Figure 66.1. Zonisamide.
MECHANISM OF ACTION
There are several pharmacologic effects of zonisamide that may be responsible for its activity as an AED. Results from several studies demonstrate the most likely mechanism of action for zonisamide to be through blockade of T-type calcium channels, inhibition of slow sodium channels, and possibly inhibition of glutamate release (1–5). Zonisamide differs from ethosuximide in that zonisamide does not inhibit G protein–activated inwardly rectifying K+ channels (6). Zonisamide does have activity as a carbonic anhydrase inhibitor, but this is not responsible for its antiepileptic activity (7). In animal models, zonisamide demonstrates broad spectrum as an AED (8–11). Beside its antiepileptic activity, zonisamide has some effect as a neuroprotective agent in ischemia (12,13). Additionally, other pharmacologic activities may make zonisamide useful in the treatment of Parkinson disease and essential tremor (14,15).
PHARMACOKINETICS
Absorption
Zonisamide is rapidly absorbed following oral administration with maximum concentrations achieved within 2 to 5 hours (16). The absolute bioavailability in humans is unknown, due to the lack of a parenteral product. Nagatomi et al. (17) measured the absolute bioavailability of orally administered zonisamide at 81% in rats. In the same study, the bioavailability of zonisamide in a rectal preparation was 96%. Zonisamide is metabolized by cytochrome P450 3A4 (CYP 3A4) (18). Intestinal CYP 3A4 may account for decreased bioavailability of the oral preparation.
Distribution and Protein Binding
Like many sulfonamide drugs, zonisamide has a dose-dependent decrease in volume of distribution (Vd/F) (19). The volume of distribution for a 200-mg dose is 1.8 L/kg and for an 800-mg dose is 1.2 L/kg. Saturable binding to erythrocytes, especially to intracellular carbonic anhydrase, is the most likely explanation for this phenomenon (20–22). Additionally, 40% to 60% of zonisamide is bound to plasma proteins, especially albumin (22,23).
Therefore, zonisamide is concentrated in the erythrocytes compared to plasma. With saturable binding to erythrocytes, the whole blood zonisamide concentration is nonlinear as the dosage increases. However, the plasma zonisamide concentration is linear with increased doses (16). Care must be taken in laboratory analysis and interpretation of zonisamide concentrations. Results should be identified as coming from whole blood or plasma.
Metabolism and Clearance
Following oral administration, the half-life (t1/2) of zonisamide is estimated at 50 to 69 hours (16,24). Apparent oral clearance (Cl/F) following single and repeated oral doses is 0.6 to 0.71 L/hour (24). Less than 30% of zonisamide is eliminated unchanged in the urine, and most of the drug undergoes extensive hepatic metabolism (25). The relatively long t1/2 and slow clearance allow for once-daily dosing of zonisamide.
Early studies of the pharmacokinetics of zonisamide suggested that concentrations increased in a nonlinear relationship to doses (19,26). Following an 800-mg dose, zonisamide clearance was 22% lower than clearance estimates following 200- and 400-mg doses. Clearance estimates at steady state with doses ranging from 400 to 1200 mg daily were 40% lower than those seen following a single 400-mg dose (16,27). One study showed steady-state zonisamide concentrations to be higher than predicted from single-dose data, but steady-state plasma concentrations did increase in a linear relationship to daily dose (28). These observations were considered to be related to the saturable, preferential binding of zonisamide to erythrocytes. However, an analysis of zonisamide doses and concentrations in children using a nonlinear mixed- effects model and population pharmacokinetic methodology demonstrated dose-dependent, Michaelis–Menten pharmacokinetics of zonisamide with a mean Vmax of 27.6 mg/d/kg and Km of 45.9 μg/mL (29). Because the Vmax is well above the typical range of daily zonisamide doses, it is unlikely that the nonlinear nature of zonisamide clearance will profoundly impact clinical practice.
The major metabolite of zonisamide is 2-sulfamoylacetylphenol (SMAP), formed under anaerobic conditions by liver microsomal enzymes (18,30,31). The formation of SMAP appears to be primarily through cytochrome P450 3A4 (CYP 3A4) (18,30). In these studies, metabolism of zonisamide to SMAP was inhibited by cimetidine and ketoconazole, known CYP 3A4 inhibitors. Zonisamide is metabolized to a much lesser extent by CYP 2C19 and CYP 3A5 (32). Studies of the effect of genetic polymorphisms on zonisamide metabolism have shown a 16% to 30% reduction in clearance in individuals who were CYP 2C19 heterozygous extensive metabolizers or homozygous poor metabolizers compared to homozygous extensive metabolizers (33). The clinical implications of this observation are unclear.
Serum Concentrations and Doses
The manufacturer’s recommended dose for adults is 300 to 400 mg daily, but doses of 600 mg daily have been used in clinical trials (34). Doses above 400 mg have not consistently been associated with increased efficacy. The recommended doses of zonisamide are typically associated with steady-state plasma concentrations of 10 to 38 μg/mL (24,29,35). However, a relationship between concentration and response has not been established. Other investigators have suggested that concentrations >30 μg/mL are associated with increased adverse effects (19,28). Therefore, it may be advisable to maintain zonisamide concentrations <30 to 40 μg/mL. The pharmacokinetics and dosing of zonisamide are summarized in Table 66.1.
Table 66.1 Summary of Zonisamide Pharmacokinetics and Dosing

aBased upon animal data.
bVolume of distribution is inversely related to dose, due to saturable binding to erythrocytes.
cAdditionally, zonisamide is highly and preferentially bound to erythrocytes.
dThese are typical concentrations observed with usual doses. A relationship between concentrations and response has not been established.
eHigher doses have been used in clinical trials.
Special Populations
Pediatrics
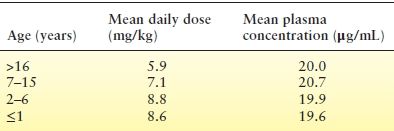
No formal pharmacokinetic studies have been done in children. In a study of zonisamide for infantile spasms by Suzuki et al. (36), daily doses of 4 to 5 mg/kg yielded plasma concentrations of 5.2 to 16.3 μg/mL. Additional work by this group substantiated these findings with zonisamide doses of 4 to 12 mg/kg/d producing plasma concentrations of 5.2 to 30 μg/mL (37). Table 66.2 summarizes typical mean plasma concentrations related to dose and age. A comparison of pharmacokinetic parameters derived from population data in children and adults shows a similar volume of distribution but more rapid clearance of zonisamide in children (29,39). Thus, children appear to require larger doses of zonisamide, based on body weight, to achieve plasma concentration similar to those seen in adults (40).
Three case reports have provided some documentation regarding transfer of zonisamide across the placenta and into breast milk. Kawada et al. (41) measured zonisamide concentrations in umbilical cord blood, infant blood, and maternal blood of two infants born to mothers taking zonisamide for epilepsy. In these infants, zonisamide concentrations were 92% of that in maternal blood. Kawada et al. also measured zonisamide concentrations in the breast milk of these mothers, showing these concentrations to be 41% to 57% of maternal plasma concentrations. In a separate case, evaluating zonisamide concentrations in breast milk to 30 days postpartum, Shimoyama et al. (42) observed breast milk concentrations to range from 81% to 100% of maternal plasma concentrations. It appears that zonisamide readily crosses the placenta. Zonisamide also appears in breast milk at concentrations similar to maternal plasma concentrations. No clinically important adverse effects related to zonisamide were documented in these case reports.
Pregnancy
A study of clearance of multiple AED, including zonisamide, in pregnant women demonstrated that changes in clearance are highly variable between women and between pregnancies. In general, doses increased during pregnancy. It is recommended that serum concentrations of zonisamide be closely monitored during pregnancy and appropriate dosage adjustments made (43).
Renal Failure
A single-dose study of zonisamide in individuals with moderate renal failure (creatinine clearance >0.6 L/hour) did not demonstrate any difference in pharmacokinetic parameters compared to normal individuals (44). Studies in severe renal dysfunction and multiple-dose studies in renal failure have not been reported.
DRUG INTERACTIONS
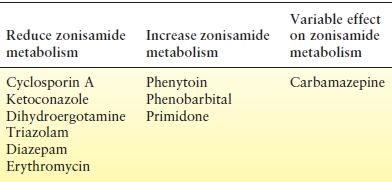
Because zonisamide is primarily metabolized through CYP 3A4 and to a lesser extent by CYP 2C19, it is potentially prone to drug–drug interactions involving these enzyme systems. Several interactions have been studied in animals and in humans (Table 66.3). However, the exact clinical implications of these interactions are poorly documented.
Table 66.3 Drug Interactions with Zonisamide
Influence of Other Drugs on Zonisamide
Using in vitro studies of the CYP 3A system, Nakasa et al. (32) showed that cyclosporin A, ketoconazole, dihydroergotamine, and triazolam profoundly inhibit zonisamide metabolism. These drugs reduced zonisamide metabolism by 85% to 95% compared to control. Other known inhibitors of CYP 3A, diazepam, terfenadine, erythromycin, and lidocaine did not produce a marked reduction in metabolism. The percent reduction in metabolism with these agents ranged from 35% to 45%. Clinical correlates to these findings have not been documented, so recommendations for dosage adjustments in patient care are not available. However, patients receiving known inhibitors of CYP 3A may require lower doses of zonisamide to reduce the risk of adverse events.
Inducers of CYP 3A have been shown to increase the metabolism of zonisamide (32). Phenytoin and carbamazepine have been shown to induce zonisamide metabolism, with phenytoin possibly having a greater influence than did carbamazepine (45,46). In a study of 12 patients receiving phenytoin or carbamazepine concomitantly with zonisamide, the mean oral clearance (Cl/F) of zonisamide was 33.9 mL/h/kg with phenytoin and 20.6 mL/h/kg with carbamazepine (45). However, some researchers have observed inhibition of zonisamide metabolism by carbamazepine (32). Other known inducers of hepatic metabolism, especially phenobarbital and primidone, can also increase the metabolism of zonisamide (16). When zonisamide is used in combination with known CYP 3A inducers, doses of zonisamide may need to be increased to achieve seizure control. In the case of carbamazepine, care must be taken to determine if induction or inhibition is predominant in a given patient and zonisamide doses adjusted accordingly.
Zonisamide Influence on Other Drugs
Studies with zonisamide have shown that it does not induce or inhibit hepatic enzymes (47,48). A study of zonisamide’s effects on ethinyl estradiol–norethindrone oral contraceptives demonstrated no alteration of hormonal effect or loss of contraceptive efficacy (49). A survey of interactions between zonisamide and cancer chemotherapy agents demonstrated no known interactions (50). It appears that zonisamide does not cause clinically significant alteration of the pharmacokinetic disposition of other drugs.
Drug–Food Interactions with Zonisamide
As a substrate for CYP 3A4, zonisamide is a candidate for drug–food interactions. Within the intestinal wall, are high concentrations of CYP 3A4 that can metabolize drugs before they are absorbed into systemic circulation. Several foods, especially grapefruit juice, lime juice, and Seville orange juice, contain substances that inhibit the activity of intestinal CYP 3A4. When these foods are eaten with drugs that are metabolized by CYP 3A4, there is increased absorption of the drug and a potential for adverse effects. Although this potential interaction with zonisamide has not been documented, it should be of concern. In a study of rectal administration (a route that bypasses intestinal CYP 3A4) of zonisamide, Nagatomi et al. (17) consistently demonstrated increased bioavailability and absorption of zonisamide.
CLINICAL TRIALS
Clinical studies of zonisamide have evaluated its use in several different types of epilepsy and epilepsy syndromes. Additionally, zonisamide has been used extensively in Japan and has gained increasing use in the remainder of the world. Despite this history, there have been no direct comparisons of zonisamide to other AEDs in specific seizure types. The best published comparison has been in two meta-analyses of clinical trials of other newer AEDs, including zonisamide (51,52).
Focal-Onset Epilepsies/Partial Seizures
Clinical studies of zonisamide have evaluated its use in several different types of epilepsy and epilepsy syndromes. The best published comparisons are in meta-analyses of clinical trials of other newer AEDs, including zonisamide (51–54). In the first study, Marson et al. (51) evaluated the odds ratio of zonisamide producing a ≥50% reduction in seizure frequency compared to placebo. Combining data from two clinical trials, zonisamide was shown to be significantly better than was placebo in controlling seizures. In a second meta-analysis, Marson et al. (52) identified the five most common adverse effects patients on zonisamide experienced. In a study designed to compare intention to treat to last observation carried forward methodology, zonisamide had a 3% seizure-free rate compared to 0.8%, 2.6%, 7.1%, and 1.4% for lamotrigine, oxcarbazepine, levetiracetam, and pregabalin, respectively (53). A Cochrane review of zonisamide for refractory partial epilepsy concluded that it is effective as adjunctive therapy in patients who have failed other pharmacotherapy (54).
Several clinical trials of zonisamide for partial seizures have been published (27,28,55–59). Each of these studies demonstrated that zonisamide was significantly more effective than was placebo in reducing seizures. For adults, a reduction in seizures occurs with doses ranging from 100 to 500 mg/day, and increasing doses in this range increases the number of patients who respond.
A summary of Japanese studies using zonisamide in pediatric patients with partial seizures estimated that 34% of children responded to zonisamide (39). Guerrini et al. (60) reported a randomized clinical trial of adjunctive zonisamide in 207 children, aged 6 to 17 years. Responder rates were 50% for zonisamide compared to 31% for placebo, a statistically significant difference. There was no difference in the incidence of adverse events between zonisamide and placebo treatments. Decreased appetite, weight loss, somnolence, vomiting, and diarrhea were more frequently associated with zonisamide. In a safety study of zonisamide in 107 patients, there was a significant reduction in all seizure types, with 7 patients discontinuing therapy due to serious adverse events (61).
Generalized Epilepsies
A small clinical trial suggested that zonisamide decreases cortical excitability in patients with idiopathic generalize epilepsies (62). Henry et al. (63) report two cases of progressive myoclonic epilepsy where zonisamide use was associated with reduced seizure frequency and improved functioning. A case series of patients with juvenile myoclonic epilepsy indicated that zonisamide was well tolerated and associated with reduced seizures compared to valproate (64). A similar retrospective study of juvenile myoclonic epilepsy indicated that zonisamide was easily titrated and had a rapid onset of action (65).
More extensive evaluation of zonisamide in primary generalized epilepsies has been done in children. In children with newly diagnosed infantile spasms, Suzuki et al. (36) used 3 to 10 mg/kg/d of zonisamide in an open-label trial. Of the children who started on zonisamide, four had complete seizure control and cessation of hypsarrhythmia with doses of 4 to 5 mg/kg/d. Kishi et al. (66) reported their experience with zonisamide in children with hypsarrhythmia. In three patients, zonisamide resulted in elimination of hypsarrhythmia and seizures. A larger study in 54 patients, newly diagnosed with West syndrome, was done (37). Zonisamide doses ranged from 4 to 14 mg/kg/d with a mean dose and serum concentration of 7.2 mg/kg/d and 15.3 μg/mL, respectively. Eleven infants had complete elimination of seizures and hypsarrhythmia, 7 children had >50% reduction in seizure frequency, and 14 with cryptogenic West syndrome responded. Of those who the authors categorized as not responding, 4 were seizure free transiently, 6 had a <50% reduction in seizure frequency, and 33 had no change in seizure frequency. The 11 individuals in this study who had elimination of seizures and hypsarrhythmia were entered into a long-term follow-up study, evaluating their response out to 79 months (mean duration of 53 months) (67). Seven of the infants who had an initial cessation of seizures continued to be seizure free. Presence of epileptiform activity on the EEG at the end of 3 weeks was predictive of recurrence of seizures. Yanagaki et al. (68) studied the use of zonisamide starting at 10 mg/kg/d, demonstrating this scheme was well tolerated in children with West syndrome.
Although case series reports and open-label studies suggest that zonisamide may be effective in patients with generalized epilepsies, it has not been well studied in this patient population. The most extensive information on zonisamide use in generalized epilepsies is in children with West syndrome and suggests that zonisamide may be an alternative treatment.
Monotherapy
Few clinical trials have evaluated the use of zonisamide in monotherapy for the treatment of epilepsy. The most extensive studies have been in children with West syndrome (36,67). Additionally, Kumagai et al. (69) studied zonisamide as a single agent in 44 children with epilepsy. In this open-label trial, 30 children with various seizure types became seizure free, and 6 children had to discontinue the drug due to adverse effects. A systematic review of adjunctive and monotherapy for partial seizures in children concluded that there was insufficient evidence to support zonisamide monotherapy (70).
The only published study of zonisamide monotherapy in adults was done by Wilensky et al. (28). Eight adults with partial seizures and receiving phenytoin were randomized to carbamazepine or zonisamide and then crossed over in an open-label design. Two subjects had improved seizure control with zonisamide compared to carbamazepine, and a third individual had a similar response but had to discontinue zonisamide due to the development of Stevens–Johnson syndrome.
The limited available data on zonisamide monotherapy treatment indicate that zonisamide may be effective as a single agent for epilepsy. However, larger, double-blind clinical trials must be done before zonisamide monotherapy can be recommended.
Nonepilepsy Indications
Preliminary clinical trials of zonisamide in disorders other than epilepsy indicate that it may be useful for other indications. One study of zonisamide in patients with mania and acute psychotic conditions indicated that 71% responded at least moderately to treatment (71). In an open-label trial of zonisamide in 35 patients with neuropathic pain, mean pain scores showed little or no improvement after 8 weeks of therapy (72). A trial in nine patients with Parkinson disease demonstrated that seven of the nine patients had improvement in their symptoms, especially wearing-off phenomenon, when zonisamide was added to their other medications (73). Preliminary data suggest that zonisamide is at least as effective as is propranolol in patients with head tremor or essential tremor (14,74).
ADVERSE EFFECTS
Common Adverse Effects
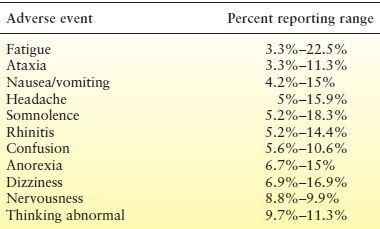
In the initial and major clinical trials of zonisamide as adjunctive therapy, several adverse effects were commonly reported (Table 66.4) (27,28,55–57). Schmidt et al. (55) reported the statistical evaluation of adverse events reported in their trial. Dizziness, somnolence, anorexia, abnormal thinking, ataxia, and confusion were more common with zonisamide compared to placebo. A meta-analysis showed that patients on zonisamide were more likely to experience anorexia, ataxia, dizziness, and fatigue compared to patients receiving placebo (52). These adverse events and their frequency are similar to those reported with other new AEDs.
Table 66.4 Most Frequently Reported Adverse Effects in Clinical Trials
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree