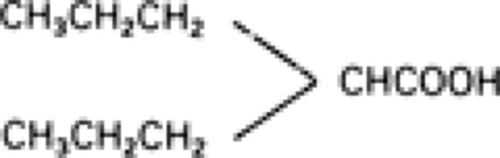Valproate
Ahmad A. Beydoun
Kevin Farrell
Wassim M. Nasreddine
Introduction
Valproic acid was first synthesized in 1882 as an organic solvent in research laboratories.59 Its anticonvulsant effects were discovered serendipitously in 1962, when it was used as a lipophilic vehicle for dissolving water-insoluble khellin derivatives. A significant anticonvulsant effect against pentylenetetrazol-induced seizures was observed in the vehicle control.94 The first clinical trials of valproic acid were reported in 1964.24 It was initially marketed in France in 1967, and later approved by the U.S. Food and Drug Administration (FDA) as a treatment for epilepsy in 1978. Since then, valproic acid has established itself as one of the major antiepileptic drugs (AEDs) with efficacy against multiple seizure types.
Chemical Structure and Formulations
Valproic acid is a simple, branched-chain carboxylic acid with a structure unlike other AEDs77 (Fig. 1). It is a colorless liquid with a pKa value of 4.56 and a molecular weight of 144.21.76 Because it is highly ionized at a pH of 7.4, valproic acid is less lipophilic than the standard AEDs.78 This results in a low volume of distribution, because only the nonionized, lipid-soluble part of a drug distributes from blood to tissues via passive diffusion. Therefore, the rapid penetration of valproic acid into the brain cannot result from its chemical properties and is thought to be mediated by active transport mechanisms across the blood–brain barrier.77
Valproate (VPA) is available in multiple forms. Sodium valproate is the sodium salt and dissociates rapidly in the body to valproic acid. Divalproex sodium is a stable coordination compound, comprised of sodium valproate and valproic acid in a 1:1 molar relationship, which dissociates in the gastrointestinal tract into valproic acid.
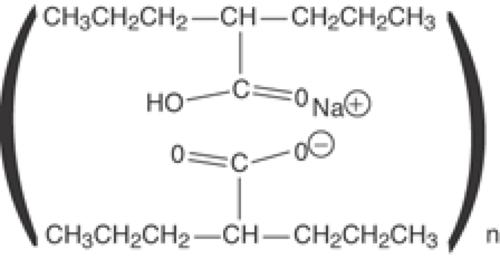
The extended release divalproex (Divalproex ER) is a newer formulation (Fig. 2) consisting of a hydrophilic polymer matrix, controlled-release tablet system, which allows for the slow release of the drug in the stomach, small intestine, and large intestine over an 18- to 24-hour period.2 This extended-release product is intended for once-a-day oral administration.
Pharmacology
Activity in Experimental Models and Mechanisms of Action
Experimentally, VPA has an anticonvulsant effect on almost all animal models of seizures including different types of generalized and partial seizures.77 The cellular mechanisms of action of VPA include primarily potentiation of γ-aminobutyric acid (GABA)ergic mechanisms and, less importantly, blockades of voltage-dependent sodium (Na) channels and glutamatergic mechanisms.77
VPA increases cerebrospinal fluid (CSF)79 and whole-brain GABA levels.52,100 This increase in GABA level may be due to an inhibitory effect on GABA degradation or an enhancement of GABA synthesis.77 Another potentially important mechanism is direct potentiation of the neuronal responses to GABA.82,105 VPA suppresses glutamate responses and N-methyl-D-aspartate (NMDA)-evoked transient depolarization in rat neocortex.148 This attenuation of NMDA receptor–mediated excitation may be an essential mode of action for the anticonvulsant effect of VPA. VPA was also shown to diminish the high-frequency repetitive firing of action potentials of cultured central neurons.77 It was suggested that the most likely explanation for this effect is through use-dependent reduction of the inward Na current.91 Although this mechanism is somewhat similar to that of carbamazepine and phenytoin, the reduction in sustained repetitive firing in the case of VPA might be due to enhancement of potassium (K) channels involved in action potential repolarization.46,95
VPA was shown to block low-threshold T-type calcium (Ca) channels in peripheral ganglion neurons but not in thalamic neurons.32,71
Clinical Pharmacokinetics
Absorption, Protein Binding, and Distribution
Valproic acid bioavailability after oral administration ranges from 70% to 100% in humans.77 The bioavailability of divalproex ER is approximately 8% to 20% less than divalproex. Proportional dosing with 8% to 20% higher daily doses of divalproex ER compared with divalproex was shown to produce equivalent serum VPA levels in healthy volunteers and patients with epilepsy.41,129
Following oral administration, peak plasma VPA concentrations (Cmax) are achieved within 2 hours (Tmax) for valproic acid and sodium VPA, and within 3 to 8 hours for divalproex and divalproex ER.70
Valproic acid is highly protein bound (∼90%), and this binding is saturable at therapeutic levels. For example, when the total VPA serum level increases from 50 to 150 μg/mL, the serum level of the free fraction of VPA increases from 3.5 to 45 μg/mL.33 The apparent volume of distribution of VPA is 0.13 to 0.19 L/kg, with the central nervous system (CNS) concentration averaging 20% of the serum concentration.77
Metabolism and Elimination
VPA is primarily metabolized by three primary pathways in the liver to a number of metabolites, some of which are
biologically active.70 Less than 5% of the parent compound is excreted unchanged in the urine. The primary pathway is through mitochondrial β-oxidation to three metabolites: 2–en-VPA, 3–OH-VPA, and 3–oxo-VPA. The 2–en-VPA is biologically active, with a long half-life.140 The second pathway is via the cytochrome P450 system to toxic intermediary metabolites known as 4–en-VPA and 2,4–en-VPA. The concomitant use of hepatic enzyme inducers activates this pathway and increases the toxic metabolites of VPA. The third pathway consists of glucuronidation of VPA to a number of inactive compounds.
biologically active.70 Less than 5% of the parent compound is excreted unchanged in the urine. The primary pathway is through mitochondrial β-oxidation to three metabolites: 2–en-VPA, 3–OH-VPA, and 3–oxo-VPA. The 2–en-VPA is biologically active, with a long half-life.140 The second pathway is via the cytochrome P450 system to toxic intermediary metabolites known as 4–en-VPA and 2,4–en-VPA. The concomitant use of hepatic enzyme inducers activates this pathway and increases the toxic metabolites of VPA. The third pathway consists of glucuronidation of VPA to a number of inactive compounds.
Efficacy
Absence Seizures
Typical absence seizures can occur in a number of epilepsy syndromes, including childhood absence (pyknolepsy), juvenile absence, juvenile myoclonic, and myoclonic absence epilepsies. VPA is considered to be a first-line agent for the treatment of absence seizures despite a paucity of data derived from well-designed clinical trials. Three small studies compared the efficacy of VPA and ethosuximide for children and adolescents with absence seizures.23,87,123 Two were open-label, parallel group trials that randomized patients with newly,23 or recently diagnosed87 simple absence seizures to VPA or ethosuximide monotherapy. The third was a randomized double-blind conditional cross-over study that randomized 45 children and adolescents with newly diagnosed or refractory absence seizures to monotherapy or add-on treatment with VPA or ethosuximide.123 All trials found a comparable efficacy between the two AEDs, with seizure-free rates ranging between 40% and 86%.111 However, none of those trials was powered to detect equivalence. The most common side effects reported in those studies consisted of nausea, vomiting, drowsiness, thrombocytopenia, and leukopenia.
A more recent open-label trial compared VPA with lamotrigine in 38 children newly diagnosed with childhood or juvenile absence seizures.30 The dose of VPA was initiated at 10 mg/kg per day and, if needed, increased by 5 mg/kg per day every 3 days up to a maximum dose of 30 mg/kg per day. Lamotrigine was started at 0.5 mg/kg per day for 2 weeks, titrated to 1 mg/kg per day for 2 weeks with increments, if needed, of 1 mg/kg per day every 5 days up to a maximum dose of 12 mg/kg per day. The percent of seizure-free patients at 1 month were 52.6% and 5.3% in the VPA and lamotrigine groups, respectively (p = 0.004). At 3 months, those percentages were 63.1% and 36.8%, respectively, a difference that did not reach statistical significance. After 12 months of treatment, the percentages of seizure-free patients were 68.4% and 52.6% in the VPA and lamotrigine groups, respectively. Adverse events occurred in 10.6% of children treated with VPA and 31.8% of those treated with lamotrigine. This study showed that both VPA and lamotrigine can be efficacious against absence seizures, but that VPA’s efficacy is much faster, partly due to its shorter titration schedule.30 It is, however, important to note that this trial was not powered to detect equivalence, and that the lack of a significant difference in efficacy between the two drugs could be due to the small sample size.
Juvenile Myoclonic Epilepsy
VPA is often considered the drug of choice in juvenile myoclonic epilepsy, an epilepsy syndrome characterized by myoclonus, generalized tonic–clonic seizures, with or without absence seizures. This is mostly based on clinical experience, because the evidence derived from clinical trials is scant and anectodal.35,54,107
Partial-Onset Seizures and Generalized Tonic–Clonic Seizures
The fact that VPA possesses anticonvulsant properties as monotherapy and add-on therapy against partial-onset seizures was unequivocally demonstrated in double-blind, parallel group, multicenter superiority trials.13,142 The monotherapy study was a concentration-dependent trial that randomized 143 patients with medically refractory partial-onset seizures to high (80–150 μg/mL) or low (25–50 μg/mL) plasma VPA groups. The efficacy results were significantly in favor of the high plasma VPA, with a 30% median reduction in complex partial seizure frequency compared to baseline, versus a 19% increase for patients randomized to the low plasma VPA group.13 Adverse events that occurred significantly more frequently in the high plasma VPA group included tremors, thrombocytopenia, alopecia, asthenia, diarrhea, vomiting, and anorexia.13
The adjunctive trial randomized 144 patients experiencing at least eight complex partial seizures over an 8-week period while maintained on carbamazepine or phenytoin monotherapy to add-on treatment with VPA or placebo.142 Add-on treatment with VPA resulted in a median reduction of 7.9 complex partial seizures per 8 weeks compared with 2.5 in the placebo group (p = 0.001). In addition, the 50% responder rates were 38% and 19%, in the add-on VPA group and placebo group, respectively (p = 0.011).
The comparative trials that evaluated the relative efficacy and effectiveness of VPA versus other standard or newer AEDs have yielded conflicting results, especially with regard to efficacy against partial seizures. A number of open-label, active-control trials compared the relative efficacy of VPA, carbamazepine, phenytoin, and phenobarbital in adults and children with newly diagnosed epilepsy.22,36,58,80,115,117,133,136 Most trials only enrolled patients with newly diagnosed epilepsy, including those with partial-onset and generalized tonic–clonic seizures,22,36,57,117,133,136 whereas one trial only enrolled patients with primarily generalized tonic–clonic seizures.115 All trials reported similar efficacy between VPA and the other AEDs on the outcome variables that included time to first
seizure, percent of seizure-free patients, and time to seizure remission. Those results were similar when data from patients with partial-onset seizures and primarily generalized tonic–clonic seizures were separately analyzed.
seizure, percent of seizure-free patients, and time to seizure remission. Those results were similar when data from patients with partial-onset seizures and primarily generalized tonic–clonic seizures were separately analyzed.
The only double-blind comparative trial was an active-control study that compared VPA to carbamazepine in 480 patients with partial-onset seizures.88 Patients were stratified into two groups, one consisting of those with predominantly secondarily generalized seizures and the other of those with predominantly complex partial seizures. For patients with predominantly secondarily generalized tonic–clonic seizures, no significant efficacy differences were noted between VPA and carbamazepine. On the other hand, the efficacy of carbamazepine was reported to be significantly better than VPA for patients with predominantly complex partial seizures, based on multiple outcome measures, including time to first seizure and a composite score of seizure severity.88 However, the percentages of patients who remained seizure-free at 1 year were comparable between the two groups. A number of factors were suggested to explain the discrepancy of results between this trial and the open-label comparative trials, including that the majority of patients in the VA cooperative trial were males, and that only approximately 50% of the participants were newly diagnosed with epilepsy.116 However, a meta-analysis of trials comparing carbamazepine and VPA found that, for partial-onset seizures, carbamazepine was significantly superior for time to 12 months of seizure remission and time to first seizure.86 A separate meta-analysis failed to find significant difference in efficacy between VPA and phenytoin for partial seizures or primarily generalized tonic–clonic seizures.132
Two comparative trials evaluated VPA with one of the newer AEDs. In a double-blind, parallel-group trial, 249 patients with newly diagnosed epilepsy, aged 15 to 65 years and experiencing partial-onset seizures or generalized tonic–clonic seizures, were randomized to treatment with VPA or oxcarbazepine.26 The study consisted of an 8-week titration followed by a 48–week maintenance period. The results showed no significant efficacy difference between the two groups, with 53.8% and 56.6% of patients randomized to VPA and oxcarbazepine, respectively, remaining seizure-free throughout the 48–week maintenance period. The numbers of premature discontinuations due to adverse events were also comparable between the two groups.26
A more recent active-control, double-blind trial compared the efficacy of topiramate with the investigator’s choice of carbamazepine or VPA as initial treatment for patients with newly diagnosed epilepsy.113 The investigators were asked to select carbamazepine (600 mg/day) or VPA (1,250 mg/day) based on the clinical presentation. Within each group, patients were randomized in a double-blind fashion to treatment with one of the two standard AEDs versus topiramate administered at 100 mg/day or 200 mg/day. Patients continued double-blind treatment until exiting the study or until 6 months after the last patient was randomized. Of the 78 patients randomized to VPA, 42% were considered to have partial-onset seizures, 63% primarily generalized tonic or clonic or tonic–clonic seizures, and 1% could not be classified. In that subgroup, 145 patients were randomized to topiramate (100 mg/day or 200 mg/day), with 41% considered to have partial-onset seizures, 65% primarily generalized tonic or clonic or tonic–clonic seizures, and 2% could not be classified. The results indicated no significant difference in time to exit, adverse events, or inadequate effect between the VPA and topiramate groups.113
Infantile Spasms
Two uncontrolled, open-label, prospective studies suggested the efficacy of VPA against infantile spasms45,126 at daily doses ranging from 25 to 100 mg/kg per day. Cessation of spasms occurred in 72% to 73% of children within 2 weeks to 3 months after initiation of therapy, with a 23% relapse rate reported in one of the studies.126 Consequent to the high doses used, approximately one-third of the children developed thrombocytopenia.126 Long-term outcome of children with infantile spasms was evaluated in two retrospective studies, with conflicting results.61,103 One study, which compared treatment with adrenocorticotropic hormone (ACTH), pyridoxine, and VPA reported that none of the VPA-treated patients had a normal IQ, but that all were seizure-free.104 In the other study, which compared ACTH to VPA, only 26% of children were seizure-free, with no significant difference between the two groups.61
The data on VPA in infantile spasm are largely anecdotal and insufficient to recommend VPA as initial treatment of infantile spasms83 but may be considered in patients with spasms that are refractory to ACTH and vigabatrin.
Febrile Seizures
A number of studies evaluated the efficacy of continuous treatment with VPA in the prevention of recurrent febrile seizures (FS).73,84,101,138,141 In an open-label, randomized trial of 58 children who experienced their second simple febrile seizure, the recurrence rate did not differ between the VPA-treated group and the no-treatment group.141 The other trials comparing the relative efficacy of VPA to that of phenobarbital and placebo or no treatment found that VPA was significantly better than no treatment or placebo in preventing further FS, and that its efficacy did not significantly differ from that of phenobarbital.73,84,101,138 For example, 73 children with their first generalized febrile seizure were randomized in a single-blind, placebo-controlled clinical trial to treatment with VPA, phenobarbital, or placebo.84 The recurrence rates were 4%, 19%, and 35% in the VPA, phenobarbital, and placebo groups, respectively. Similar results were reported in another, double-blind, placebo-controlled trial conducted in children enrolled after their first simple febrile seizure.101
Those data provide evidence that continuous treatment with VPA significantly reduces the likelihood of further FS and that its efficacy in that regard is overall at least comparable to that of phenobarbital. However, the Practice Parameter developed by the American Academy of Pediatrics does not recommend the use of daily anticonvulsant treatment, because it does not influence the long-term outcome in children with simple FS.7 In addition, the risk of serious hepatotoxicity is greatest in the age group in which FS usually present, and studies of valproic acid in FS were insufficiently powered to detect a risk of serious hepatotoxicity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree