45 Treatment of Thoracic Vertebral Fractures
KEY POINTS
Introduction
Thoracic fractures account for approximately 16% of all spinal fractures.4 Multiple classification systems have been developed in an attempt to characterize thoracic fractures as stable or unstable. While it is important to realize that no classification is perfect, these classification systems aid in making sound clinical decisions. They range in simplicity from the Denis three-column classification to the complicated Magerl (AO) classification.7 Regardless of the type of classification system employed, the presence of neurologic deficits, ligamentous injury, and a significant loss of height, angulation, translation, distraction, and/or rotation at the level of the vertebral injury must always increase suspicion for spinal instability.5
Basic Science
The incidence of neurological deficits from thoracic fractures is about 10% or greater; this occurs for several reasons. First, the diameter of the thoracic spinal canal is smaller than the canal of the cervical or lumbar region, being narrowest at T3-T9.9 Second, the midthoracic cord is located in a watershed region between the blood supply to the cervicothoracic and thoracolumbar spines. Last, the high-energy mechanism of injury required for most thoracic fractures is transferred to the underlying cord and spinal nerve roots.
Clinical Practice Guidelines
Stable Thoracic Vertebral Fractures
A significant percentage of thoracic compression fractures fail to heal within 3 to 6 weeks. Such fractures are prone to a progression in the kyphotic deformity and may cause severe back pain. In some instances, the pain is so debilitating that patients remain sedentary, placing them at increased risk for deep vein thrombosis, pneumonia, and bone resorption. Initially developed to treat painful vertebral hemangiomas, vertebroplasty and kyphoplasty offer marked to complete pain relief in 63% to 90% of nonhealing thoracic compression fractures.8
While there are few absolute contraindications to vertebroplasty and kyphoplasty, these interventions are strongly discouraged in the presence of systemic infection, bleeding diathesis, and spinal canal or neural foraminal stenosis leading to myelopathy or radiculopathy, respectively. Patients with pathologic compression fracture resultant from an underlying neoplasm are also candidates for vertebroplasty or for kyphoplasty; however, surgery must be coordinated with chemotherapy and/or irradiation.
Once the cannulated needle is satisfactorily positioned in the vertebral body using radiographic guidance, polymethyl methacrylate (PMMA) cement is instilled. In the case of kyphoplasty, a balloon is first inflated through the cannulated needle to create a cavity for the cement. This maneuver enables a 50% restoration in vertebral body height and alignment in two thirds of patients undergoing kyphoplasty.6
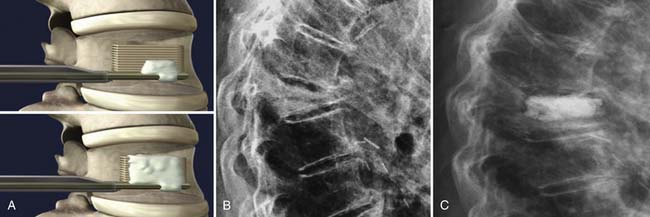
The StaXx kyphoplasty is a newer system that allows the firing of a series of PEEK wafers into the vertebral body through a device secured just inferior to the pedicle and at its lateral edge (Figure 45-1). This is performed under fluoroscopic guidance. The number of PEEK wafers required in the fractured vertebral body is determined once endplate reduction is obtained and appropriate vertebral body height correction is established. The wafers provide structural support to the vertebral body. A smaller amount of PMMA is then injected through the same channel around the PEEK construct, as compared to both vertebroplasty and kyphoplasty. The StaXx kyphoplasty system potentially offers an advantage over kyphoplasty alone, in which the space opened by the balloon may undergo some collapse before the PMMA is injected. In vertebroplasty, the PMMA simply flows to the spaces of least resistance, but does not offer height restoration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree