so using two antipsychotics simultaneously can increase the side effect burden. A recent study by Langle et al. [57] suggested that patients with schizophrenia on antipsychotic polypharmacy have a worse clinical course compared to those on monotherapy. However, it is unclear if worse clinical outcome was caused by antipsychotic polypharmacy or if it is simply a matter of more treatment-resistant or otherwise difficult patients being the most likely to require more extreme treatment measures such as polypharmacy. Earlier studies also suggested that antipsychotic polypharmacy was associated with increased mortality; however, subsequent studies do not support this idea, and, in fact, a more recent analysis suggests that antipsychotic polypharmacy may actually be associated with reduced mortality as well as fewer psychiatric hospitalizations [51,53,72,73].
If polypharmacy is attempted, antipsychotics should be combined in a rational manner, based on the binding profiles of each antipsychotic for various receptors [48,54]. The logic of combining two antipsychotics should take into account not only the desired boost in D2 antagonism, but also the potential therapeutic and adverse effects of recruiting additional non-dopamine receptors. Combinations of antipsychotics that have similar side effect profiles should be avoided, and potential interactions of antipsychotics should be considered, especially with respect to the cytochrome P450 system [48,74]. Interestingly, antipsychotic polypharmacy may actually be preferable as a way to increase D2 receptor occupancy while avoiding particular adverse effects that may occur with high-dose monotherapy [47,54]. For example, a recent study showed that the addition of aripiprazole to clozapine treatment resulted in a reduction in clozapine-induced cardiometabolic effects [75]. Although this particular study did not show improvement in symptoms (measured using the Positive and Negative Symptom Scale [PANSS]) using a combination of clozapine and aripiprazole, other studies of clozapine and aripiprazole have found some symptom improvement [72,76]. It is also important to note that combining aripiprazole with a non-clozapine antipsychotic may actually worsen symptoms of psychosis due to actions of aripiprazole as a partial agonist with high binding affinity for D2 receptors [54].
Antipsychotic combinations that include clozapine have the most evidence for efficacy [48,67]. When clozapine is not an option, most clinicians who utilize antipsychotic polypharmacy appear to prefer a second-generation antipsychotic (SGA) in combination with a first-generation antipsychotic (FGA), and there are some data to support this [56,67]. Often the rationale for antipsychotic polypharmacy involves combining an antipsychotic with relatively weak binding affinity for D2 receptors (such as clozapine or olanzapine) with an antipsychotic that binds more strongly to D2 receptors (such as sulpiride or amisulpride); in this way D2 receptor occupancy can be maximized while taking advantage of the vast molecular non-D2 binding affinities inherent to many SGAs [47,56].
The rationale for attempting polypharmacy should be carefully documented, along with any therapeutic or side effects that occur. Throughout the course of treatment, plasma levels of both antipsychotics should be monitored in order to ensure treatment adherence and rule out pharmacokinetic issues.
High-Dose Monotherapy
High-dose monotherapy is another strategy for increasing D2 receptor occupancy, although this strategy may also increase the risk of intolerable side effects (notably EPS and akathisia) and, as with antipsychotic polypharmacy, can be associated with substantially higher medication costs, especially for newer agents, than standard dose monotherapy. As with all off-label practices, dosing of antipsychotics above standard therapeutic levels warrants informed consent and increased monitoring of the patient. As the pharmacodynamic and pharmacokinetic characteristics vary from patient to patient, it is virtually impossible to predict what daily dose will be needed in order to achieve an antipsychotic effect [77]. Antipsychotic dosing should be started at the low FDA-approved dose and then titrated upward accordingly until therapeutic efficacy or intolerable side effects occur; thus antipsychotic plasma levels should be continuously monitored as the dose is escalated [78]. The standard dose ranges for atypical antipsychotics and special considerations for high dosing are summarized in Table 21.1. In the following sections, we review the art and science of prescribing each of the FDA-approved atypical antipsychotics at high doses. As antipsychotics are dosed at a level that blocks 60%–80% of D2 receptors (with the exception of clozapine), it is important to note that any receptor binding that is stronger than that of D2 receptors will also be occupied at levels greater than 60% and will likely cause additional therapeutic and adverse effects [10]. It is essential to keep the relative receptor binding affinities in mind when dosing an atypical antipsychotic at higher-than-usual levels to attain >80% occupancy of D2 receptors, so that potential effects of binding to receptors other than D2 can be anticipated and monitored.
| Medication | Usual dose range (mg/day)* | Recommended plasma levels (ng/mL)** | Considerations for high dosing |
|---|---|---|---|
| Clozapine | 300–450 | 350–600 | Maximum dose is usually 900 mg/day. Doses above 550 mg/day may require concomitant anticonvulsant administration to reduce the chance of a seizure |
| Risperidone | 2–8 | 20–60 | FDA-approved up to 16 mg/day. Very high doses usually not tolerated |
| Paliperidone | 3–6 | 20–60 | Maximum dose is generally 12 mg/day |
| Olanzapine | 10–20 | 20–80 | Some forensic settings up to 90 mg/day |
| Quetiapine | 400–800 | 100–500 | Some forensic settings up to 1800 mg/day |
| Ziprasidone | 40–200 | 50–200 | Must be taken with food. Positron emission tomography (PET) data support > 120 mg/day. Some forensic settings up to 360 mg/day may be appropriate |
| Aripiprazole | 15–30 | 150–500 | Higher doses usually not more effective and possibly less effective |
| Iloperidone | 12–24 | 5–10 | High dosing not well-studied and may be limited due to risk of orthostatic hypotension |
| Asenapine | 10–20 | 2–5 | High dosing not well-studied |
| Lurasidone | 40–160 | >70*** | Must be taken with food. Nightly administration may improve tolerability. High dosing not well-studied, but some patients may benefit from doses up to 160 mg/day |
Clozapine
Clozapine is not recommended as a first-line treatment strategy due to the risk for serious adverse effects, most notably agranulocytosis; however, in patients who have failed several first-line atypical antipsychotic monotherapies, a trial of clozapine is warranted. Clozapine has been well-documented for treatment-resistant patients and those who are violent or aggressive, and is therefore recommended for such patients [6,79]. Interestingly, the anti-aggressive effects of clozapine are somewhat independent of its ability to improve positive symptoms [7]. Usual doses of clozapine (plasma levels of 400–600 ng/ml) actually bind less than 60%–80% of dopamine D2 receptors; however, clozapine often has antipsychotic effects at 20%–67% D2 occupancy, suggesting that the antipsychotic effects of clozapine go beyond its ability to block D2 receptors [31]. This is not surprising given the vast molecular binding profile of clozapine. Clozapine has relatively weak affinity for dopamine D2 receptors compared to its affinity for many other receptors, including histaminic H1, adrenergic alpha-1, serotonin 5HT2B, and muscarinic M1 receptors, as well as a host of other receptors. Owing to these high binding affinities for receptors other than D2, high dosing of clozapine may cause sedation (due to antagonism of M1, H1, and alpha-1 receptors), hypersalivation and constipation (due to antagonism of M1), cardiometabolic issues (due to antagonism of H1 and 5HT2C receptors, as well as the hypothesized receptor “X”), and seizures (mechanism unknown) [34]. A meta-analysis by Davis and Chen [43] showed that patients with high plasma levels of clozapine responded more frequently than those with low plasma levels, indicating that doses above 400 mg/day may be required by many patients. Titration of clozapine to high doses should be done by increasing the dose every 5–7 days [29,43].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







