Chapter 37 Vertebral Body Reconstruction in the Thoracic Spine
GOALS OF CORPECTOMY RECONSTRUCTION
The purpose of vertebral body reconstruction is to restore anterior and middle column weight-bearing function and to obtain the intended bony fusion.1 Solid body fusion appears to be the most reliable means to achieve this goal, but fibrous union also may provide a long-term favorable clinical outcome. Several instrumentation options can be used depending on the extent of the vertebral body resection.
RECONSTRUCTION OPTIONS
AUTOGRAFT ILIAC CREST
Autograft yields very high rates of fusion, as long as mechanical stability is provided. It shows rapid healing and modulus similar to vertebral body. After fusion has occurred, the construct shows extreme durability (Fig. 37-1). Even though the fusion bed is irradiated, the fusion rate is very good. When survival is expected to be more than 6 months, autograft is the most favored graft material. However, in anterior reconstruction involving at least one vertebral body and its adjacent discs in thoracic and lumbar spine, the size of the defect to be filled necessitates the harvesting of a large amount of autograft, often associated with a significant morbidity at the graft harvesting sites. Furthermore, the mechanical resistance of such a large reconstruction with autograft is usually not sufficient to allow full weight-bearing without external orthosis. Other problems are pseudoarthrosis, necrosis after adjuvant radiation, transplant loosening, and morbidity at the donor site.
VASCULARIZED RIB AUTOGRAFT
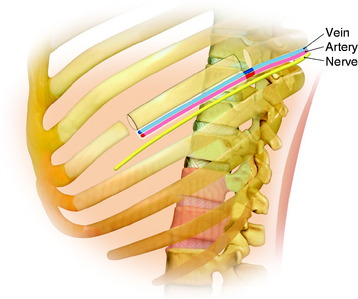
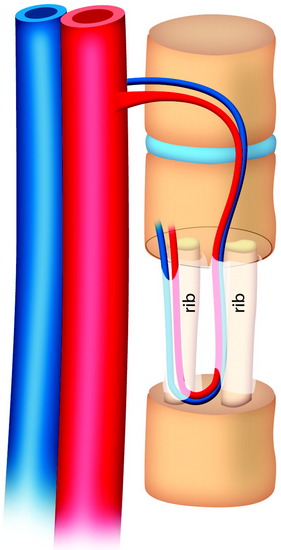
During the thoracic approach, the periosteum of the rib is neither divided nor rasped. Intercostal muscles are divided above and below the selected rib to protect the periosteum and the vessels running below the distal edge of the rib (Fig. 37-2). At the front, vessels are ligated and divided along with the rib. At the back, the periosteum is incised all around the posterior arch of the rib and is rasped with care, pushing away the pedicle in one block. The rib is elevated with its accompanying artery and vein. The intercostal nerve is separated from the vascularized rib. The vascularized rib is isolated. Anteriorly the end is near the costochondral junction, and posteriorly it is near the costal angle. The rib is wrapped up in a wet pad. The mean length of the resected segment is about 10 cm.
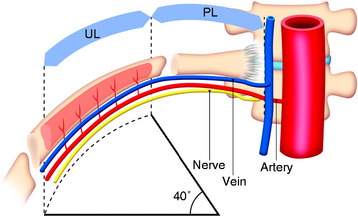
The length of the useful segment can vary according to the number of levels to be fused. In thoracic levels, the length of 10–12 cm can cover four or five segments. The average length of the vascular pedicular segment is 5–6 cm. The useful segment usually has a 40-degree curvature (Fig. 37-3).
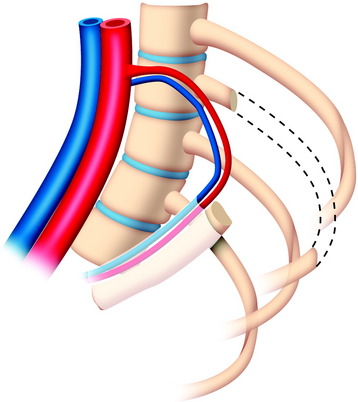
The useful segment of the rib is mobilized around the vascular pedicle. The surgeons must be sure that the pedicle is not twisted, elongated, or tight, and not folded (Fig. 37-4). The whole length of the rib is sectioned into two or three pieces. The length of each fragment adjusts to match the defect in the corpectomy. The rib is folded cautiously to prevent injury to the accompanying vessel (Fig. 37-5). The natural progression of non-vascularized bone graft is a first stage of necrosis, then a second stage of creeping substitution. When the vascularized graft is used, the creeping substitution is avoided, and fusion and bone healing are obtained within 3 months.
TITANIUM MESH CAGE
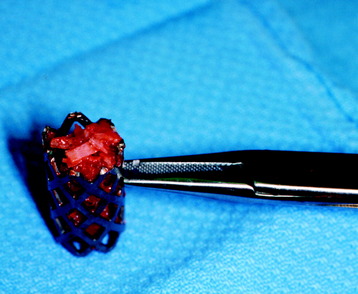
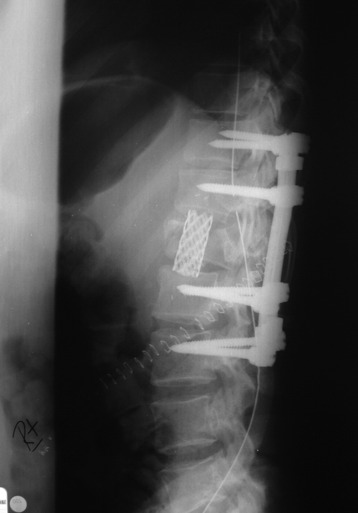
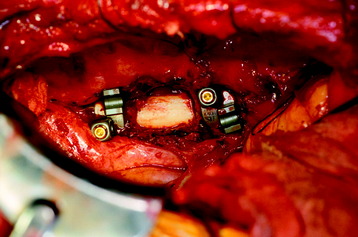
Recent studies on the titanium mesh cage (TMC) have reported excellent stability rates compared with the traditional tricortical iliac crest grafts or fibular grafts because they provide immediate immobilization and a larger healing surface when packed with cancellous bone (Fig. 37-6).2 The mesh cage allows anchoring by bone because its end tip has spikes. Unlike bone grafts, both ends of a titanium mesh cage have sharp edges that allow it to be anchored into the adjacent vertebral bodies, which results in torsional stability (Fig. 37-7). Early stabilization could be achieved by making the cage ends “bite” into the adjacent vertebral bodies.
It is postulated that preservation of the endplates may prevent potential telescoping of the graft when placing cages.3 End caps, designed to prevent telescoping, increase the cage’s maximum load-bearing ability but do not affect the construct’s stiffness. They may interfere with the bone-bone interface or the transference of stress lines, which is needed for fusion, through the TMC. The plate/TMC combination results in the strongest, stiffest construct, preventing flexion and extension, rotation, and parallelogram effect. The inability of plain radiography to evaluate bone growth within the TMC can be overcome with computed tomography (CT) scanning. The presence or extent of bone growth within the TMC can be determined from CT scanning. On CT images, hyperdense areas are seen, which suggests bone growth within the TMC, and indirect evidence of fusion is presumed based on radiographically documented stability. The TMC/plate construct has been safe and effective as a vertebral replacement in the spine.
ALLOGRAFT
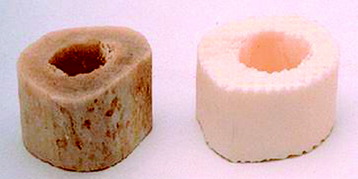
Allografts include femoral cortical rings, tibial rings, fibular rings, and humeral cortical rings (Figs. 37-8 and 37-9). Some surgeons advocate the use of an allograft-autograft composite by packing the medullary canal of the allograft with autografts. Deep-frozen cortical rings and freeze-dried cortical rings are available. Both deep-frozen and freeze-dried cortical allografts have been strong enough to withstand loads taken by the reconstructed spine. The results of the fusion operation have been good. Infection and implant failure are rare.
Allografts are of multiple sizes and provide reliable fusion with the host bone with partial bone remodeling, preventing fatigue fracture. The major disadvantage is the risk of transmission of the disease. The risk can be reduced with several physical and chemical treatments, including washing, lipid extraction, prion inactivation, freeze drying, packing, and sterilization by 2.5 Gy gamma irradiation.4
Two-dimensional (2D) CT evidence of bony ingrowth into the central canal of a fibula strut allograft provides an adequate means of quantifying the extent of fusion (Fig. 37-10).
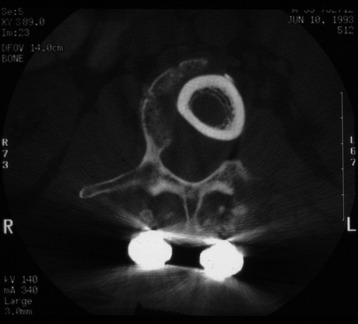
Fig. 37-10 Femoral ring allograft provides a strong cortical construct with a large contact surface.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree