CHAPTER 190 Ventricular Shunting Procedures
The development of effective shunt procedures represents a landmark accomplishment in neurosurgery. Despite a somewhat notorious failure rate that has spawned a sinister reputation within neurosurgery, the fact remains that no other frequently performed neurosurgical procedure has saved more lives in western society than the placement and revision of ventricular shunts. Although comprehensive epidemiologic studies have never been performed, it is estimated that approximately 25,000 shunts are placed each year in North America and twice as many are revised (total of 75,000 shunt operations).1–5 Shunt operations remain the most frequently performed operation by pediatric neurosurgeons, and they are the most common reason why children undergo any form of brain surgery.3,4 Even though endoscopic third ventriculostomy (ETV) has demonstrated promising and significant clinical utility, its overall role in managing hydrocephalus remains controversial; it appears fair to conclude that less than 50% of patients with hydrocephalus can be successfully managed over the long term with ETV.6–8 Accordingly, it appears that there will continue to be a significant role for ventricular shunts for the foreseeable future.
A wide range of conditions may result in hydrocephalus in adults, including subarachnoid hemorrhage, head trauma, meningitis (or other infections), and normal-pressure hydrocephalus.9,10 Hydrocephalus is more common in children (1 in 2000 births) and most often arises from intraventricular hemorrhage of prematurity, congenital anomalies of the CSF pathways, or spinal dysraphism/myelodysplasia or as a complication of childhood brain tumors, head injuries, or infections. In general, children are more absolutely dependent on their shunts and frequently have a more complicated clinical course than that of adults. This chapter reviews established principles and presents recent developments in ventricular shunt procedures.
Background Physiology and Pathophysiology
Composition of Cerebrospinal Fluid
CSF exists in a fluid continuum with the other important intracranial fluid space, which is the interstitial fluid (ISF). ISF appears to be secreted by vascular endothelial cells, and it moves freely within the perivascular spaces and along white matter tracts to interface with CSF at the ependymal surface. Approximately 10% to 30% of the total CSF volume is contributed in this fashion, which is independent of the CP. Consequently, surgical removal of the CP as a singular intervention is insufficient to control hydrocephalus.11
Shunts—Overview
A simple shunt is one in which a single ventricular catheter connects to a valve and single distal catheter. Approximately 90% of shunts are simple constructs. A complex shunt has more than one ventricular catheter and may have a highly complex layout. Complex shunts are characteristically necessary in situations involving loculated hydrocephalus or posterior fossa cystic CSF collections such as the Dandy-Walker anomaly or Dandy-Walker variant.12 Loculated hydrocephalus most often follows either intraventricular hemorrhage or intraventricular infection. In this situation, dense sheets of thickened fibrotic glia and arachnoid partition the ventricles into multiple CSF-filled cavities. Each may dilate independently unless adequately drained. Before the development of contemporary endoscopic techniques that allow fenestration of the loculated spaces, multiple ventricular catheters were necessary and repeated obstructions were frequently problematic.13
Historical Development of Effective Shunt Systems
Comprehensive reviews of the historical treatment of hydrocephalus have been published, and a detailed discussion is beyond the scope of this chapter.14,15 It is relevant, however, to place the evolution of effective ventricular shunts into a historical context because it is in this context that the critical thought processes that went into developing effective shunt systems are best appreciated.
Broadly, evolution of the understanding and treatment of hydrocephalus can be divided into three periods. The first period extends from antiquity to the end of the 18th century of the common era. This time was a period in which hydrocephalus was recognized and some early anatomic and morphologic observations were made. The earliest observations are attributed to Hippocrates, who recognized the presence of disease, recorded physical findings, and may have tried ventricular puncture as a means of treating it. Other anatomists and naturalists made independent descriptions and observations of the disease, including Galen, Vesalius, and Al-Zahrawi, the great Arab physician of the Middle Ages.15 Pachioni described arachnoid granulations in 1701 but erroneously thought that they were the site of CSF production rather than reabsorption. It was not until 1768 that hydrocephalus was described as a distinct illness by Robert Whytt.15
The second historical period in the development of effective treatment of hydrocephalus occurred between the early 19th century and the midportion of the 20th century. It was during this time that the early anatomic observations were extended and focused on the disease process and that the pathophysiologic principles of hydrocephalus were developed. These insights led to meaningful but insufficient efforts at treatment in the early 20th century. In 1825 Magendie and in 1859 Luschka described the crucial foramina in the fourth ventricle that today bear their names as eponyms and described the circulatory patterns of CSF within the brain. In 1876 the Swedish anatomist Gustaf Retzius published a two-volume encyclopedic anatomic study of the central nervous system that has been claimed to have intellectually set the stage for the explosive developments that were to occur in late 18th and early 19th century neurology and neurosurgery in Europe. The development of radioactive tracers in the early part of the 20th century made studies of the dynamic qualities of CSF circulation possible and led to further insight into hydrocephalus. A steady progression of therapeutic ideas followed that were based on the emerging and evolving ideas of CSF physiology and the pathophysiology of hydrocephalus. Quincke in 1891 first recommended serial lumbar puncture as a treatment of hydrocephalus.15 Keen recommended continuous ventricular drainage, but it was Miculicz in 1896 who first recommended diversion of CSF from the ventricles to another space. He recommended diversion to the subgaleal, subdural, and subarachnoid space, none of which showed long-term effectiveness. Payr in 1908 recommended diversion of CSF into the sagittal sinus and jugular veins with the use of autologous vein grafts. Kausch in 1908 recommended diversion of CSF to the peritoneum but used rubber tubes to accomplish this. Heile similarly advocated diversion of CSF with vein grafts or rubber tubes. He also attempted to treat hydrocephalus by sewing bowel serosa to the dura mater in the hope of increasing CSF absorption.
Several concepts other than diversion of CSF were attempted during this era as well.15 First, medical attempts that included thyroid extracts and diuretics yielded uniform failure. Dandy and Blackfan’s seminal work published in 1914 supported two key ideas in the pathophysiology of hydrocephalus. The first was that the CP was the singular source of CSF production. This observation led Dandy to recommend choroid plexectomy as the preferred treatment of hydrocephalus, and this technique became the preferred method of treating hydrocephalus for years. Endoscopic techniques were added decades later. The second key idea from their early experimental work was that obstruction to outflow of CSF was the central event in hydrocephalus. Consequently, therapeutic efforts were targeted at eliminating the resistance to outflow or providing an alternative pathway for CSF flow. These ideas led to the development of several surgical operations designed to treat hydrocephalus. One such operation was third ventriculostomy, in which the lamina terminalis at the anterior extent of the third ventricle was opened surgically after performing a craniotomy. A similar operation in which the corpus callosum was divided and the ventricle exposed had been proposed earlier in 1908 by Anton and van Brauman and called the “Balkenstich method.” This procedure, like third ventriculostomy, gradually fell out of favor because of high operative morbidity despite success in controlling the hydrocephalus.
The third era in the development of successful shunts began in the midportion of the 20th century.16,17 Although pioneers such as Matson continued making progress with diversion of CSF to such novel target sites as the ureter, the critical event in development occurred not in medicine, surgery, or neurophysiology. Rather, developments in polymer chemistry led to the production of biocompatible products made of silicone that would retain their inert qualities over long periods while implanted. Simultaneously, the development of functional valve systems ushered in the true beginning of the shunt era in the early 1950s. Valves and biocompatible material permitted avoidance of many of the problems that had plagued early efforts toward unrestricted shunting of CSF with either biologic (e.g., vein grafts) or nonbiocompatible (e.g., rubber) shunts.16
From the beginning, great attention has been paid to valves and valve design.18 The story of the development of the first functional VP shunt valve is a classic in neurosurgical history. John Holter was an engineer who had a child born with spina bifida and hydrocephalus. At the time there were no functional valve systems, and shunts were not widely available. Daily ventricular taps through the open fontanelle kept the child alive. Holter worked with the neurosurgeons Nulsen and Spitz and together developed the Spitz-Holter valve in 1952. This valve was implanted into Holter’s child as a ventriculojugular shunt.19 Cardiac problems ensued and resulted in an anoxic brain insult to Holter’s son. Despite these setbacks, the Spitz-Holter valve became the prototype of the more widely used CSF shunt valves. Virtually simultaneously, Pudenz and colleagues created a silicone slit–valve system that was similarly developed and marketed. Since that time there has been a plethora of valve systems devised and developed for the treatment of hydrocephalus.20 Many have been named for the developer (eponymic), but they share conceptual similarities that can be organized according to flow and resistance-to-flow characteristics.
Every shunt system consists of three central components—a ventricular catheter, a valve, and a distal catheter. Additional components such as tapping reservoirs, on-off devices, pressure transducers, and antisiphon devices may also be present, and there may be more than one ventricular catheter. Simple shunts have only a ventricular catheter, valve, and distal catheter, whereas complex shunts have more elaborate arrangements. Most commonly this consists of multiple ventricular catheters, but many different arrangements are possible. Loculated hydrocephalus occurs when fibrotic adhesions develop within the ventricles and cause isolated pools of CSF to arise that maintain independent capability of dilating and expanding. Before the development of contemporary endoscopic fenestration techniques, each of these loculated spaces required a ventricular catheter to ensure adequate drainage. Multiple ventricular catheters are still occasionally required for complex multiloculated hydrocephalus and are commonly required for some well-established congenital anomalies such as the Dandy-Walker variant or anomaly. Some commercially available systems have fused one or more of the aforementioned elements together to form a unishunt (to prevent potential disconnection21), but these systems still contain the same three conceptual components as all others.
Ventricular Catheter
The ventricular catheter is the component of the shunt that traverses the space from the ventricle to the valve. A variety of designs of ventricular catheters have been used over the years, but they share similar design principles. The rostral end of the catheter has a rounded tip and multiple holes along the proximal shaft of the catheter. Different design models have featured holes of different size. Proximal ventricular catheter obstruction is the most common cause of mechanical shunt failure, and the tissue implicated in such failure is an ingrowth of CP and gliotic neural tissue.22 Initially, it was thought that larger holes in the ventricular catheter would result in fewer shunt obstructions; however, experience with catheters with large holes showed that proximal obstruction still occurred. More importantly, the larger holes provided an opportunity for more robust ingrowth of CP and a secondary risk for hemorrhage when the catheters needed to be removed for revision. Other types of ventricular catheters were developed with mechanical adjuncts to keep CP away from the holes.23 The Portnoy catheter had a series of Silastic ribs that gave it an appearance similar to a honey dipper and were designed to deflect the CP. Similarly, the Carrea cage had a series of four rib-like Silastic structures that laid in parallel with the shaft of the catheter and were designed to deflect CP away. Both systems were associated with higher rates of hemorrhage and revisions and did not prevent proximal catheter obstruction. As a result, most current systems have multiple rows of very small holes arranged concentrically along the axis of the ventricular catheter. The exact number of holes or volume necessary to ensure adequate flow has never been firmly established, and it appears to be an area of modest investigation. Ginsberg and colleagues demonstrated that a single 500-µm-diameter hole permits flow and relieves the associated hydrostatic pressure.24 However, these investigators were seeking to understand the minimum volume that needed to be opened to ultrasonically clean an obstructed catheter rather than determine the necessary or optimum criteria for shunt flow. Lin and associates demonstrated that approximately 60% of flow occurs through the first row of holes in a shunt catheter and that more than 80% flows through the first two rows. Further understanding of the mechanisms contributing to proximal catheter function may substantially reduce morbidity from proximal shunt malfunction.25
Another important recent technologic advance in ventricular catheter design is the antimicrobial impregnation of ventricular catheters with antibiotics (antimicrobial-impregnated shunts). Two systems are commercially available. In the Codman Bactiseal system, the catheter is impregnated with clindamycin and rifampin as part of the manufacturing process. This patented process results in the gradual release of antimicrobials over a period of approximately 4 weeks after implantation of the device. Clindamycin and rifampicin are the preferred antimicrobials because of technical issues involving incorporation into the polymer, even though other antibiotics have greater effectiveness against organisms that commonly cause shunt infection. Results have varied widely, with some groups reporting nearly complete elimination of shunt infection after incorporation of antimicrobial-impregnated shunts. Other series have demonstrated a modest or nil impact of antimicrobial impregnation. The second commercially available system is the Medtronic BioGlide system, in which the ventricular catheter is impregnated with a polymer that gradually absorbs antibiotic when the device is soaked in a solution of antibiotic. The advantage of the system is that it affords the operating surgeon the choice of antibiotics to use. The disadvantages are that the catheter becomes very slippery and can readily be dropped or disconnected. Furthermore, the antibiotic migrates off the device more rapidly, and there is markedly less published experience with this system.26
Shunt Valves
Since development of the first Spitz-Holter and Pudenz valves, valve design has received the greatest attention in the overall development of effective shunt systems (Table 190-1).27,28 Although this empirically appears logical because the valve is mechanically the most complex (and hence potentially most vulnerable) part of the shunt, several important studies have shown that valve mechanics play a limited role in the overall rate of shunt failure or success. The first of these landmark studies was the Shunt Design Trial, a multicenter prospective survey of the effectiveness of different widely used shunt systems in the 1990s.29,30 In this trial the three most common types of commercially available shunts were compared with regard to shunt function. No difference was noted in the initial report, nor with longer term follow-up.30 Other subsequent studies have repeated this finding.31
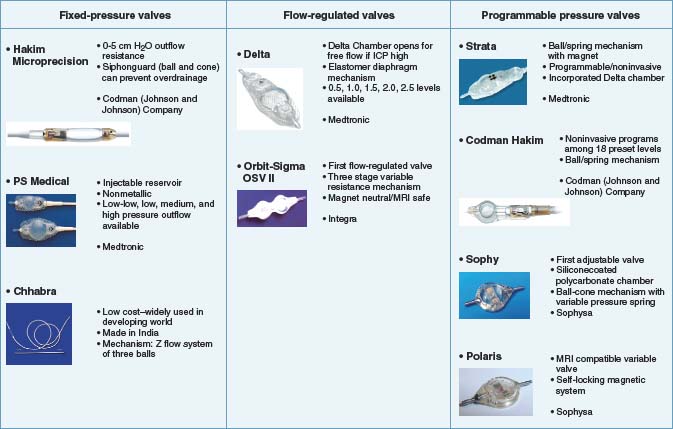
Fixed-pressure valves were the first valves developed for ventricular CSF shunts and overcame a significant barrier to successful CSF shunting, which was the development of low-pressure headaches. There are four different design styles of fixed-pressure valves: silicone rubber slit valves, silicon rubber diaphragm valves, silicon rubber miter valves, and metallic spring and ball valves. Each of these designs shares a common technical goal, which is to provide fixed resistance to outflow of CSF from the ventricle. If such resistance is not provided, the majority of patients will suffer from overdrainage, low-pressure chronic severe headaches. The resistance values are manufactured to three broad levels (low, medium, and high) that reflect different ranges within the normal spectrum of intracranial pressure. Examples of fixed-pressure valves that were widely used include the (first-generation) Hakim valves, the PS Medical (differential pressure) valve, the Denver valve, and the Chhabra valve, which is manufactured in India and still used widely throughout the world.32,33 Generally, these valves functioned well, but over time it became evident that debilitating overdrainage headaches still developed in up to 10% to 12% of patients with fixed-pressure valves. Clinically, this was manifested as an unyielding and life-altering headache that was accompanied by radiographic evidence of successful and sufficient drainage (typically small or nonvisible ventricles on computed tomography [CT]).34–36 This led to the development of flow-regulated valves, which regulated the amount of flow through the system when they were functioning within the normal physiologic range. The two most widely used systems of this design are the PS Medical Delta valve and the Orbis Sigma valve.31,37
Programmable valves can be adjusted noninvasively to vary resistance to outflow across a physiologically normal range. Two clinical observations drove the development of variable-pressure programmable valves. The first was that a significant (although smaller than with fixed-pressure valves) subset of patients treated with flow-regulated valves suffered from chronic headache. The second was the belief among selected groups of neurosurgeons that the ideal outflow resistance for an individual patient was a dynamic rather than a static system. Thus, the ideal outflow pressure for a patient varies with time, and periodic manipulation of outflow pressure ensures optimal function and the fewest symptoms of discomfort. For a persistently symptomatic patient plagued with endless headaches, outflow resistance can be varied to minimize or eliminate symptoms. Controversy still surrounds these ideas, and there are broad variations in clinical practice with regard to the utility of externally programmable valves. Proponents of the less costly flow-regulated valve systems contend (1) that the vast majority of pediatric patients do not require variable outflow resistance and the additional cost is unnecessary and (2) that manipulation of outflow resistance can distract one from the far more common problem of proximal catheter obstruction and can lead to delays in diagnosis of shunt failure. An additional vulnerability of programmable valves is the requirement that they be retested and reset after magnetic resonance imaging (MRI) because the magnetic field of the scanner characteristically affects settings of the programmable valve. Proponents of programmable valves consider the capability of varying outflow resistance of the shunt an important and nearly essential tool in the armamentarium used in treating hydrocephalus.38–41 The only prospective trial that compared programmable (Strata) and nonprogrammable valves showed similar survival across groups.42 Another important prospective study compared conventional flow-regulated valves with the markedly less expensive Chhabra system that is produced in India and found no difference in shunt survival in groups of shunted children in sub-Saharan Africa.33
There appears to be greater consensus that programmable valves play a valuable role in treating adults with hydrocephalus. This is particularly so for older patients, who are at risk for the development of normal-pressure hydrocephalus and thereby at increased risk for the development of subdural hematomas.43,44 In this patient group, the programmable valve allows outflow resistance to be initiated at a higher level to prevent sudden ventricular decompression with concomitant ventricular collapse, cortical infolding, and tearing of the draining veins. Once an initial period of drainage is completed and a period of adjustment to ventricular shunting has occurred, outflow resistance can be decreased or adjusted as needed to optimize the clinical impact of ventricular drainage.45
Distal Catheter
The distal catheter is by far the largest and longest component of a ventricular shunt, but it generally functions the best and has the fewest problems of all the shunt components. The most common problem related to distal catheter function is fracture of the catheter.46 Fracture characteristically occurs at points where movement across the shunt is maximized. In practical terms, this means that most ventricular shunts that fracture do so between the mastoid and the clavicle. This is the region of greatest movement because the shunt is secured to and moves with the skull above and the chest wall and abdomen below. The distal catheter may also pull out of the atrium or peritoneum with growth of the patient, or an infected pseudocyst may develop around the distal catheter tip, but these are not problems in which the catheter itself can be implicated as being causative.47 Hernia or varicoceles with migration of the distal catheter into the scrotum of a male patient have also been repeatedly observed and reported.48,49 Infection of the shunt may result in the development of an abdominal pseudocyst around the distal catheter. This was originally thought to be focal ascites, but focal distal CSF accumulation is now thought to strongly suggest infection.50,51 Tunneling for placement of the distal catheter can cause traumatic injury to adjacent structures.52 Very rarely, perforation of a hollow viscus may occur.53–55 Each of these complications has spurred innovative approaches in design that have resulted in a number of structural variations in distal catheter construction.56
The proximal end of the distal catheter is always open to enable coupling with the shunt valve, but the distal end of the shunt catheter may be either open or closed with a distal slit.57,58 Other distal tip designs have incorporated basket-like projections around the distal tip to prevent obstruction to outflow from adjacent tissues (e.g., omentum) within the peritoneum. One system that was initially popular in the 1970s incorporated a spring into the distal catheter tip. The intent of the spring was to prevent distal kinking and obstruction, but an unfortunate side effect (which led to U.S. Food and Drug Administration [FDA] notification and virtual elimination from widespread use) was a pronounced increase in the incidence of viscus perforation.
The distal catheter may be homogeneously impregnated with barium or may have only a single stripe of barium within its wall. Barium impregnation allows the catheter to be visualized radiographically and fractures or placement problems (e.g., tight coiling within the abdomen, which may suggest preperitoneal placement) to be detected. Some authors contend that solid barium catheters tend to leach barium salts over the extended life span of the shunt. The barium in turn is thought to precipitate locally within tissues as barium salts, which increases the focal tethering effect and risk for catheter fracture.59 Catheters with single barium stripes leach less barium, are associated with a reduction in local tethering from the deposition of barium salts, and consequently appear to have a lower risk for fracture. The practical tradeoff is that catheters with barium stripes may be more difficult to see on routine radiographs, particularly in large or obese patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree