Vigabatrin
Elinor Ben-Menachem
Olivier Dulac
Catherine Chiron
Introduction
Vigabatrin is a novel antiepileptic drug (AED). It differs from most other agents in that it was designed to have a specific effect on brain chemistry and was subsequently proved to produce that effect. Thus, it is still one of the few AEDs whose mechanism of action is presumably completely known. The mechanism consists of irreversible inhibition of γ-aminobutyric acid (GABA)-transaminase, the enzyme that degrades the inhibitory neurotransmitter GABA in the central nervous system (CNS). A great deal of information has been obtained about this unique compound from clinical trials and also from the large number of patients receiving vigabatrin as a registered drug in nearly 50 countries worldwide. It is not, however, available in the United States because of its tendency to cause irreversible visual field defects (VFDs). The possibility exists, nevertheless, that vigabatrin will one day be available in the United States for use in children with infantile spasms. This chapter reviews the current preclinical data, efficacy, safety, and clinical use of vigabatrin.
Chemical Characteristics, Formulations, and Methods for Determination in Body Fluids
Chemical Characteristics
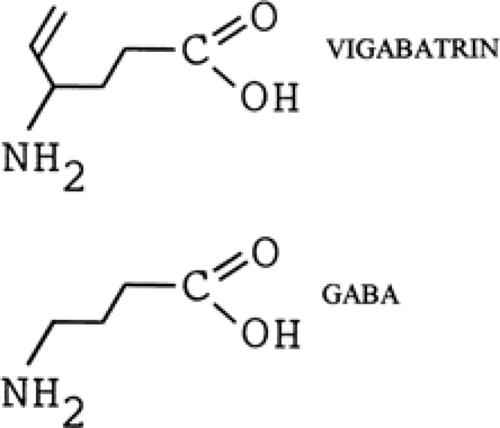
Vigabatrin (4-amino-5-hexenoic acid, γ-vinyl GABA, GVG) is a structural analog of GABA with a vinyl appendage (Fig. 1). It is a rationally designed, enzyme-activated, irreversible selective inhibitor of GABA-transaminase.38,76 Vigabatrin is highly water-soluble, only slightly soluble in ethanol and methanol, and insoluble in hexane and toluene. It is a white to off-white crystalline solid with a melting point of 171° to 177°C. The molecular weight is 129.16, and the conversion factor (CF) is 7.75 (mg/L × CF = μmol/L).
Vigabatrin is a racemic mixture of R-(–) and S-(+)-enantiomers in equal proportions and exhibits no optical activity. The pharmacologic activity and toxic effects of vigabatrin are associated only with the S-(+)-enantiomer; the R-(–)-enantiomer is entirely inactive.40,87 The duration of effect is determined by the half-life of the enzyme rather than by that of S-(+)-vigabatrin because GABA-transaminase has a much longer half-life than S-(+)-vigabatrin itself.8,50
Formulations
The only available forms of vigabatrin are oral formulations (500-mg tablets and 500-mg sachets). Sachet contents may be placed in a beverage (e.g., water, fruit juice, or milk) immediately before oral administration.
Methods of Determination in Body Fluids
Vigabatrin is a highly water-soluble amino acid and can be measured using standard automatic amino acid analysis, high-performance liquid chromatography (HPLC), or gas chromatography-mass spectrometry.
HPLC is the most commonly used method of analysis for vigabatrin in body fluids. A reversed-phase HPLC assay was developed by Smithers et al.101 Tsanaclis et al.111 described a single-step protein precipitation with subsequent precolumn derivatization with o-phthaldialdehyde and direct injection into a Microsorb C18 column. The isocratic HPLC method developed subsequently by Chollet et al.20 is more rapid than previous methods. Since only the S-(+)-enantiomer is pharmacologically active, it is generally preferable to utilize assays that differentiate between enantiomers, such as that described by Vermeij and Edelbroek.113
Pharmacology
Activity in Experimental Models of Seizures/Epilepsy
Vigabatrin has been tested in numerous seizure models and has been shown to be effective in some, but not all. It is inactive in models such as maximal electroshock (MES), bicuculline (GABA antagonist), and pentylenetetrazol unless injected directly into the midbrain of rats.31 However, after an intravenous injection of vigabatrin, seizure protection was observed against bicuculline-induced myoclonic activity,54 strychnine-induced tonic seizures,98 isoniazid-induced generalized seizures,98 audiogenic seizures in the mouse,96 light-induced seizures in the baboon,71 and amygdala-kindled seizures in the rat.76,103
Stereotaxic injections of small amounts of vigabatrin into certain areas of rat brain gave seizure protection in relation to the locally increased GABA levels.31 Seizure protection in the MES model was most prominent with local GABA increases in the midbrain tegmentum, including the substantia nigra and the midbrain reticular formation. Injections of vigabatrin into the thalamus, hippocampus, and cortex did not protect against seizures in this model. The duration of seizure protection was as long as 72 hours after a single injection to the substantia nigra. Only by the fifth day did the rats respond normally to MES. This finding supports the observation that the rate of recovery of GABA-transaminase is 5 days,50 and suggests that the anticonvulsant effect is a consequence of the local increase in GABA levels rather than of a direct effect of vigabatrin itself.
Mechanisms of action
Studies in Animals
The pharmacokinetics in the CNS and effects on GABA, GABA-transaminase, glutamate decarboxylase, and other neurotransmitters and amino acids in animals demonstrate the specific effects of vigabatrin in the brain. In one of the first experiments studying the effects of vigabatrin, 1,500 mg/kg of the compound were injected intraperitoneally in mice. The brain content of GABA, GABA-transaminase, and glutamate decarboxylase was measured over time. By 4 hours, a fivefold increase of whole-brain GABA was noted.50 At the same time, a sharp decline occurred in GABA-transaminase activity, with a recovery to 60% of baseline after 5 days. Glutamate decarboxylase was also affected, with a 30% reduction of activity. However, this decrease was seen only at very high doses (1,500 mg/kg), and the probable cause is considered to be feedback following the rise in GABA concentration.
In addition to GABA, vigabatrin also influences other amino acids and transmitters, at least at high doses.82 An increase in brain β-alanine (an alternative substrate for GABA-transaminase), as well as homocarnosine and hypotaurine, was demonstrated, whereas glutamine and threonine levels decreased.
Studies in Humans
Observing effects on GABA and other neurotransmitters in the cerebrospinal fluid (CSF) has been an important method to study the effects of vigabatrin on GABA and other neurotransmitters and amino acids in the human brain. The first study to investigate the relationship between vigabatrin and GABA in the CSF was carried out by Grove et al.38 Patients with various neurologic conditions were given 0.5, 1, 2, or 6 g daily for 3 days. Free and total GABA, β-alanine, homocarnosine, and vigabatrin increased in a dose-responsive manner, except at a dose of 0.5 g/day, with which no changes in the parameters studied were recorded. Schechter et al.96 studied ten patients given 0.5 g vigabatrin twice daily for 2 weeks followed by 1 g twice daily for 2 weeks and then placebo for 2 weeks. When parameters were measured at the end of the treatment period, there were no changes from baseline in homovanillic acid (HVA; the metabolite of dopamine) and 5-hydroxyindolacetic acid (5-HIAA; the metabolite of serotonin), but dose-related increases were seen in free and total GABA, and in homocarnosine. At the end of the placebo period, the levels of GABA and homocarnosine had declined to baseline levels.
No changes in CSF levels of acetylcholine, somatostatin, β-endorphins, prolactin, cyclic adenosine monophosphate, or cyclic guanosine monophosphate were observed during long-term treatment.84,99 No consistent changes have been found in amino acids, HVA, or 5-HIAA with long-term treatment of vigabatrin at 50 mg/kg up to 3.5 years either in tissue or CSF.7,99 In a single-dose study, however, HVA and 5-HIAA concentrations increased initially up to 100%, but they returned to the baseline level or slightly below baseline level after 1 month of treatment.8
At a dose of 50 mg/kg, vigabatrin causes a 200% to 300% increase in GABA in the CSF and brain tissues.6 A reduction of vigabatrin dose from 3 g/day to 1.5 g/day caused a proportional reduction of GABA concentrations in the CSF.99 Dose and percentage increase in CSF GABA concentrations show a good linear relationship, but the relationship between dose and efficacy appears more complex and depends on the nature of the epilepsy. Nuclear magnetic resonance spectroscopy in patients treated with vigabatrin have confirmed the observations seen using CSF GABA analysis.69
The effects of vigabatrin are also seen in blood GABA and platelet GABA-transaminase levels. Administration of vigabatrin causes a marked reduction in platelet GABA-transaminase at therapeutic doses of 2 to 3 g/day. It appears that a dose of 2 g/day maximally inhibits platelet GABA-transaminase, with mean enzyme inhibition at approximately 70%.89 The concentration of plasma vigabatrin is almost tenfold that seen in the CSF and, because platelets cannot regenerate GABA transaminase, the effect of vigabatrin on this test system is also influenced by platelet regeneration.
There is no clear-cut correlation between blood concentration of vigabatrin and efficacy.57
Clinical PHARMACOKINETICS
Absorption
The only available forms of vigabatrin are oral. There are no intravenous or rectal formulations, so the absolute bioavailability of the drug has not been determined in humans. Studies in animals, however, indicate that the areas under the plasma concentration versus time curves (AUCs) are similar when intravenous, intraperitoneal, subcutaneous, intramuscular, and oral doses are administered.50,101
All pharmacokinetic studies39,40,92 in humans have demonstrated that absorption is rapid, with the peak concentration reached in the first 2 hours after doses of between 0.5 and 3 g. Absorption half-life ranges from 0.18 to 0.59 hours. In a single-dose kinetic study that used an enantioselective assay,40 peak plasma concentrations for both enantiomers were reached between 0.5 and 2 hours after a 1,500-mg dose.
The bioavailability of vigabatrin in tablet form and in solution has been studied in healthy volunteers.45 Although there was a slightly lower, delayed peak concentration for the tablet, the pharmacokinetic profiles were very similar. Thus, it can be concluded that the vigabatrin tablet is bioequivalent to the oral solution. Approximately 60% to 80% of the drug can be recovered unchanged in the 0- to 24-hour urine. This indicates that bioavailability is at least 60% to 80%.
The effect of food on the bioavailability of vigabatrin in tablet form has been studied.30,45 The AUC for fasted and fed volunteers was not significantly different. This indicates that food does not have an effect on the extent of absorption. The half-life was 7.15 hours, and renal clearance was 95.0 mL/min for the fasted group, and 9.15 hours and 99.9 mL/min for the fed group. Therefore, the time of administration and what and
when a person eats should not influence the clinical response to vigabatrin.
when a person eats should not influence the clinical response to vigabatrin.
Plasma Protein Binding and Distribution
Plasma Protein Binding, Volume of Distribution, and Penetration into the Cerebrospinal Fluid
Vigabatrin does not bind at all to plasma proteins.89 The distribution of vigabatrin in the body is very wide. This is not surprising, because vigabatrin is not protein-bound and is a highly water-soluble compound. The apparent volume of distribution is 0.8 L/kg (total body water is 0.6 L/kg) in volunteers, with the half-life of distribution at 1 to 2 hours.
Vigabatrin levels in CSF have been analyzed in patients with epilepsy. The concentration of vigabatrin in the CSF was approximately 10% of that in the blood.8 In this study, patients taking concomitant AEDs were given 50 mg/kg as a single dose. CSF and blood samples were taken for up to 5 days after dosing. The highest concentrations of vigabatrin were found in CSF after the first sample. By 24 hours, only a trace was detectable in the CSF, and no vigabatrin was found at 72 hours or thereafter. The peak concentration in the blood was reached by 1 hour and decreased thereafter, with only trace amounts detectable at 72 hours. After a 3-year follow-up at doses of 50 mg/kg per day, the levels of vigabatrin in CSF were not significantly increased when compared with the 6-month levels.7
Distribution in Placenta
Passage of S-(+)-vigabatrin and R-(–)-vigabatrin across the human placenta in vitro was studied by Challier et al.16 The transfer from maternal to fetal blood across the placenta was low and comparable with that of other acidic α-amino acids. Clearance for both of the enantiomers was about 27% that of phenazone.
Distribution in Special Populations
To determine whether vigabatrin has a different distribution profile in the elderly, 1.5 g vigabatrin was given as an oral dose to healthy volunteers between the ages of 60 and 75. Steady-state volume of distribution was not markedly different from that in the younger volunteers.39 However, in volunteers between the ages of 76 and 97 years, a decrease of the apparent volume of distribution occurred.
In renally impaired patients, the volume of distribution was about one-half to one-third that in healthy volunteers.39 Because most of the renally impaired volunteers were elderly (mean age, 86.4 years), the reduced muscle mass probably contributed to the reduced volume of distribution.
Metabolism
Vigabatrin is not significantly metabolized in humans. Up to 82% of the oral dose is excreted unchanged in the urine (data on file, Sanofi-Aventis, Paris, France).
Elimination
Half-Life and Clearance
The elimination half-life is 5 to 8 hours, and the total clearance is about 1.7 to 1.9 mL/min per kilogram, with renal clearance accounting for 70% of the total apparent oral clearance. Elimination is not influenced by dose or duration of treatment.37 Again, it should be stressed that the biologic half-life—that is, the half-life for inhibition of GABA-transaminase—is measured in the order of days, not hours.
Elimination parameters have also been assessed separately for the two vigabatrin enantiomers. The pharmacokinetics of the S-(+)-enantiomer is independent of that of the R-(–)-enantiomer. After dosing with pure S-(+)-vigabatrin, the mean terminal half-life was 386 minutes. The half-life for the R-(–)-enantiomer was 485 minutes, and 447 minutes for the S-(+)-enantiomer in the racemate.40 The AUC was 39.2 μmol/mL per minute for the R-(–)-enantiomer and 30.1 μmol/mL per minute for the S-(+)-enantiomer. The AUC for the pure S-(+)-vigabatrin was 30.6 μmol/mL per minute. An explanation to account for the lower AUC value for the S-(+)-enantiomer is that some of the S-(+)-enantiomer is bound irreversibly to the enzyme GABA-transaminase and is unavailable for analytical measurement. The R-(–)-enantiomer is inactive and therefore does not bind to the enzyme. No chiral inversion exists in humans.
Elimination in Children
In a preliminary study, children and, particularly, infants showed a lower AUC than historical adult controls, but renal clearance was comparable.87 This might indicate that infants and children may have a lower bioavailability (which could explain why infants may need higher doses of vigabatrin to achieve seizure control), although more studies are required to characterize differences in vigabatrin pharmacokinetics between adults and children.
Elimination in the Elderly and the Renally Impaired
In elderly patients, both renal and total body clearance are slower. Terminal half-life showed an inverse relationship to renal function.39 The elimination of vigabatrin is slower in elderly patients because of their reduced renal function. A direct correlation exists between renal clearance of vigabatrin and creatinine clearance. Renally impaired patients have higher plasma concentrations of vigabatrin, and the half-life is longer. The half-life in renally impaired patients with reduced creatinine clearance is approximately twice that of normal healthy volunteers. Analysis of AUC-to-body weight ratio versus creatinine clearance shows a nonlinear increase in the ratio of AUC to body weight as creatinine clearance falls below 60 mL/min.39
Efficacy
Adjunctive Therapy in Adults
Partial Seizures
The largest number of controlled clinical trials of vigabatrin have been performed in adults with previously intractable seizures taking the drug as add-on therapy. Table 1 outlines the designs of the six single-center, double-blinded European trials. Although most of the trials enrolled patients with other seizure types, the majority of patients had partial seizures. All trials demonstrated a statistically significant superiority of vigabatrin over placebo. In all but one, an overall mean or median seizure reduction of 40% or greater was obtained when patients were on vigabatrin in comparison with placebo. In a somewhat different type of trial design,88 33 patients were placed on open-label vigabatrin. Only those achieving a seizure reduction greater than 50% were studied further in a double-blinded phase and randomized to placebo or continued vigabatrin. Patients randomized to vigabatrin maintained a mean 54.7% seizure reduction, whereas patients randomized to placebo had a relatively rapid return of seizures.
Table 1 Summary of the double-blind placebo-controlled add-on trials of vigabatrin for refractory (mainly partial onset) seizures | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The European trials were performed primarily on patients with partial seizures, but patients with other seizure types were also included. A meta-analysis was performed to examine results among the 98 patients with partial epilepsy. In 46%, seizure reduction was greater than 50% after vigabatrin treatment in comparison with placebo, whereas in 11%, seizures worsened.34,59,86,90,106,108
Unlike the European trials, the two U.S. double-blind trials were of parallel rather than crossover design, and both were multicenter trials.10,23,28 They enrolled exclusively patients with complex partial seizures, with or without secondary generalization. Details of these trials are outlined in Table 1. Both trials demonstrated a statistically significant reduction in seizures when vigabatrin was compared to placebo.
In the Canadian double-blind trial,12 111 patients with refractory partial seizures were given either placebo or vigabatrin at doses of 2 to 4 g per day. Magnetic resonance imaging (MRI) and evoked potentials and neuropsychological testing were done at regular intervals for a 36-week period. In the vigabatrin group, 48% of patients achieved a 50% or more seizure reduction compared with 26% on placebo. However, seizure-free rates were not presented, only seizure-free days. Vigabatrin was well tolerated, and no changes were noted in the MRI or visual evoked potential (VEP) in this short-term study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree