Vein of Galen Aneurysmal Malformation
Vein of Galen aneurysmal malformation (VGAM) is a rare vascular malformation involving the embryonic precursor of the vein of Galen. “Vein of Galen malformations” are reported to account for fewer than l% of all arteriovenous malformations (AVMs) in the cooperative study of subarachnoid hemorrhage.1–3 However, the true incidence is difficult to determine because significant diagnostic confusion exists among the various malformations that involve or drain into the vein of Galen or its embryonic precursor, the median vein of the prosencephalon. We define VGAM as a purely fistulous AVM draining to the median vein of the prosencephalon. Other vascular lesions that cause dilatation of the true vein of Galen are defined as vein of Galen aneurysmal dilatation (VGAD) or vein of Galen varix (VGV). VGADs are a group of malformations that drain pial or dural shunts into the true vein of Galen or its tributary and are associated with dilatation of the vein of Galen. VGV is a dilated vein of Galen without arteriovenous (AV) shunts. We describe the vascular anatomy, types, clinical manifestations, natural history, treatment, and outcome of VGAM and its distinctions from VGAD and VGV.
69.1 Vascular Anatomy
Raybaud et al4 reported in 1989 that the dilated midline vein in VGAM is not the true vein of Galen but its embryologic precursor, the median vein of the prosencephalon. Based on analysis of the vascular anatomy of VGAM and vascular embryology, a VGAM likely forms within an embryonic stage of 21 to 23 mm (6 weeks) and 50 mm (11 weeks), during the time when the vein of Galen and straight sinus normally develop from a transient precursor, the median vein of the prosencephalon.4
Therefore, a true vein of Galen does not exist in VGAM. Moreover, in VGAM, the median vein of the prosencephalon drains only AV shunts. In VGAM, the normal deep cerebral structures drain through alternate venous pathways, typically through a vein that has an epsilon shape on the lateral view of a cerebral angiogram.5,6 Further drainage of the malformation is in most cases through the embryonic falcine sinus (precursor of the straight sinus) to the superior sagittal sinus. A straight sinus is usually absent, and persistence of other embryologic sinuses, such as occipital and marginal sinuses, is frequently observed in patients with VGAMs. Persisting arterial anomalies such as a limbic arterial ring involving the anterior and posterior choroidal arteries and pericallosal arteries are also frequently present.
The AV fistula in VGAM can be single or multiple. Arterial anastomoses between feeding pedicles are often seen. Two subtypes of VGAM, choroidal and mural, can be distinguished.
69.2 Classification
69.2.1 Choroidal Vein of Galen Aneurysmal Malformation
In choroidal VGAM (▶ Fig. 69.1), multiple fistulas are located in the subarachnoid space in the choroidal fissure. These multiple fistulas communicate with the anterior aspect of the median vein of the prosencephalon. The arterial feeders are usually bilateral anterior and posterior choroidal arteries, a subforniceal branch, pericallosal arteries, and subependymal branches of thalamoperforators. This type of VGAM usually causes high-output cardiac failure in newborns because of the presence of multiple high-flow fistulas with less outflow restriction than in the other type of VGAM. This type of VGAM can be recognized by its complex arterial network and is the more aggressive manifestation of the disease. These lesions are more challenging to treat because of comorbid severe cardiopulmonary failure.
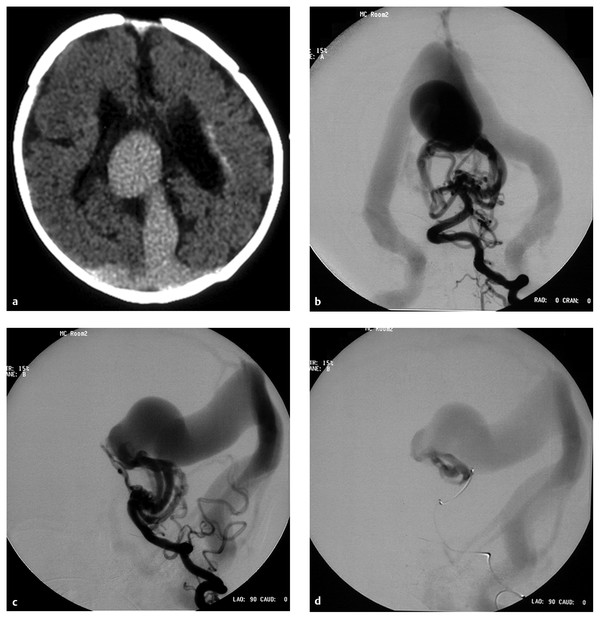
Fig. 69.1 A male patient presented with severe congestive heart failure (CHF) and acidosis at birth. His Apgar score was 5, with severe respiratory distress. The head circumference was 50 cm and the weight was 3.3 kg at birth. The patient was intubated and placed on dopamine and dobutamine drip as well as on furosemide. (a) A computed tomographic scan at 2 days old demonstrates a dilated midline vessel suggesting a vein of Galen aneurysmal malformation (VGAM) and mild hydrocephalus. There are early periventricular calcifications (arrow). Because of progression of the CHF, the patient underwent cerebral angiography and endovascular treatment at 4 days of age. Left vertebral artery injection in (b) anteroposterior (AP) and (c) lateral views demonstrates a choroidal-type VGAM draining to the median vein of the prosencephalon, then to the embryonic falcine sinus. Multiple fistulas to the anterior portion of the median vein of the prosencephalon are supplied by bilateral posterior choroidal arteries and thalamoperforators. No straight sinus is seen. (d) After the left vertebral angiogram, the left posterior choroidal feeder was superselectively catheterized. Superselective angiogram shows a high-flow fistula. This was embolized with N-butyl-cyanoacrylate (NBCA) under systemic hypotension.
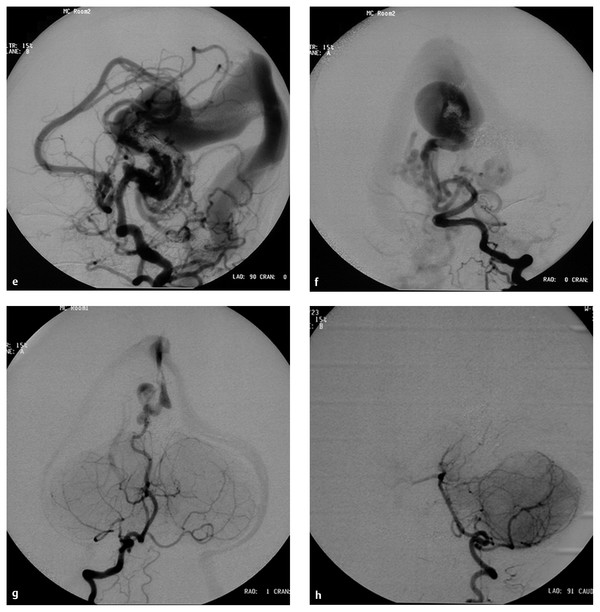
(e) After embolization of the left common carotid artery, the lateral view demonstrates the remaining fistulas supplied by the posterior choroidal arteries and the left anterior cerebral artery. (f) After embolization of the left vertebral artery, angiogram demonstrates decreased but remaining fistulas. The patient underwent another embolization at 11 days old because of persistent CHF. At 11 months old, the patient underwent a third-stage embolization. At this time, the patient had no CHF but had moderate developmental delay with hypotonia. (g) Right vertebral artery angiogram in the AP view demonstrates a significant decrease in the size of the fistula and the median vein of the prosencephalon. The remaining fistula is supplied by the posterior thalamoperforator, which is increased in size compared with the previous angiogram [compare with (f)]. Complete occlusion of the VGAM was accomplished by the third embolization. (h) The right vertebral artery in the AP view demonstrates no opacification of the remaining fistulas. Distal posterior cerebral arteries are not opacified in this injection.
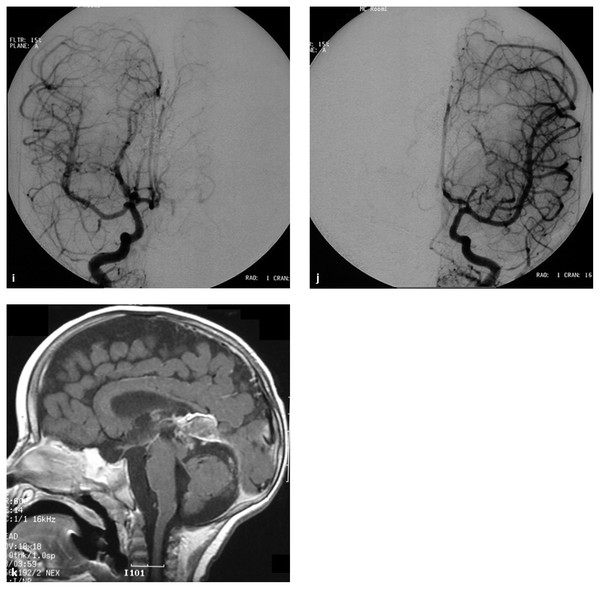
(i) Right and (j) left common carotid artery angiograms in the AP view demonstrate no opacification of the fistulas. Distal posterior cerebral arteries were opacified through the posterior communicating artery bilaterally. The patient remained with moderate developmental delay. (k) T1-weighted sagittal magnetic resonance imaging with contrast 2 days after the third embolization demonstrates thrombosis and shrinkage of the median vein of the prosencephalon and the embryonic falcine sinus.
69.2.2 Mural Vein of Galen Aneurysmal Malformation
In mural VGAM (▶ Fig. 69.2), the fistula is located in the subarachnoid space in the wall of the dilated median vein of the prosencephalon, usually along its inferolateral margin. The vessels supplying the shunt are usually the collicular and/or the posterior choroidal arteries and may be unilateral or bilateral. In contrast to the choroidal-type VGAM, the mural type has fewer fistulas and typically has more outflow restriction, which leads to dilatation of the median vein of the prosencephalon but may protect against high-output cardiac failure. The mural type of VGAM, therefore, clinically presents later in infancy with macrocephaly or failure to thrive and may be associated with mild cardiac failure or asymptomatic cardiomegaly.
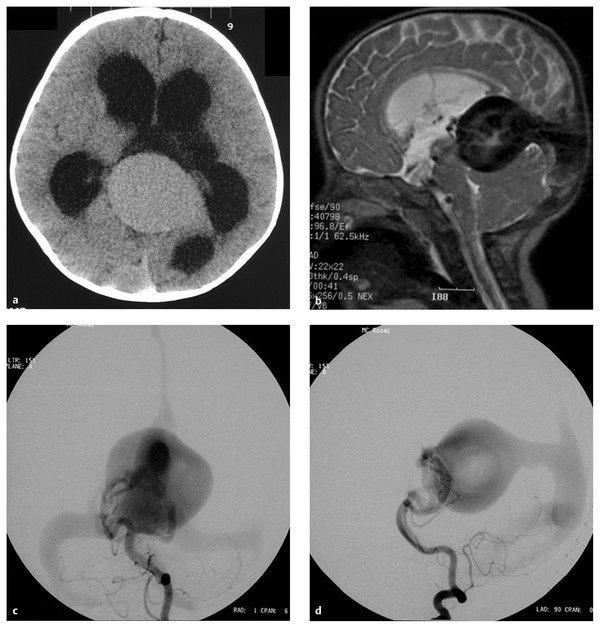
Fig. 69.2 A 6-month-old female patient presented with macrocephaly. The patient was developing normally. (a) Computed tomographic scan without contrast demonstrates hydrocephalus and a large midline vascular structure. (b) T2-weighted sagittal magnetic resonance (MR) image demonstrates a large midline vein draining to the embryonic falcine sinus and hydrocephalus. Left vertebral artery in the (c) anteroposterior (AP) and (d) lateral views demonstrates a mural- type vein of Galen aneurysmal malformation (VGAM) supplied by bilateral posterior choroidal arteries. The dilated median vein of the prosencephalon is draining to the embryonic falcine sinus with reflux to the superior sagittal sinus.
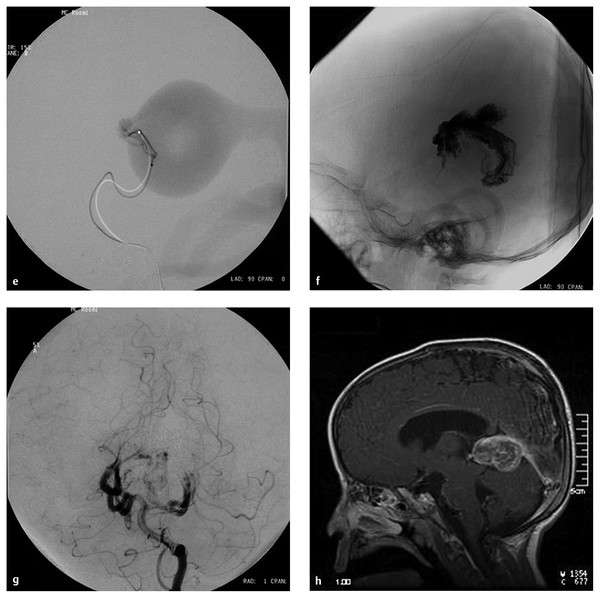
(e) Superselective angiogram of the left posterior choroidal artery showing a high-flow fistula. This was embolized with N-butyl-cyanoacrylate (NBCA). (f) Lateral skull film showing the NBCA cast penetrating into the venous side. (g) Left vertebral angiogram in the AP view after one NBCA embolization demonstrating almost complete obliteration of the fistulas with minimal residual shunting, showing that this VGAM was a single-hole mural-type fistula. (h) T1-weighted sagittal MR image with contrast 5 months after the embolization demonstrating complete thrombosis and shrinkage of the dilated vein. The ventricular size also decreased without ventriculoperitoneal shunt. The patient remained neurologically normal with normal development.
69.2.3 Vein of Galen Aneurysmal Dilatation
In contrast to VGAM, the midline ectatic vein in this group of lesions is the true vein of Galen. The vein of Galen, therefore, receives drainage from normal brain as well as from the malformation. Two types of VGAD, parenchymal and dural, can be distinguished.
Parenchymal Arteriovenous Malformation with Vein of Galen Aneurysmal Dilatation
Parenchymal VGADs (▶ Fig. 69.3) are subpial brain AVMs that drain into a tributary of the vein of Galen. Aneurysmal dilatation of the vein of the Galen is secondary to an outflow obstruction. Outflow obstruction develops in many pediatric fistulous malformations of the brain. The etiology of this outflow obstruction is unknown but may be related to underdevelopment of the jugular bulb, abnormal skull base maturation, and high-flow angiopathy of the venous system causing kinking or thrombosis at the tentorial or dural edge at the skull base. Because of this outflow restriction, the vein of Galen dilates and blood is refluxed into other, normal cerebral veins (internal cerebral, vermian, hippocampal, basal, medial ventricular, parietal, and occipital veins or other normal tributaries of the vein of Galen).
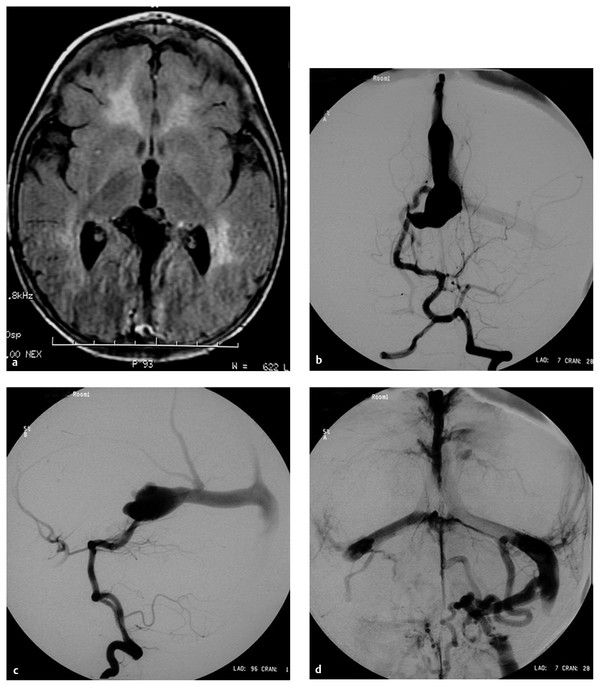
Fig. 69.3 A 12-month-old male patient presented with a 1-month history of seizures. (a) Magnetic resonance (MR) image demonstrates evidence of diffuse brain congestion and midline vascular malformation. The night before the angiography, the patient became obtunded with opisthotonic posturing. (b) Frontal and (c) lateral angiograms demonstrate an arteriovenous fistula from the right posterior choroidal artery to the dilated vein of Galen, which drains to the embryonic falcine sinus. There is reflux of venous drainage to the right basal vein of Rosenthal and the superior and inferior sagittal sinuses. (d) Late venous phase demonstrates significant venous stagnation and occlusion of both sigmoid sinuses with suboptimal collateral venous drainage. Acute venous hypertension due to sinus occlusion was thought to be the cause of acute deterioration.
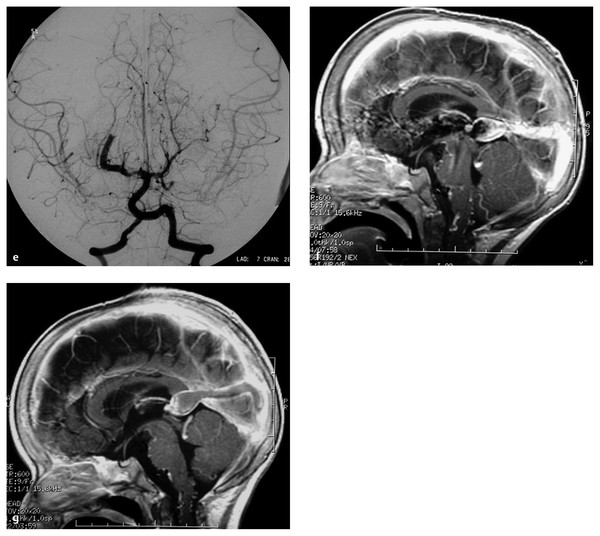
(e) The patient underwent endovascular embolization with N-butyl-cyanoacrylate (NBCA), resulting in complete occlusion of the single-hole fistula. T1-weighted sagittal MR imaging with contrast (f) before and (g) after the embolization showing decreased congestion of the brain and thrombosis of the vein of Galen and embryonic falcine sinus. Note the increased cerebrospinal fluid space and decreased size of the veins in the posterior fossa after embolization. The patient was discharged neurologically intact without seizures.
This type of VGAD usually presents in childhood or young adulthood with intracerebral hemorrhage, focal neurologic deficit, or seizures. The differentiation between a VGAM and a tectal AVM based only on angiography can sometimes be difficult, but demonstration of transmesencephalic feeders by magnetic resonance (MR) imaging confirms a tectal location and VGAD.7 For treatment, transvenous embolization is contraindicated in VGAD because it may produce extensive venous thrombosis in deep cerebral veins.
Dural Arteriovenous Malformation with Vein of Galen Aneurysmal Dilatation
Dural AVMs with VGAD are acquired lesions, usually presenting in the fourth or fifth decade of life, in which AV shunts are located in the wall of the vein of Galen itself. The dilatation of the vein of Galen is secondary to straight sinus stenosis or thrombosis. Reflux is always noted into afferent cerebral veins from the vein of Galen. The clinical presentation is similar to that of other dural AVMs draining into cerebral veins. Although the number of patients is small, the risk for progressive dementia in this group is higher than in patients with other dural AVMs. The arterial supply of a dural AVM with VGAD is predominantly from dural falcotentorial arteries of the carotid or vertebral system and from the vasa vasorum to the wall of the vein of Galen, which arise from pial arteries.8
69.2.4 Vein of Galen Varix
VGV is dilatation of the vein of Galen without the presence of an AV shunt. Two types have been encountered in children. One is transient dilatation of the vein of Galen in neonates presenting with cardiac failure from a cause other than VGAM. This dilatation is usually noticed on an ultrasound study and disappears in several days following improvement of cardiac conditions. The dilatation itself does not cause symptoms. The second type of VGV occurs as an anatomical variation in which venous drainage of the brain converges toward the deep venous system. It is asymptomatic, but the lack of compliance of this type of venous drainage may predispose to future venous thrombosis and resultant ischemic symptoms.
69.3 Clinical Presentation
Characteristic clinical manifestations of VGAM occur in each age group, neonatal and infant. In neonates, cardiac failure is usually the most prominent clinical feature, whereas in infants hydrodynamic macrocephaly and neurologic symptoms are more frequent.
69.3.1 Neonatal Cardiac Failure
In the neonate, cardiac failure is the most pressing symptom, ranging from asymptomatic cardiomegaly to multiple-organ failure. The reasons for this wide spectrum of cardiac manifestations are still poorly understood. The size of the shunt, the type of venous drainage, the degree of outflow restriction, the complexity of the arterial supply to the VGAM, and the different responses of individual patients to cardiac stress are contributory. After a brief initial period of stabilization, in most cases the neonate’s congestive heart failure (CHF) worsens during the first 3 to 5 days of life, then stabilizes and improves with appropriate medical management. Severe heart failure can cause neurologic dysfunction in newborns with VGAM. Cyanosis can be associated with the heart failure because of a right-to-left shunt from a patent ductus arteriosus, from a patent foramen ovale, or from pulmonary hypertension that may be irreversible, even after treatment. A patent ductus arteriosus can worsen the clinical situation and may require cardiac surgery. Cardiac failure has been diagnosed prenatally in a few patients and is usually associated with major neonatal cerebral ischemia and encephalomalacia.
Cardiac manifestations have been reviewed for antenatally diagnosed VGAM.9 In the series of Rodesch et al9 of 18 patients with antenatally diagnosed VGAM, 17 were born with cardiac failure. During antenatal ultrasound examination, cardiac enlargement was noted in 4 of 17 patients. These 4 patients all had a low neonatal score because of systemic failure or because of encephalomalacia. Treatment was withheld in these 4 patients, and they soon died. The others were medically managed and underwent embolization at between 2 and 13 months. Their neurologic outcome was reported to be excellent at 2-year follow-up. The antenatal diagnosis of VGAM is not an absolute indication for emergency embolization in the neonatal period. Macrocrania in uterus has no negative significance in our experience.
The pathophysiology of cardiac failure in neonates with high-flow AV fistulas starts with increased venous return and subsequent right-sided heart overload. The AV shunts therefore lead to right-sided heart dilatation, pulmonary arterial hypertension, and increased pulmonary blood flow. The resultant greater pulmonary venous return to the left side of the heart increases the left ventricular diastolic volume, which may be further increased by a patent ductus arteriosus if present. The increased left ventricular preload results in increased myocardial oxygen requirements. Coronary perfusion to the left ventricle occurs mainly during diastole and depends on the systemic arterial–intramyocardial diastolic pressure difference as well as the duration of diastole. Therefore, a reduction in arterial diastolic pressure (as observed in an AV shunt), an increase in end-diastolic pressure (due to increased preload), and a reduction in the diastolic period (tachycardia) are all detrimental to myocardial perfusion and hence oxygen delivery, which precipitates left ventricular failure. Thus, even if only the right ventricle fails initially, biventricular failure frequently follows depending on the size of the left-to-right shunt.
The neonatal heart is limited in its reserve capabilities and cannot cope with the extra work imposed by the fistula. These factors explain the rapid progression toward cardiogenic shock observed in neonates with high-flow fistulas. Furthermore, the transition from a fetal circulatory pattern to an adult circulatory pattern is complex, and both the pulmonary circulation and the systemic circulation remain highly unstable during the first week after birth. This can explain a persistent transitional circulation with shunts through a patent ductus arteriosus and a foramen ovale and pulmonary hypertension, which both worsen the systolic and diastolic wall stress.
Hepatomegaly with hepatic dysfunction, prerenal azotemia followed by oliguria, and metabolic and lactic acidosis are also present in severe cardiac failure and are negative predictive factors.
At present, in utero diagnosis has become more frequent, and we have developed a multidisciplinary strategy to optimize the care of these challenging patients. After the diagnosis is made, usually by prenatal ultrasound, we obtain MR imaging in utero to confirm the diagnosis and search for any obvious brain damage. The mother then undergoes an evaluation by a pediatric cardiologist, which includes fetal ultrasound and echocardiography. Fetal echocardiography is performed when a VGAM is diagnosed prenatally primarily to assess the fetal heart size and determine the presence of associated heart defects. The fetal heart size is assessed by measuring the cross-sectional area of the fetal heart in a four-chamber view and the cross-sectional area of the fetal chest. Normally, the cross-sectional area of the fetal heart is one-third of the cross-sectional area of the chest. This ratio may be increased to more than one-half in a fetus with VGAM. When the ratio of the cross-sectional area of the fetal heart to the cross-sectional area of the chest is 0.5 or more, the mother is started on digoxin. This is given under cardiac monitoring at doses of 0.5 mg intravenously every 6 hours until a maternal serum digoxin level of close to 2 ng/dL is reached. At that point, the patient is switched to an oral maintenance dose of digoxin, usually about 0.5 mg twice daily, and a digoxin level of approximately 2 ng/dL is maintained until delivery.
Neonatal Care
When possible, the patient is born at our hospital and transferred to the neonatal intensive care unit for immediate evaluation by the pediatric cardiologist and intensivist. The opportunity is taken to obtain umbilical arterial and venous access, which is critical. If needed, respiratory support with positive-pressure ventilation or intubation is initiated. A cardiovascular examination, echocardiography, and transcranial ultrasound are obtained on day 1 of life. Heart failure, when present, is managed with medications. If the patient cannot be weaned from ventilator support or intravenous medications or is progressing toward multiple-organ failure, then endovascular intervention to close as much of the fistula as possible and control the heart failure is considered. Otherwise, a stable patient is followed with a plan for treatment in 5 to 6 months. As our experience with newborns increases, our threshold for intervention has been lowered toward earlier intervention, which avoids the need to deal with a very sick newborn who has a greater likelihood of irreversible damage. We have found certain early echocardiographic findings to be prognostic indicators of the necessity of early intervention. In addition to generalized cardiomegaly and hypercontractility, these echocardiographic findings include pulmonary hypertension, frequently with suprasystemic pressure in the pulmonary artery as measured by tricuspid insufficiency velocity. There is persistent fetal circulation, with right-to-left shunting at the level of the patent ductus arteriosus and the patent foramen ovale. Almost all patients requiring neonatal intervention have dilatation of the ascending aorta and the carotid arteries, with Doppler findings of severe diastolic reversal of flow in the descending aorta. Patients with severe heart failure and pulmonary hypertension are maintained on mechanical ventilation, at times with the use of nitric oxide. They are started on intravenous inotropic agents, such as dopamine, dobutamine, and milrinone, as well as furosemide and digoxin. All patients undergo neuroimaging with MR imaging techniques, including diffusion-weighted imaging, MR angiography, and MR venography, before intervention. Neuroimaging assesses the brain parenchyma for underlying permanent damage or ischemic changes and delineates the vascular anatomy and venous outflow. High flow through the sinuses, without outflow restriction, also correlates with uncontrolled heart failure and the need for early intervention. Evidence of parenchymal damage is used for the prognosis and in the decision to proceed with or withhold treatment.
With the present availability of noninvasive imaging, there is no role for diagnostic angiography; the only reason to undertake invasive intervention is for emergent treatment, and this should be done in very specialized centers. All patients are treated with transarterial N-butyl-cyanoacrylate (NBCA) liquid embolic material, which is used to target the fistula sites of the malformation. Although in the past we have used transumbilical access to the cerebral circulation,24 at present we routinely use ultrasound-guided percutaneous femoral artery access. Transvenous access is used in older children who have no femoral access or the residual arterial supply is collaterals, distal perforators secondary to proximal pedicle occlusion versus fistula closure. In neonates, the venous approach was not necessary in any cases. The goal of embolization in the neonate is to reduce AV shunting to permit control of the CHF.
Endovascular Treatment
The treatment of a newborn in heart failure must be very efficient, with the extremely limited use of liquids and contrast material, which may be restricted by the patient’s weight (up to 10 mL/kg). If the treatment goal cannot be achieved in one session, the patient will undergo a second session of treatment another day. After the resolution of severe heart failure, patients return for further treatment sessions at approximately 6 to 8 months of age or earlier, if necessary. The final goal is complete obliteration of AV shunting without neurologic injury.
In 2012, we reported our results of the management of 16 patients who presented to the INN during the antenatal or neonatal period with a diagnosis of VGAM. Of 16 patients, 10 had an antenatal diagnosis of VGAM by prenatal ultrasound in the third trimester. VGAM was diagnosed in the remaining 6 patients after birth. Of the 16 patients, 7 responded to medical management in the neonatal intensive care unit, were discharged from the hospital, and returned for endovascular treatment at a later date. Nine patients required endovascular intervention because of refractory heart failure within the neonatal period. All 9 patients had a choroidal-type VGAM and required treatment within the first 10 days of life. Associated fetal cardiac defects were seen in 4 patients, who had sinus venosus, atrial septal defect, or a mild aortic coarctation (1 patient). Of 8 patients, 5 required more than one endovascular treatment session during the neonatal period to adequately control heart failure. Control of heart failure was achieved in 8 of 9 patients. One premature baby who weighed 1.5 kg died after treatment because of diffuse intracranial hemorrhage. One technical complication occurred, in which a microcatheter rupture resulted in stroke and a permanent neurologic deficit of mild hemiparesis. After endovascular embolization, routine cross-sectional imaging demonstrated that 3 patients had asymptomatic intracranial hemorrhage. These included 2 patients in the series who were treated despite parenchymal abnormality on pretreatment MR imaging. In these patients, asymptomatic petechial hemorrhagic conversion occurred in the preexisting area of diffusion abnormality. After embolization, 8 of 9 patients were managed medically and discharged from the hospital on oral medications.
Long-term Outcomes
Of the 8 patients who survived the neonatal period, angiographic obliteration has been documented in 6 at an average age of 20 months after an average of 4.2 treatments (▶ Fig. 69.1). The remaining 2 patients have an estimated 95% closure of the AV shunting with a significant reduction in the size of the vein of Galen. Clinical outcomes are as follows: One premature baby died. Two of the 8 patients have minor neurologic deficits. In one, a microcatheter rupture resulted in an intraprocedural left middle cerebral artery stroke during his second treatment. He has a mild left hemiparesis and undergoes physical therapy. The other patient had focal posterior cerebral artery territory infarcts on pretreatment MR imaging because of hypotension in the neonatal period and has mild motor and language delay. One of the 8 patients has severe developmental delay and seizures. This patient had bilateral laminar necrosis on pretreatment MR imaging. Five of the 8 surviving patients have normal neurologic development and are achieving normal developmental milestones. In our series, one patient developed chronic occlusions of the femoral arteries with the formation of collaterals seen on angiography. There were no clinical sequelae. There are also technical considerations in the embolization of these lesions. The usefulness of the transvenous approach in neonates has been reported, given the difficulty with transarterial access.17,29 In true VGAM, the transvenous approach may be a viable option. However, it has been our experience that a vein of Galen malformation cannot be distinguished from a vein of Galen dilatation with normal cerebral veins draining into the aneurysm with certainty in a neonate. A transvenous occlusion of the aneurysm in a patient with a vein of Galen dilatation can have devastating consequences, such as postprocedure venous infarct and hemorrhage, by obliterating the venous outflow of the normal brain. A recent literature review of VGAM treatment supports this, with patients treated transarterially exhibiting a higher rate of “good” clinical outcome.34 Although in the past we reported the use of a transumbilical approach in neonates with VGAM,24 since the initiation of the use of ultrasound for femoral artery cannulation, we have had no failures with this approach. A unique complication of femoral artery occlusion due to repeated femoral artery access occurs in pediatric patients in comparison with adults. After the procedure, careful compression and hemostasis are essential because the long-term patency of the vessels may become compromised. With advancements in our treatment strategy over the past two decades, as discussed above, our mortality rate has dropped to 11% in this current series. Normal neurologic outcome has been achieved in 66.7% of patients and mild delay in 11%, with a good outcome rate of 77.8%.10 The importance of an interdisciplinary team starts at the very time of diagnosis. In utero management by an experienced high-risk obstetric team and pediatric cardiologist is essential. Fetal and neonatal echocardiography can identify patients in whom refractory heart failure is likely to develop. At present, because of significant advances in endovascular techniques, it is possible to intervene much earlier, before end-organ damage occurs.
69.3.2 Infant Hydrodynamic Disorder
In infants, the presenting symptoms are usually caused by hydrodynamic disorders. Hydrodynamic disorders, however, can manifest in fetuses, neonates, and infants. They manifest as macrocrania, which results from enlargement of the cerebrospinal fluid space, hydrocephalus, and increased intracranial pressure and can be associated with developmental delay and mental retardation. Decompensation can occur when cranial sutures close. The drainage of the AV shunt increases the pressure in the torcular and superior sagittal sinus, thereby creating a hemodynamic obstacle to the cortical and deep venous drainage of the brain. This condition is aggravated by the fact that the venous drainage of the brain converges to the torcular at this age. Further compromising the cerebrospinal fluid drainage, the pacchionian granules do not mature until infancy. Distal venous stenosis or secondary venous occlusion can occur and further compromise the cerebral venous drainage. The direction of flow in the cerebral veins is usually reversed and rerouted toward the cavernous sinus and ophthalmic veins, causing dilatation of the facial veins or epistaxis, or to the basisphenoid sinus and pterygoid veins. If these alternative pathways of venous drainage are not yet well developed, cerebral venous congestion can lead to brain edema, hypoxia, and hydrocephalus, aggravating neurologic conditions.11 If the sylvian veins and the cavernous sinus develop with adequate extracranial outflow, spontaneous stabilization of the hydrodynamic disorder can occur.
Hydrocephalus results from the impairment of cerebrospinal fluid resorption due to venous hypertension. Although the VGAM may compress the aqueduct of Sylvius, obstructive hydrocephalus is rare. Ventricular shunting interferes with the water balance between the extracellular and intravascular compartments and may cause brain edema, enlargement of the VGAM, seizures, subdural hematoma, and overshunting with slit ventricles.
69.4 Natural History
69.4.1 Cerebral Calcification
Cerebral calcifications are typically bilateral, symmetric, and located in the transcerebral venous watershed area between the superficial and deep venous system. When present, cerebral calcifications are objective anatomical evidence of a venous hypoxic disorder and its hydrodynamic consequences. Calcification is often associated with subependymal brain atrophy and may secondarily produce seizures.
69.4.2 Dural Sinus Occlusion and Venous Hypertension
Progressive dural sinus occlusion is common in the evolution of VGAM, but its cause is unknown. This phenomenon is not specific to VGAM but is frequently seen in high-flow fistulas in young children. We are not aware of any experimental studies that have investigated the etiology of progressive sinus occlusion. We speculate that this is related to a combination of underdevelopment of the jugular bulb, abnormal skull base maturation due to macrocrania, and high-flow angiopathy of the venous system due to high-flow fistulas that may lead to kinking or thrombosis at the tentorial or dural edge at the skull base. The symptoms depend on the timing between sinus occlusion and capture of the sylvian veins by the cavernous sinus and on the patency of the jugular bulbs and veins. When the jugular bulbs are occluded, the veins of the foramen ovale or the ophthalmic veins will drain the cavernous sinus, which may cause dilatation of the facial veins. If both the VGAM and the brain drain into the same cavernous sinus, facial vein dilatation will be more prominent and epistaxis can occur because of nasal vein congestion. If collateral venous outlets are insufficient, cerebral pial venous congestion may develop. The long-term result of pial venous congestion is chronic cerebral ischemia, manifested by development of calcifications and the peculiar appearance of the chronically congested cortical veins. If further outflow restriction of the venous drainage of the VGAM occurs, pial cortical venous reflux will develop, which can cause acute focal neurologic deficits, seizures, and hemorrhages. When these occur, they represent rare examples of hemorrhages seen in children or young adults with true VGAM.
Prolapse of the cerebellar tonsils is a manifestation of infratentorial pial venous congestion due to sinus occlusion. If the prolapse has not been present for a long time, it may disappear with occlusion of the AV shunts. Prolapse of the cerebellar tonsils usually does not cause any specific symptoms at this age. The prolapse is not related to a global increase in intracranial pressure and is not an indication for emergency ventricular shunting, but rather for endovascular embolization to decrease venous hypertension.
69.4.3 Hemorrhage
Intracranial hemorrhage is rare but possible in patients with VGAM. Intracranial hemorrhage can occur if venous stenosis or occlusion reroutes the venous drainage of the AV shunt into pial veins. This usually occurs in older children and young adults as a secondary manifestation. We have not encountered hemorrhage as the initial presentation in true VGAM. In contrast, hemorrhage is a frequent manifestation of VGAD. Most cases of so-called vein of Galen malformation presenting with hemorrhage in the literature are actually VGAD, not true VGAM. VGAM can also bleed after partial treatment with venous outflow occlusion, which is more common after transvenous than transarterial embolization. Other types of bleeding, such as hemorrhagic infarction, intraventricular hemorrhage, and subdural hematoma, may occur following ventricular shunting or transvenous embolization.
69.5 Treatment
Depending on the patient’ age and the type, onset, and predominant symptoms of VGAM, the objectives of treatment will vary. A major breakthrough in treatment began in the early 1980s with endovascular embolization in the management of VGAMs.12–16 Before the introduction of endovascular embolization for the treatment of VGAM, surgery had been performed with uniformly poor results.17 Mickle and Quisling18 introduced the neurosurgical transtorcular venous approach to control severe CHF in 1986, and Lasjaunias et al reported the first complete transarterial occlusion and cure in 1989.19–21 If treatment is properly timed and performed by a group well trained in pediatric interventional neuroradiology and endovascular neurosurgery, embolization of VGAM is the present treatment of choice and can lead to good clinical outcomes. Medical, surgical, and/or radiosurgical treatment is also used, either alone or combined with endovascular therapy. The indication for radiosurgery is limited to the last stage of treatment for a small residual in older children.
69.5.1 Indications and Goal
The ultimate goal of treatment for VGAM is complete obliteration of the lesion leading to normal development of the patient without neurologic deficits. The immediate treatment goal, however, is different and depends on the age of the patient. The primary goal of treatment in neonates is obliteration of the major portion of the fistulas to allow the resolution of CHF. The combination of neonatal macrocephaly or moderate hydrocephalus with CHF that is not life-threatening does not mandate urgent treatment. Conversely, if significant brain damage is demonstrated by computed tomography (CT), ultrasound, or MR imaging, treatment is not indicated because the clinical outcome is poor even if the lesion is anatomically cured (▶ Fig. 69.4).22–24 The extent of angiographic evaluation before treatment should be limited in neonates because they usually have compromised cardiac and renal function and cannot tolerate a significant volume and contrast medium load. Immediate complete occlusion of the malformation is not always possible, but it is usually not necessary in neonates to achieve the immediate goal.
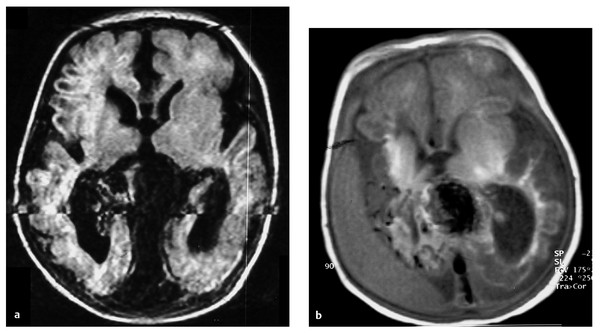
Fig. 69.4 This male patient presented with severe congestive heart failure (CHF) at birth. (a) FLAIR (fluid-attenuated inversion recovery) magnetic resonance (MR) imaging at 1 day old demonstrates severe brain tissue loss and midline vascular malformation with a dilated vein of Galen. The patient underwent transarterial and transvenous embolization at 2 days and at 12 days old at an outside institution with technical success in partial obliteration of the vein of Galen aneurysmal malformation, although treatment was not indicated because of significant brain damage. (b) T1-weighted MR imaging at 25 days old demonstrates the progression of brain atrophy and a subdural fluid collection. Arrow indicates coils in the draining vein. At 18-month follow-up, the patient had no CHF but was on tracheostomy and tube feeding with spastic quadriparesis. The patient underwent embolization at this time with complete occlusion of the malformation and relief of apparent headaches.









