Tuberculosis
The tide appears to be turning against tuberculosis (TB) once again; although there were 8 million new cases worldwide in 2010, with 1.1 million deaths in HIV-negative patients and 350,000 deaths from HIV-associated TB, there has been a fall in the absolute number of cases since 2006, sustaining hope that the Millennium Development Goal of falling TB incidence rates will be achieved by 2015.1 The number of new cases in 2011 fell among both U.S.-born and foreign-born persons in the United States.2
TB is caused by infection with an aerobic, nonmotile, rod-shaped bacterium readily identified on Ziehl-Neelsen (acid-fast bacillus) stain, almost invariably Mycobacterium tuberculosis. There is an important distinction between asymptomatic infection, which is common, and disease, which occurs in a minority of cases.
Pulmonary infection results from the inhalation of aerosolized droplets; phagocytes carry bacilli from the alveoli to regional lymph nodes, which enlarge and form the so-called primary complex with the lung lesion. Lymphatic and hematogenous dissemination to other organs also occurs in the first few weeks, which in its most severe form presents as miliary TB. Macrophages are the primary cells infected by M. tuberculosis, and mycobacterial antigens trigger T helper cells to produce interferon-γ, which activates the macrophages to become bactericidal. Activated macrophages secrete tumor necrosis factor (TNF) and produce the epithelioid cells and giant cells typical of tuberculous granulomas.3
In approximately 95% of cases, infection is controlled by this activation of cell-mediated immunity in the host, but in some patients, the lung lesion gives rise to progressive pulmonary disease or extrapulmonary spread, resulting in systemic miliary TB or isolated TB in any of the organs seeded.3 Secondary TB may follow many years later as a consequence of reduced host immunity, with reactivation of a latent focus or exogenous reinfection. CNS involvement occurs in approximately 1% of persons with TB4 and takes the form of tuberculous meningitis (TBM), focal parenchymal lesions, such as tuberculomas or tuberculous abscesses, or tuberculous osteomyelitis, either of the skull or vertebrae (Pott disease, discussed in Chapter ▶ 81).
80.1.1 Tuberculous Meningitis
TBM is the most lethal form of systemic tuberculosis, and in some parts of the world it is the most common form of bacterial meningitis; in sub-Saharan Africa, this has largely happened on the back of the HIV pandemic.5,6 In general, TBM is typically a disease of young children in countries with a high incidence of the disease, whereas in countries with a low incidence, it is seen more often in adults, arising from reactivation of a dormant focus.5
Rich and McCordock proposed that mycobacteria spread via the blood to the CNS, where a parenchymal or subpial focus develops and the cerebrospinal fluid (CSF) space is entered when the so-called Rich focus ruptures into the subarachnoid space.7 Other possible routes into the CSF include rupture of a focus in the choroid plexus or elsewhere in the ventricle, spread from a contiguous bony structure, and delayed rupture of a previously controlled caseous focus. The characteristic feature is a thick, gelatinous exudate in the basal cisterns, common consequences of which are hydrocephalus and cerebrovascular involvement; both of these contribute to ischemia, raised intracranial pressure (ICP), and parenchymal injury.8,9
More than half of all cases may have infarcts on magnetic resonance (MR) imaging or at autopsy, and although vasospasm and external compression of the basal arteries of the circle of Willis and their perforators may play a role, histologic studies show infiltrative, proliferative, and necrotizing changes as well as thrombosis in arteries and veins.10 Infarcts are found in the diencephalon or the cortex11–13 and may develop even after treatment has been started and the ICP is normalized.14 Stroke in TBM occurs in up to 56% of cases, with an even higher prevalence in childhood.15,16 Vascular complications in TBM are therefore common and often fatal, making ischemia the most important reason that patients with tuberculous hydrocephalus (TBH) fail to improve clinically or even deteriorate further despite aggressive medical treatment and the early treatment of hydrocephalus.17
Hydrocephalus is present in 70% of TBM cases18 and is due mainly to blockage of the basal cisterns by tuberculous exudate in the acute stage and adhesive leptomeningitis in the chronic stage. Although typically communicating hydrocephalus develops, noncommunicating hydrocephalus is reported to occur in approximately 17% of cases as a result of obstruction at the outlet foramina of the fourth ventricle or the aqueduct by meningeal exudate, edema, or a tuberculoma,12 or a combination of these factors.
The diagnosis of TBM is made in the appropriate clinical context on the basis of CSF analysis results supported by appropriate imaging findings. This is more easily said than done, and clinical research in this area has been hampered by the lack of standardized diagnostic criteria.19 Perhaps the greatest challenge is to make the diagnosis early, which is difficult because of the nonspecific early features of TBM; a review of 554 pediatric cases seen over a 20-year period found that 91% of patients had poor weight gain or weight loss, and this may be an important early clue.18 The prevalence of the disease in the community determines the degree of clinical suspicion. The diagnosis is suggested by a history of a TB contact and a positive tuberculin skin test or Mantoux test result; the chest X-ray may show findings typical of pulmonary TB, and the organism may be identified or cultured from sputum or gastric washings. New molecular diagnostic tools, such as the fully automated amplification system Xpert MTB/RIF, have been introduced into clinical practice and are being validated for CNS disease, but major challenges in scale-up exist in countries where the need is greatest.20
The gold standard for the initial diagnosis of TBM is the demonstration of acid-fast bacilli under the microscope, but the yield is low unless large volumes of CSF are collected. Microscopy typically shows pleocytosis, with a cell count seldom greater than 500/mm3. Lymphocytes predominate, although polymorphonuclear leukocytes may on occasion be more numerous, particularly in the early phase of the disease. Typically, the CSF glucose is low and the protein is elevated. M. tuberculosis is particularly slow-growing, so culture plays no role in the initial diagnosis and management of the disease, but it becomes important should one not see an adequate clinical response, because multidrug resistance or extended drug resistance should be considered.
The typical appearance of TBM on computed tomography (CT) is often described as a triad of contrast enhancement of the basal meninges with hydrocephalus and infarcts21 (▶ Fig. 80.1). Hyperdensity may be noted in the basal cisterns before the administration of contrast, and this represents the cisternal exudate.22 Low densities related to cerebral ischemia are common, particularly in the vascular territory of the middle cerebral artery, involving the lenticulostriate and thalamoperforating vessels in the so-called TB zone adjacent to the basal ganglia.15,17 Occasionally, accompanying tuberculomas or tuberculous abscesses may be noted. It has been shown that there is no direct correlation between ventricular size and ICP in TBM.23
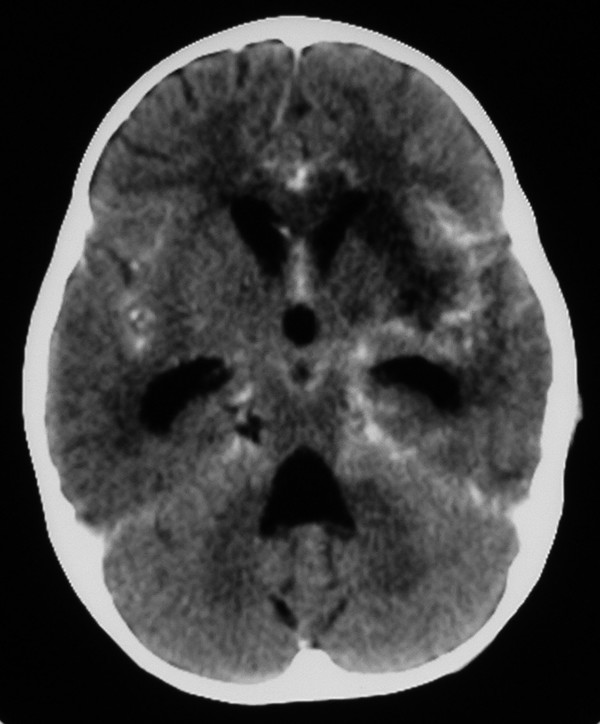
Fig. 80.1 Tuberculous meningitis. Axial computed tomographic scan following the intravenous administration of contrast showing hydrocephalus, low-density lesions in the basal ganglia, and leptomeningeal enhancement in the basal cisterns.
Antituberculous medication comprises an initial intensive phase with four drugs; isoniazid (INH), rifampicin (RIF), and pyrazinamide (PZA) are recommended together with either streptomycin, ethambutol, or ethionamide. Some authors advocate treatment with four drugs for 6 months,18 whereas others prefer 9 to 12 months.24–26 Pyridoxine should be prescribed with INH to prevent peripheral neuropathy. The emergence of strains resistant to two front-line drugs, INH and RIF (multidrug-resistant TB), has been followed by the emergence of extended drug-resistant TB.6
Steroids are of proven benefit in reducing mortality and possibly morbidity in children.27,28 Hyponatremia is commonly seen and may be due to either the syndrome of inappropriate antidiuretic hormone (SIADH) secretion or cerebral salt wasting. Distinction between the two causes may be difficult; fluid restriction must be avoided unless there is clear evidence of fluid overload, and sodium replacement with hypertonic saline may be indicated.9 Follow-up imaging is of value in detecting complications, such as hydrocephalus and infarcts.29
The optimal management of TBH is controversial with respect to the indications for surgery and the choice of operation, with most institutions developing their own treatment algorithms.30,31 A logical point of departure is determining whether the patient has communicating hydrocephalus or noncommunicating hydrocephalus. Because axial CT is unreliable in this respect,32 a number of authors have advocated the use of air encephalography to demonstrate communication between the ventricular and subarachnoid CSF.18,33 This must be done under carefully controlled circumstances, with the option of immediate surgical intervention in cases of noncommunicating hydrocephalus.
Nonsurgical treatment will be effective in about 70% of children with communicating hydrocephalus. It entails the use of diuretics, such as acetazolamide (50 mg/kg) and furosemide,18 and/or repeated lumbar punctures.34 Those who fail medical management will require a shunt. Bhagwati35 reported the use of ventriculoatrial shunts in TBH in 1971, but ventriculoperitoneal shunts soon became the preferred option,36 although various authors have reported a high rate of complications.37,38 Because some patients do not respond despite control of hydrocephalus, some authors advocate a selective approach, inserting an external ventricular drain as a temporary measure and then placing a shunt in those who respond.39,40
Endoscopic third ventriculostomy (ETV) as a treatment option for noncommunicating hydrocephalus in acute TBH was first reported in 2003,41 and subsequent analysis of the experience at this center disclosed a success rate of about 60% in cases in which the procedure was successfully completed.31 ETV in this setting is technically challenging because the floor of the third ventricle is usually thickened, and copious fibrinous exudate in the basal cisterns makes identification of the cisternal space and the major vessels difficult. Some authors report greater success with ETV in chronic TBH when the acute exudate has resolved.30,42
With appropriate antituberculous therapy, the majority of children with TBM survive, but the long-term neurologic, psychometric, and behavioral outcome is often poor. A wide range of outcomes has been reported,18 with death in 7 to 57%, neurologic sequelae in 13 to 75%, and normal outcome in only 11 to 61%. There is an urgent need for improved diagnostic tools that will enable earlier diagnosis, as well as biological markers that reflect extent of disease and response to therapy.
80.1.2 Tuberculoma
Tuberculomas are discrete lesions that represent focal infection; multiple lesions may be present in half of all cases43 and may be seen with or without concomitant TBM. In the preantibiotic era, tuberculomas accounted for 3.6 to 50.8% of intracranial masses encountered by neurosurgeons,44 but these lesions have declined in neurosurgical importance since the advent of effective antituberculous drugs and modern imaging.
Tuberculomas can occur anywhere in the brain but are rare in the spinal cord.45 They are found more commonly in children, in whom they have a predilection for the cerebellum. A tuberculoma most commonly comes to medical attention either as a large lesion with mass effect causing neurologic symptoms, hydrocephalus, or diagnostic uncertainty or as a smaller supratentorial lesion causing seizures.
A mature tuberculoma is typically a well-defined, lobulated, avascular mass with a yellowish, gritty, caseating central core surrounded by firm collagenous tissue or softer pink granulation tissue.44 The wall tends to be thicker and more crenated or nodular than those of a typical bacterial abscess,46 and the mass may be attached to adjacent dura, leading to the mistaken intraoperative impression of a meningioma. Immature lesions may consist of multiple small tubercles, some with caseating or cystic centers, and there may be intense white matter edema.44 Microscopically, a central zone of necrosis is surrounded by tuberculous granulation tissue comprising epithelioid cells, Langerhans giant cells, lymphocytes, polymorphonuclear cells, and plasma cells; acid-fast bacilli are often identified.
The typical imaging appearance is a lesion that is isodense or hyperdense on CT and is isointense on T1 and hypointense on T2 MR imaging; T2 hypointensity is noteworthy because this is seldom found in other intracranial masses.47,48 Studies correlating the imaging appearance with pathology suggest that the most common type of necrosis seen in CNS tuberculomas is not caseous but gummatous necrosis, in which inflammatory granulation tissue undergoes ischemic necrosis. By contrast, caseous necrosis is hypodense or isodense on CT and is isointense or hypointense on T1 and hyperintense on T2 MR imaging (similar to a bacterial abscess).46 The lesion enhances avidly with contrast, showing a dense unbroken ring of enhancement, in some cases an enhancing nodule or disk, or a combination of rings and disks, which may coalesce.43
The clinical presentation is similar to that of other slowly evolving intracranial space-occupying lesions. Features that may point to the diagnosis are constitutional symptoms (e.g., weight loss, fever, malaise); a history of TB or a TB contact; a high frequency of seizures, even with a cerebellar lesion; and a positive Mantoux test.43 Young children may have macrocrania.
The old clinical observation that patients with tuberculomas are often better nourished than those with TBM may point to better immunologic function. Small lesions that present with seizures alone may be difficult to distinguish from cysticercal granulomas, and in the absence of active tuberculosis elsewhere, they may not require treatment other than anticonvulsants. This is discussed in more detail later in the chapter. Larger lesions should be treated with the standard four-drug regimen of INH, RIF, PZA, and either ethionamide or ethambutol for up to 4 months, followed by two drugs for up to 18 months or longer, depending on clinical and radiologic response.49 Steroids may be indicated should there be significant edema, and anticonvulsants are often required.
Most tuberculomas resolve with medical treatment alone, and surgery is indicated only if the lesion causes life-threatening raised ICP or fails to respond to medical treatment, or if the diagnosis is in doubt. A clear plane of cleavage is typically found between the lesion and the brain,50 and complete excision may be possible for lesions that are small enough or superficial (▶ Fig. 80.2). Larger lesions may require piecemeal removal, but this does carry a risk for inciting meningitis.44 Stereotactic aspiration51 has been reported for deep-seated lesions, but this may be difficult owing to the firm nature of the lesions.43 Hydrocephalus due to a posterior fossa lesion may be very effectively managed with an ETV (▶ Fig. 80.3). Despite appropriate and effective drug therapy, tuberculomas may sometimes enlarge,52 a phenomenon termed paradoxical expansion, but eventually most do respond.
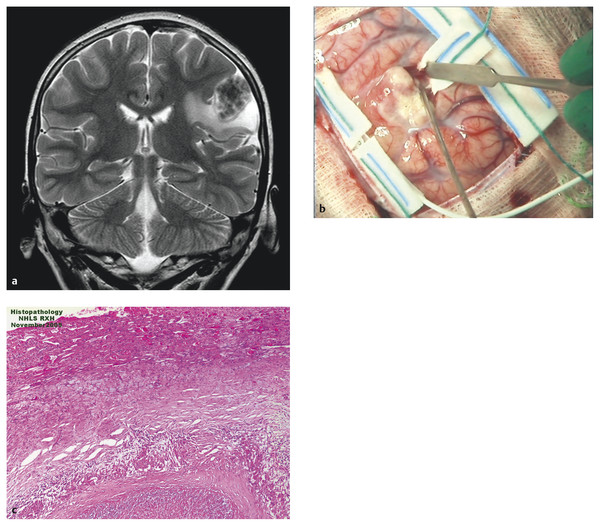
Fig. 80.2 Cortical tuberculoma. (a) A 12-year-old HIV-positive patient with a cortical lesion that did not respond despite 12 months of antituberculous treatment. Initial magnetic resonance image shows a T2 hypointense, inhomogeneous cortical tuberculoma in the left parietal lobe. (b) At surgery, the lesion was easily separated from the surrounding brain. (c) Histology showed typical features of a tuberculous granuloma.
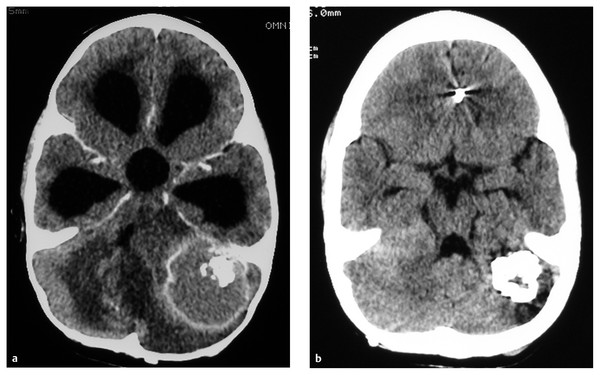
Fig. 80.3 Posterior fossa tuberculoma. (a) This patient presented with hydrocephalus, which was treated with a ventriculoperitoneal shunt, although our current practice is to consider an endoscopic third ventriculostomy. (b) The lesion responded well to antituberculous treatment.
80.1.3 Other Tuberculous Lesions
Tuberculous Abscess
Tuberculous abscess is an encapsulation of pus containing viable tubercle bacilli and must be distinguished from a tuberculoma with central liquefaction.53 Tuberculous abscesses may be refractory to treatment despite repeated surgical drainage, and in one small series, the addition of thalidomide to the antituberculous treatment regimen resulted in significant improvement.54 This effect is mediated via cytokines, such as TNF-α.4 Another immunologically mediated condition is the immune reconstitution inflammatory syndrome (IRIS) seen in HIV-infected patients following the commencement of combination antiretroviral therapy (cART).55
Tuberculous Osteitis
Tuberculosis may on rare occasions involve the bones of the skull base or the vault (the lesions are often referred to as caries).56 Tuberculous osteitis may present as a fluctuant scalp swelling (cold abscess) or as an ulcerated lesion that is easily mistaken for an infected scalp laceration. If the correct diagnosis is made, surgery may not be indicated because the lesion will usually respond to antituberculous drugs.
Solitary Small Enhancing Cerebral Lesions
The small solitary cerebral lesion in a patient with seizures remains a subject of considerable interest. Although the differential diagnosis for a solitary cerebral enhancing lesion is extensive, the two most common inflammatory diagnoses, particularly in regions where they are endemic, remain tuberculosis and cysticercosis.57 The relevant clinical and imaging features of these two conditions are summarized in ▶ Table 80.1. An Indian series comparing the features of these two inflammatory lesions listed some of the differentiating characteristics.57 Active tuberculosis requires the commencement of antituberculous medication, whereas cysticercosis is usually self-limiting, requiring only single-agent antiepileptic medication with regular clinical and imaging follow-up to monitor resolution of the lesion.58,59
| Neurocysticercal lesion | Tuberculous lesion | |
| Number | Usually single, can be multiple | May be single or multiple |
| Location | Usually frontal and parietal lobes | Can occur supratentorially or infratentorially |
| Edema | Minimal, depends on phase of development, midline shift unusual | Extensive surrounding edema, often with midline shift |
| Size | Dimension < 20 mm | Dimension > 20 mm |
| Enhancement pattern | Usually ring-enhancing, may have central hyperdensity (scolex); calcification usually indicates inactive lesion | Heterogeneous enhancement |
| Clinical features | Usually presents with seizures, raised intracranial pressure or progressive neurologic deficit rare | Usually features of raised intracranial pressure and/or progressive neurologic deficit |
| Disease course | Usually self-limiting, may require antiepileptic drugs for seizure control | Antituberculous medication required for progressive disease |
| Systemic involvement | None | May have systemic or pulmonary tuberculosis |
80.2 Parasitic Infestations
Parasitic infestation of the CNS affects millions of people, mostly in the developing world, where poverty and related conditions prevail, although a change in the geographic distribution of these diseases has become more evident in the era of globalization. Parasitic infestations can be broadly classified as protozoan (caused by single-celled organisms) and metazoan/helminthic (caused by complex, multicellular organisms).
80.2.1 Cysticercosis
Cysticercosis was initially described by the ancient Greeks, who identified cysticerci (meaning “bladder tails”) in pork.60 Neurocysticercosis results from infestation of the CNS by larvae the helminthic tapeworm Taenia solium. Cysticercosis is the most common CNS parasitic infestation in humans and a leading cause of acquired epilepsy.61
Cysticercosis is widely endemic in those parts of the developing world where pork is consumed and sanitation is poor.62 T. solium infection is endemic in Central and South America and in non-Islamic regions of Asia and sub-Saharan Africa. In certain developed countries, the prevalence appears to be rising as a consequence of the increasing migrant workforce,63–65 with some authors suggesting that the screening of potentially “at-risk” household employees be considered before employment.66
The life cycle of T. solium involves pigs as intermediate hosts, after they ingest the eggs, and humans as definitive hosts, when they harbor the egg-producing adult tapeworm. The cycle is completed when a human inadvertently consumes measly pork contaminated with viable T. solium eggs. The oncospheres enter the mucosa of the small intestine, mature over a 2- or 3-month period, and then spread hematogenously, displaying a predilection for muscle, brain, and skin.67 Humans may also be autoinfected, either externally (fecal–oral route) or internally (reverse peristalsis). Between 5 and 40% of adult carriers of T. solium will develop cysticercosis.68
Once an oncosphere enters the brain parenchyma, it develops through four identifiable phases: (1) vesicular phase (viable cyst with minimal host response); (2) colloidal phase (rupture of a degenerating cyst into surrounding parenchyma, inciting a strong immune response); (3) granular–nodular phase (further degeneration, with formation of a nodular cyst); and (4) calcified phase (death and calcification of the cyst).69
Immunocompromised patients appear more susceptible to multiple-organ involvement, with HIV coinfection in neurocysticercosis increasing the risk for basilar meningitis and the formation of giant cysts.70,71 The effect of HIV infection on the natural history of neurocysticercosis, however, remains unclear.72 Carpio et al73 have proposed a useful classification for neurocysticercosis based on viability and location: active when the parasite is alive, transitional if it is degenerating, and inactive if the parasite is dead. Each of these categories can be subclassified as parenchymal or extraparenchymal (i.e., meningeal, intraventricular, and subarachnoid forms).73,74 Traditionally, the term cysticercus cellulosae is used to describe thin-walled, 3- to 20-mm cysts (containing scolices) occurring within the parenchyma, and cysticercus racemosus to describe larger, “grapelike” vesicles (without scolices), usually located in the basal cisterns, ventricles, or sylvian fissure.69,75
The clinical manifestations of neurocysticercosis vary, depending on the number, size, location, and developmental stage of the cysticercal lesions, as well as the host immune response.76 The most common clinical manifestations are seizures, headache, and focal neurologic deficits. The parenchymal form presents with seizures or focal neurologic deficits caused by direct compression. The meningeal form may present with signs of raised ICP that is due to widespread arachnoiditis and adhesions leading to hydrocephalus. Cranial nerve palsies may be caused by vasculitis or fibrous adhesions. The intraventricular or cisternal form presents mostly with raised ICP due to hydrocephalus resulting from the obstruction of CSF flow. This form tends to be rapidly progressive.77 Rarer neurologic manifestations include neurocognitive impairment, altered mental state, brainstem dysfunction, strokelike symptoms, and extrapyramidal signs.59
Diagnostic criteria based on objective clinical findings, imaging features, and immunologic and epidemiologic data have been proposed.78 In a hospital-based setting, however, neuroimaging remains the mainstay, with a definitive diagnosis made on the basis of histopathologic evidence. Lateral X-rays of the thigh and calf, in suspected cases of cysticercosis, often demonstrate cigar-shaped calcifications and may be useful in confirming the diagnosis. CT scans in the active phase of the disease demonstrate hypodense lesions, which may be single or multiple and of varying size, containing a hyperdense mural nodule (▶ Fig. 80.4). Ring enhancement is suggestive of perilesional inflammation, and calcification, which denotes inactive, degenerated lesions, is present in 50% of cases. MR imaging is more sensitive in demonstrating smaller cysts within the ventricular and subarachnoid spaces as well as the various stages of development of the parasite. The cysts display distinctive features on both T1- and T2-weighted imaging79
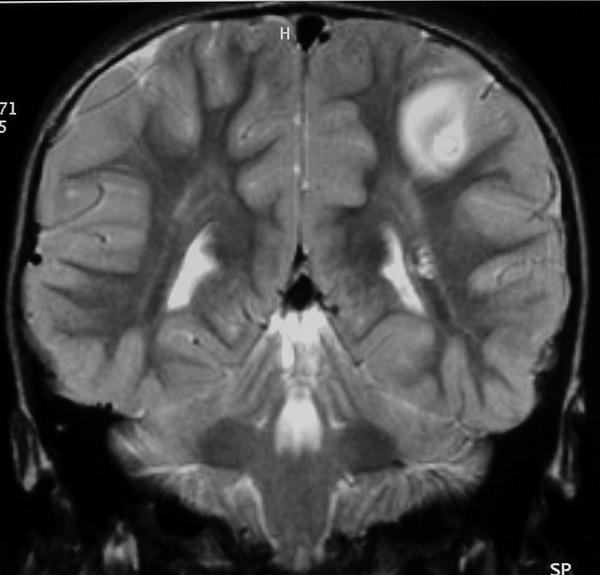
Fig. 80.4 Solitary neurocysticercus on T2 MR image (a) and anatomical pathology specimen (b) of an encysted cysticercus metacestode displaying a protoscolex. The germinal tissue forming the cyst wall is not marginated, consistent with viability and likely complete absence of contrast enhancement.
(Courtesy of Dr. Richard Hewlett.)
Laboratory studies are useful in confirming the diagnosis. CSF mononuclear pleocytosis with raised protein and low glucose levels are typical findings in extraparenchymal disease, particularly with arachnoiditis.80 Immunodiagnostic methods capable of detecting antibodies include complement fixation, indirect hemagglutination, enzyme-linked immunosorbent assay (ELISA), and enzyme-linked immunoelectrotransfer blot (EITB). ELISA and EITB are the most frequently used tests for the diagnosis of human cysticercosis, with EITB demonstrating 90% sensitivity for detecting human cysticercosis, but a much lower sensitivity for detecting single intracerebral lesions.81 In children with a single intracerebral lesion, EITB can yield false-negative results in 75% of cases.82 Serologic results, therefore, need to be interpreted together with clinical and radiologic findings.
The treatment modalities for neurocysticercosis include antiparasitic agents, symptomatic medication, and surgery. Guidelines proposed in 200283 suggest that there is insufficient evidence to support the recommendation of the antihelminthic drugs praziquantel and albendazole in neurocysticercosis and therefore recommend that treatment be individualized in terms of burden of disease and clinical presentation. Antihelminthic drugs are generally considered useful for long-term seizure control and for persisting or enlarging parenchymal lesions.83 Albendazole appears superior to praziquantel because the latter has less efficacy for extraparenchymal lesions, interacts with steroids and antiepiletics, and may increase the risk for stroke.84–86 Antiheminthics should be used with caution in patients in whom raised ICP is suspected because the inflammatory response they evoke can precipitate further neurologic deterioration.
The use of corticosteroids in conjunction with antihelminthics is generally recommended because they reduce the inflammatory reaction caused by the death of the larvae and are beneficial in cases of cysticercotic encephalitis, as well as in cases with involvement of the meninges and subarachnoid space. Antiepileptic drugs remain the principal therapy for seizure control in neurocysticercosis.83 Standard first-line antiepileptic drugs are usually adequate as single agents. The duration of treatment is controversial, however, and gradual cessation of the medication after resolution of the parasitic infection on imaging studies has been recommended.61–63 Standard treatment regimens appear effective in immunocompromised hosts, but the impact of antiretroviral therapy and immune reconstitution on the severity of the disease remains uncertain.72
Neurosurgical intervention is indicated for (1) obtaining a tissue diagnosis, (2) relieving mass effect caused by the cyst, (3) treating raised ICP, (4) diverting the CSF to manage hydrocephalus, and 5) decompressing a compressed spinal cord and roots. A tissue diagnosis can be obtained via stereotactic biopsy or open craniotomy and resection of the lesion. Patients with neurocysts causing significant mass effect may require a craniotomy for adequate exposure and removal, and those with raised ICP due to hydrocephalus may require insertion of an external ventricular drain as a temporizing measure. Rates of ventriculoperitoneal shunt failure in neurocysticercosis are reportedly as high as 67%.87 Endoscopic approaches have gained increasing popularity for removing ventricular neurocysts, either as stand-alone treatments or in combination with ETV to treat hydrocephalus.88,89 In this setting, ETV is most useful when there is a noninflammatory obstruction at the level of the aqueduct.90
Patients with parenchymal forms of neurocysticercosis have a better prognosis than those with the extraparenchymal form, particularly when the latter causes hydrocephalus and arachnoiditis.91 The eradication of cysticercosis remains dependent on prevention, which requires improvement in human sanitation and the public health infrastructure. This disease remains a biological marker of poverty and social disparity.
80.2.2 Echinococcosis (Hydatid Disease)
Hydatid disease is caused by the cestode Echinococcus. The disease is prevalent worldwide but is endemic in the sheep- and cattle-raising areas of the world, particularly Latin America, Australia, Mediterranean countries, the Middle East, and India. The most common genus found in humans is Echinococcus granulosus, with Echinococcus multilocularis and Echinococcus vogeli seen rarely. The definitive hosts for Echinococcus are canines, such as dogs, wolves, and foxes.92 Humans inadvertently serve as intermediate hosts by ingesting food contaminated with dog feces containing viable parasite eggs. The eggs form oncospheres in the human intestine, penetrate the mucosa, and spread hematogenously to the liver (75%), lungs (15%), and brain (2%).93,94 They usually grow at a rate of 1 cm per year, but growth of 5 to 10 cm within the first year has been reported,95 and the eggs are able to survive for many years. Although cerebral involvement is rare, the majority of cases of cystic hydatid disease, up to 75%, are found in children.96–98 Most cysts are single and unilocular and are located in the distribution of the middle cerebral artery, but they may also occur infratentorially.92 Rupture of the cyst is uncommon but may occur spontaneously or as a result of trauma.
There is a wide spectrum of clinical presentations in cerebral hydatid disease, with the presentation depending on the number, size, and location of the cysts as well as on the host immune response. Most patients present with symptoms and signs of raised ICP (i.e., headache, nausea, vomiting, papilledema, and focal neurologic deficit). Seizures may occur but are uncommon.
Hydatid disease can be diagnosed in a patient with an appropriate history (resident in an area where the disease is endemic) based on the imaging and clinical findings, supported by serologic tests or the definitive identification of E. granulosus on histopathology. Serologic tests have a low sensitivity in cerebral hydatid disease, and characteristic imaging findings may still be diagnostic despite negative serology.92,93,99 Cerebral hydatid cysts appear as large, unilocular, thin-walled cysts, usually without calcification or surrounding edema, containing fluid with a density similar to that of CSF on CT scans and MR imaging (▶ Fig. 80.5). Calcification on a CT scan usually signifies death of the parasite, but areas of calcification and irregularity along the cyst wall may suggest previous rupture.100
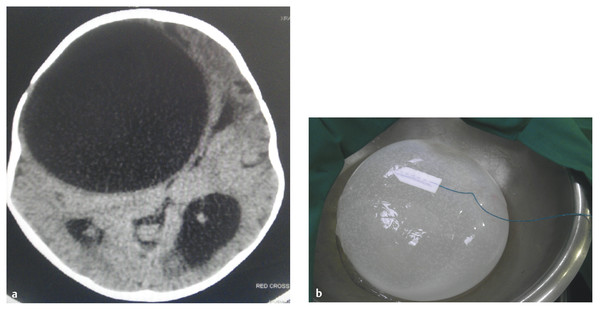
Fig. 80.5 (a) Very large frontal hydatid cyst seen on axial computed tomographic scan and (b) operative specimen following removal.









