CHAPTER 27 VESTIBULAR SYSTEM DISORDERS
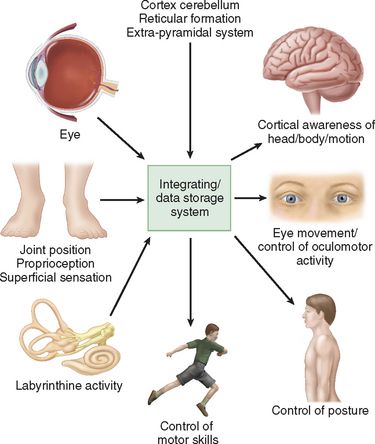
Humans have developed a sophisticated and complex mechanism for maintaining balance that relies on the integration and modulation of sensory inputs from vision, the vestibular receptors within the labyrinth, and proprioception. Within the central nervous system, the cerebellum, the extrapyramidal system, the limbic system, and the cerebral cortex facilitate processing to enable the perception of head and body position in space, eye movement control, and appropriate static and dynamic postural function (Fig. 27-1). An alteration in any one of the three sensory inputs, or within the central vestibular pathways and their connections, may give rise to disordered eye movements, disequilibrium or instability, and the perception of dizziness or vertigo. The complexity of this system is such that pathology in almost all body systems may be associated with dizziness/disequilibrium; thus, affected patients present to many different specialist departments but most commonly to otology or neurology outpatient offices (Table 27-1). Despite these ubiquitous presentations, most clinicians do not have a clear diagnostic strategy, including knowledge of detailed neuro-otological examination, to enable them to accurately diagnose and appropriately manage vestibular symptoms. This chapter provides a broad overview of peripheral and central vestibular syndromes, together with an outline of an appropriate clinical assessment based on an understanding of vestibular pathophysiology, a discussion of management strategies, and specific points with regard to common vestibular disorders.
TABLE 27-1 Causes of Disequilibrium
EPIDEMIOLOGY
Dizziness is an extremely common symptom, both in primary care and at the tertiary level. One in four healthy subjects in the community reports symptoms of dizziness, with significant effects on their daily living.1 By the age of 70, 36% of women and 29% of men have balance problems, whereas by the ages of 88 to 90, 45% to 50% of the population suffer symptoms of balance dysfunction.2
In the community, many cases of vestibular dysfunction resolve spontaneously, without recourse to medical care, although each year 5 per 1000 patients consult their general practitioners because of symptoms that are classified as vertigo, and a further 10 per 1000 are seen for dizziness or giddiness.3 In a tertiary setting, dizziness is associated with significant morbidity, and in the older population, falls and mortality are common sequelae.4 Vestibular symptoms after head/whiplash injury are the commonest cause of failure to return to work, and two thirds of patients in a tertiary neuro-otological clinic suffer psychiatric symptoms in association with vestibular pathology.5
VESTIBULAR ANATOMY AND PHYSIOLOGY
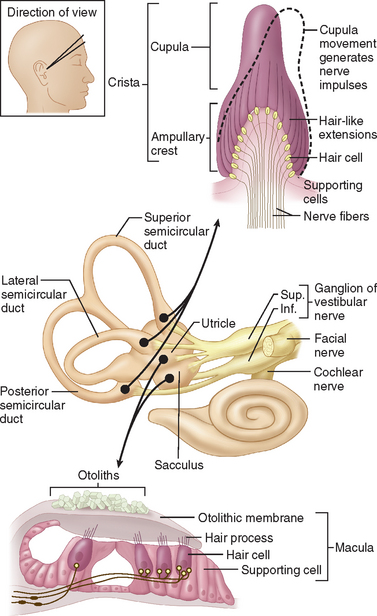
The internal ear is a minute membranous structure within the bony labyrinth, buried in the temporal bone. Within the internal ear, the cochlea is the acoustic end-organ receptor, whereas, of the five vestibular end-organs, one lies within each of the three semicircular canals and one each in the utricle and saccule within the vestibule. The vestibular sensory epithelium is composed of type 1 and type 2 hair cells, covered with a gelatinous membrane that, in the saccule and utricle, contains calcium carbonate-rich crystals termed otoconia (Fig. 27-2).
A force parallel to the surface of the sensory epithelium provides the maximal stimulus. Thus, the horizontal anterior and posterior semicircular canals are stimulated by angular acceleration in the three planes of space but are insensitive to gravity or head position.6 The saccule, which lies approximately vertically, senses vertical linear head acceleration and gravity; the utricle, which is oriented approximately horizontally, senses horizontal linear head motion and head position in space.
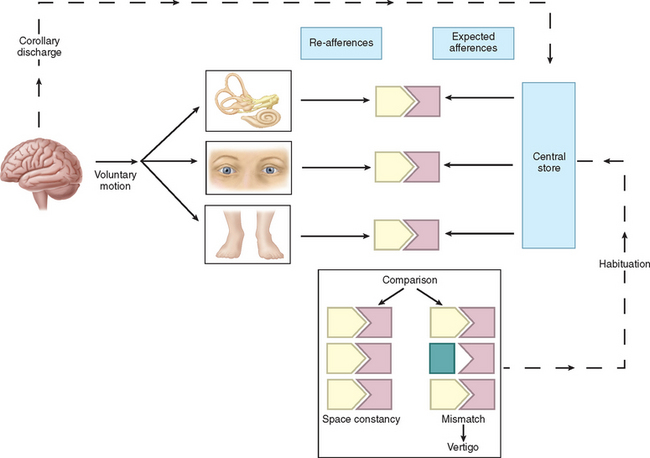
Physiologically, the vestibular apparatus can be considered in two halves, the right labyrinth and the left labyrinth, which are perfectly balanced and work in parallel. For example, when the head is turned to the right, the right horizontal semicircular canal increases its firing rate, whereas the left decreases its firing rate. This asymmetry in neural input is transmitted (1) to the vestibular nuclei and the cerebellum, which controls the amplitude and timing of movements, and (2) via the vestibular nuclei and the thalamus to the parietoinsular vestibular cortex. From birth, the vestibular, visual, and proprioceptive inputs associated with every type of movement are monitored, integrated, and stored in a “data bank,” which is considered to be the reticular formation of the brainstem.7 Subsequently, each movement generates signals that are then compared with the information in the “data bank.” Integration of movement-induced neural asymmetry with other sensory input and comparison with the “data bank” template allow for awareness of head and body position in space, together with the generation of compensatory oculomotor (vestibulo-ocular reflex) and motor (vestibulospinal) activity. In addition to the motor control, the extensive convergence of vestibular and autonomic afferent information in the brainstem and cerebellum allows for coordination of appropriate motor and autonomic responses during movement or changes in posture.
Thus, if there is any mismatch of the sensory input to the existing template, the patient senses disorientation, may develop an abnormal eye movement, frequently feels off balance, and may develop nausea lvomiting and other autonomic symptoms (Fig. 27-3). The classic physiological example of such a mismatch is motion sickness.8 However, any pathological lesion that results in a change in, for example, vestibular input to the central nervous system, as may occur in Meniere’s disease or vestibular neuritis, produces similar symptoms of disorientation, nausea, vomiting, and malaise as a consequence of the change in the vestibular signal, with no corresponding changes in visual and proprioceptive inputs. In addition, connections at various levels of the central vestibular system with the locus ceruleus, the limbic system, and other brain regions that control affective responses, mood, and arousal may underlie the observed overlap between psychiatric and vestibular disorders.9,10
AGING AND THE VESTIBULAR SYSTEM
Histopathological age-related changes in the human vestibular sensory organs include progressive hair cell degeneration, otoconial degeneration in the otolith organs, and decreasing numbers of vestibular nerve fibers,11,12 and age-dependent changes in both caloric and rotational test responses have been demonstrated.13,14 These changes alone are unlikely to generate vestibular symptoms, as they are symmetrical, and dizziness in elderly people is probably more multifactorial in origin.15 Thus, although older patients may be subject to the same common balance disorders as are younger patients, they have more problems with chronic disequilibrium and falls, and vertigo has been reported to rise with advancing age in parallel with the incidence of hearing loss.13 Correct diagnosis, prevention, and rehabilitation are particularly important in treating this group of patients.16
PERIPHERAL VESTIBULAR DISORDERS
Acute Unilateral Vestibular Deafferentation
Acute pathology of one labyrinth manifests as an acute clinical syndrome with profound motor and sensory abnormalities17 and with the same symptoms and signs irrespective of the cause. A patient with an acute total right vestibulopathy has the following signs (Table 27-2):
TABLE 27-2 Consequences of Unilateral Peripheral Vestibular Destruction
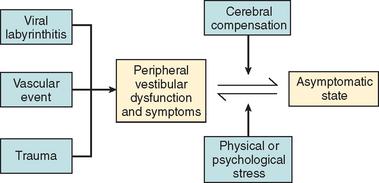
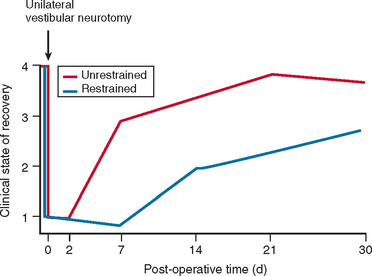
In most cases, the characteristic symptoms and signs of vestibular deafferentation abate, and the patient is rendered asymptomatic over a period of 2 weeks to several months. In this regard, the vestibular system has been shown to be extremely adaptable.18 The processes, which bring about the resolution of vestibular symptoms, are collectively known as cerebral compensation and are attributed to cerebral plasticity (Fig. 27-4; Table 27-3).
TABLE 27-3 Vestibular Compensation
The structures subserving compensation for vestibular dysfunction are unknown, but it has been shown that brainstem, cerebellar, and cortical structures are involved; the cerebellum is key to this recovery phenomenon,17,19,20 in addition to the requirement for all sensory inputs, including vision, somatosensory afferents, and remaining labyrinthine input.21–23 Furthermore, integrity of both the vestibular nerve24 and the central vestibular connections25 is required.
The physiological mechanisms on which compensation depends include physical activity26,27 and vision28 (Fig. 27-5). Moreover, Fetter and coworkers29 demonstrated that occipital lobectomy before labyrinthectomy impaired compensational recovery, and Schaefer and Meyer in 197330 also demonstrated that transsection of the cervical cord that led to loss of proprioception delayed vestibular compensation.
Bilateral Vestibular Hypofunction
 Sense of imbalance when standing or walking, especially on uneven surfaces (e.g., sand) or in the absence of vision (e.g., at night).
Sense of imbalance when standing or walking, especially on uneven surfaces (e.g., sand) or in the absence of vision (e.g., at night). Bobbing oscillopsia: that is, vertical bouncing or blurring of vision when the patient walks, runs, or moves, with degradation of visual acuity as a result of loss of the vestibulo-ocular reflex.
Bobbing oscillopsia: that is, vertical bouncing or blurring of vision when the patient walks, runs, or moves, with degradation of visual acuity as a result of loss of the vestibulo-ocular reflex. An inability to stand or walk when both vision and proprioception are removed (e.g., when standing on a foam pad with eyes closed or when attempting to walk across a foam pad with eyes closed).
An inability to stand or walk when both vision and proprioception are removed (e.g., when standing on a foam pad with eyes closed or when attempting to walk across a foam pad with eyes closed).In these cases, the cervico-ocular reflex has been implicated in recovery of function31; other authorities have suggested that slippage of the retinal image in bilateral vestibular failure may be compensated for by central visual mechanisms, as occurs in congenital nystagmus32 and oculomotor palsies.33 In general, patients with bilateral vestibular function recover significantly, although a proportion remain handicapped by oscillopsia and instability.
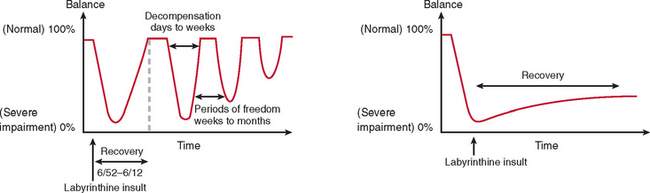
Compensation/Decompensation
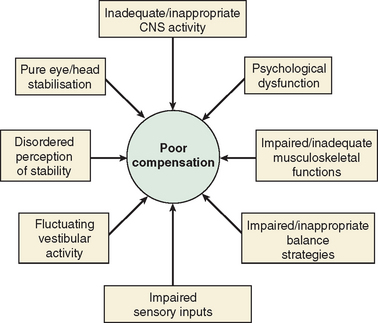
The majority of cases of a unilateral peripheral vestibular deficit recover by means of cerebral compensation. However, some patients do not recover spontaneously and require vestibular rehabilitation with physiotherapy. The basis of physical therapy intervention relies on a structured approach in promoting recovery with visual, proprioceptive, and vestibular stimulation by means of a standard or customized range of exercises. A number of factors that predispose to failure of compensation (Fig. 27-6) or decompensation from a previously recovered state (Fig. 27-7) have been identified. There is some evidence that there exists a critical period in which stimuli must be provided to the adaptive mechanisms and recalibration of the vestibular function must begin, or else the rate of recovery and perhaps the ultimate degree of recovery may decrease.34 However, Shepard and colleagues35 did not identify duration of symptoms, or age, as a negative prognostic factor of a vestibular rehabilitation program, although financial compensation, head injury, and severe postural control abnormalities have all been reported to indicate poor outcome.
DIAGNOSIS OF VESTIBULAR DISORDERS
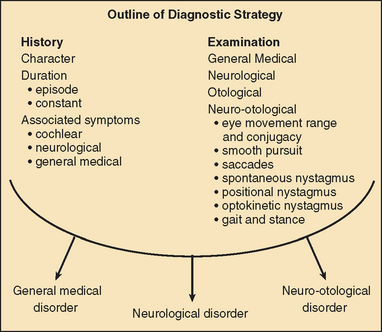
The diagnosis of vertigo is critically dependent on a clear history that includes the following:
Vertigo of less than 1 minute’s duration is most commonly associated with benign paroxysmal positional vertigo (BPPV), whereas acute rotational vertigo of several hours’ duration is most commonly associated with migraine and Meniere’s disease. Vertigo lasting several days is common in viral vestibular neuritis and in ischemic and brainstem labyrinthitis. Pathology involving the labyrinth and cranial nerve VIII is commonly associated with hearing loss and/or tinnitus, whereas vertigo arising in the central vestibular pathways is most commonly associated with disordered eye movements. In order to make a correct neuro-otological diagnosis, a clinical examination of the vestibular and oculomotor systems is key and requires a clear understanding of vestibular and oculomotor pathology, together with regular clinical practice at examination.36
COMMON PERIPHERAL VESTIBULAR DISORDERS
Acute Vestibular Neuritis
Single episodes of acute rotational vertigo associated with nausea and vomiting, with or without cochlear symptoms, are a common occurrence in all age groups. The attacks are usually unprecipitated and are commonly ascribed to a viral infection, termed vestibular neuritis, vestibular neuronitis, labyrinthitis, or acute vestibulopathy.37,38
The signs and symptoms are as described earlier for an acute unilateral vestibular disorder, and the natural history is resolution of symptoms within a few days or weeks. The majority of patients recover spontaneously, but it appears that early mobilization and vestibular rehabilitation reduce the incidence of disability from chronic vestibular symptoms, which develops in about 20% of patients with acute vestibular neuritis.39
Most cases of vestibular neuritis affect the superior vestibular nerve, with a marked canal paresis on caloric testing, which shows progressive recovery in about 50% of patients on repeat testing.40,41 Frequently, it is possible to obtain a normal saccular response, as judged by the vestibular evoked myogenic potential, which depends on normal inferior vestibular nerve function. In 25% of the patients with vestibular neuritis,43 BPPV (described later) of the posterior canal variant may develop subsequently.
Ramsay-Hunt Syndrome
The Ramsay-Hunt syndrome is the clinical presentation of herpes zoster oticus with facial palsy, auricular rash, and hearing loss, which are often associated with acute vertigo. Abramovich and Prasher44 reported vertigo in 85% of their series; conversely, vestibular dysfunction has also been described with Bell’s palsy or idiopathic facial palsy,45,46 at an incidence of between 20% and 92%. A number of mechanisms of vestibular involvement in this latter condition have been postulated, including compression of cranial nerve VIII by the edematous cranial nerve VII and involvement of both cranial nerves VII and VIII in the same disease process.
Vertigo, imbalance, ataxia, and nausea have all been reported in human immunodeficiency virus infection, although it remains unclear whether the pathology is central or peripheral in type, and vestibular dysfunction is less common than auditory involvement.47
Meniere’s Disease
Meniere’s disease remains a clinical diagnosis characterized by fluctuating hearing loss, tinnitus, and vertigo, often associated with sensation of fullness or blockage in the ear. In 60% of patients affected, both vestibular and cochlear symptoms have developed within 6 months of the onset of the disease. The literature abounds with controversy on all aspects of this condition, and the diagnosis should be based on the strict American Academy of Otolaryngology—Head and Neck Surgery Committee on Hearing and Equilibrium Guidelines.48 The vertigo attacks usually last between 1 and 8 hours, but the tinnitus, hearing loss, and sensation of fullness in the ear may last for several days. Attacks tend to occur in clusters, with attack-free intervals. Initially, both vestibular function and cochlear function recover, so that the caloric test and audiometry may be normal between attacks. Later there is a progressive low-frequency hearing loss, which, in the older patient, may be superimposed on presbycusis to yield a tent-shaped audiogram, and with continuing progression, a plateau hearing loss emerges. Moreover, with progressive attacks, interval disorientation may accompany loss of vestibular function.
Clinical examination may show spontaneous nystagmus directed toward the affected ear (i.e., an irritative response), followed by an ablative phase, in which the nystagmus beats away from the affected ear, and a recovery phase, in which the nystagmus may again beat toward the affected side.49 Late in the disease, patients may develop drop attacks called Tumarkin or otolithic crises.50
The natural history of Meniere’s disease is variable, but in general there are clusters of episodes (relapses) with attack-free periods that may last several years (remission). Other patients, however, have a progressive course, with ultimate loss of auditory and vestibular function. Bilateral involvement is reported in 20% to 50% of cases.51
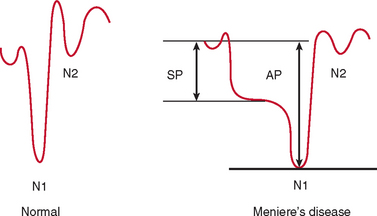
Electrocochleography with transtympanic recording at the promontory is the most sensitive and specific test for Meniere’s disease. Characteristically, there is broadening of the summating potential/action potential ratio; this ratio is often greater than 35%, in comparison with approximately 20% in normal subjects (Fig. 27-9).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree