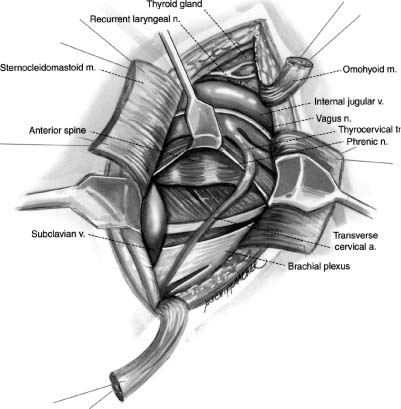
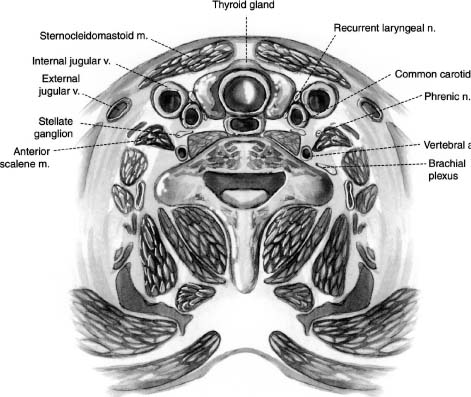
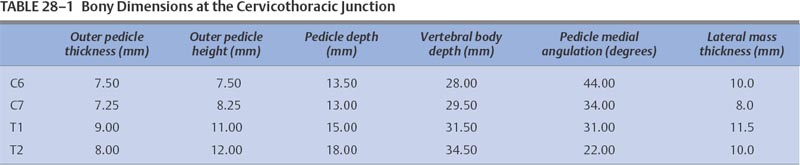
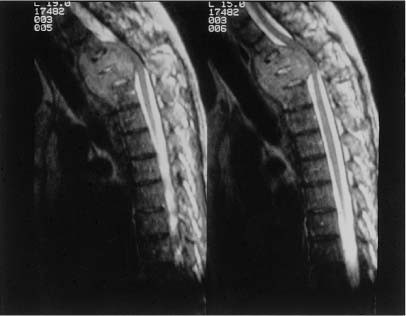
28 With the exception of Pancoast tumors, the nature and types of tumors that affect the cervicothoracic junction are the same as lesions affecting other segments of the spine; however, the anatomy, the biomechanics, and the stabilization principles of the cervicothoracic junctions make treatment of primary and secondary tumors of this region of the spine a unique consideration. Direct, anterior access to and fixation of the cervicothoracic junction are hampered by the bony structures constituting the thoracic inlet, the great vessels, and the prevertebral soft tissues, and may be further complicated by the unique configuration of this segment of the spine. This chapter reviews the anatomy of the cervicothoracic junction and thoracic inlet, the various surgical approaches to this segment of the spine for decompression, and the principles of fusion and fixation of the cervicothoracic junction. Factors such as the patient’s age, level of activity, neurologic status, operative risk, prognosis, suspected tumor type, and sensitivity to radiation or chemotherapy must be considered before deciding to do surgery on a patient with a cervicothoracic junction tumor. Surgery may be indicated when tissue is required for a diagnosis; for neck pain that is unresponsive to bracing, analgesics, or radiation; when there is neurologic deterioration, which is rapid or unresponsive to radiation; or when there is loss or impending loss of spinal stability. The evaluation of such a patient begins with a thorough history and physical exam, as well as a laboratory investigation. Computed tomography (CT) scans and magnetic resonance imaging (MRI) are complementary studies that can define the location and extent of the tumor and suggest the tumor type, the stage of disease, and the extent of bony involvement, as well as the anatomy at the region of involvement. In addition, the cervicothoracic junction represents a potential watershed zone with respect to the blood supply of the spinal cord at this level. The utility of preoperative spinal angiography to better define this blood supply and possibly embolize a potentially vascular lesion should be considered. Once the preoperative evaluation is completed, a clear surgical goal must be defined. Complete resection of primary or metastatic tumors at this region is usually not possible. As a result, the goals of treatment often shift from trying to achieve local control of the tumor to palliation. The decision as to whether to decompress the spine via an anterior and/or posterior approach depends on the location of the tumor and its extension into surrounding structures as well as the goals of the operation and the health of the patient. Decompressions at this region usually must be followed by a fusion. Internal fixation where the lordotic cervical spine meets the kyphotic thoracic spine can be technically challenging and may be further complicated by a kyphotic deformity from a pathologic compression fracture or by a paucity of good bone due to tumor erosion. If there is to be an attempt at a correction of a deformity or the possibility of postoperative radiation therapy, the use of both anterior and posterior instrumentation should be considered. A thorough understanding of the anatomy of the cervicothoracic junction and the anatomic variabilities that exist from patient to patient is necessary when determining the surgical feasibility and approach to the treatment of lesions at this region. This anatomic variability prevents a good correlation of anatomic landmarks with deep neurovascular structures.1 In addition, good intraoperative radiographic evaluation of the lower cervical and upper thoracic spine is exceedingly difficult. Safely navigating in this region, which is already unfamiliar to most spine surgeons, can be difficult. Technology such as image-guided frameless stereotaxy has already been applied safely to the cervical and thoracic spine, and it is hoped it will reduce complications and improve outcomes as this technology improves.2 The thoracic inlet is bound by the body of T1 posteriorly, the tip of the manubrium anteriorly, and the first ribs laterally. The thoracic inlet slopes inferiorly from the vertebral body of T1 to the manubrium, along the orientation of the first ribs. The muscles within and surrounding the thoracic inlet begin with the superficially located platysma muscle lying below the subcutaneous fat. Deep to this, the sternocleidomastoid (SCM) muscles arise from the sternum and clavicle. The SCM and infrahyoid strap muscles overlie the anterior aspects of the mediastinal organs, passing through the thoracic inlet. Beneath the SCM muscle lie the great veins of the mediastinum, including the facial and cephalic veins, which are joined by the internal jugular and subclavian veins. The proximal aspect of the left subclavian vein is the venous structure most commonly encountered during low anterior cervical approaches to this region. The arterial structures lie deep to the veins and include the right brachiocephalic, left internal, and left subclavian arteries. Anterior approaches to the second and third thoracic vertebrae frequently require retraction of these vascular structures. The vertebral arteries are coursing toward the C6 lateral transverse foramina through the thoracic inlet from their origins off the subclavian arteries. The surgeon must be mindful of the presence and course of the vertebral arteries when addressing cervicothoracic pathology involving the anterior aspect of C7. Deep to the vascular structures in the upper mediastinum run the trachea and the esophagus, which overlie the anterior aspect of the spinal column. Important nerves and ganglia also pass through this region. The vagus nerves course into the thorax within the carotid sheath. The stellate ganglia lie along the anterolateral aspect of the vertebral column at the C7-T1 junction (Fig. 28-1). The phrenic nerves pass from the cervical plexus along the anterior aspects of the scalene anterior muscles in the mediastinum as they descend toward the diaphragm. The recurrent laryngeal nerves arise from the vagus nerve in the neck. These nerves initially project caudally through the thoracic inlet, then reverse direction and course cephalad between the esophagus and trachea to the laryngeal musculature. The configuration of the mediastinal arterial structures determines the course of the right and left recurrent laryngeal nerves. The right recurrent laryngeal nerve arises from the vagus nerve at the level of the subclavian artery. It travels behind and then hooks around the right subclavian artery, usually at the level of the T1–2 interspace. It then passes posterior to the common carotid artery to ascend obliquely from lateral to medial in the prevertebral soft tissues toward the trachea and the esophagus.3 The left recurrent laryngeal nerve arises from the vagus nerve at the level of the arch of the aorta. This nerve hooks around the arch of the aorta, distal to the ligamentum arteriosum to ascend between the trachea and esophagus. A procedure incorporating a right low anterior cervical approach is at greater risk of injuring the right recurrent laryngeal nerve than the same approach performed on the left side; however, such a left-sided approach places the thoracic duct at risk. The thoracic duct travels along the anterolateral surface of the vertebral column as it ascends into the thoracic inlet. It opens into the left subclavian vein near the junction with the left internal jugular vein. Injury to the thoracic duct can best be avoided by limiting the dissection medial to the carotid artery. FIGURE 28-1 An illustration of the cross-sectional anatomy at the level of the C7-T1 interspace. The bony anatomy at the cervicothoracic junction can vary from patient to patient and may be furthered altered by tumor involvement; however, several studies have demonstrated some consistent trends in this region.1,4–7 Although there is a progressive enlargement of vertebral body size from C5 to T3, the lateral masses in the cervical spine and the transverse processes in the thoracic spine progressively decrease in size from C5 to T3.4 The pedicle size is greatest at T1 and T2, and then decreases above and below the cervicothoracic junction.4 At the cervicothoracic junction there is a change in the facet orientation from horizontal to coronal. The orientation of the cervical facet joints is relatively horizontal, with a posterior-inferior slope to the joint surfaces. In the thoracic spine, the facets tend to be coronally oriented, with a slight medial-to-lateral anterior curvature.8 The precise configuration of the facet joints in the upper thoracic spine varies from patient to patient, as transitional shapes are sometimes seen. In addition, the pedicle becomes more perpendicular to the vertebral body below the cervicothoracic junction; however, the pedicle angle at each level is often variable1 (Table 28-1). These factors underscore the need for careful preoperative planning when planning on stabilizing this region using posterior instrumentation. Closely related to the bony anatomy of this region are the vertebral arteries, nerve roots, and spinal cord. The vertebral artery originates from the subclavian artery proximal to the thyrocervical trunk and most commonly runs anterolateral to the body of C7 and then enters the transverse foramen of C6. This places the vertebral artery susceptible to injury during an anterior decompression at the cervicothoracic junction where the artery is not protected in the transverse foramen. Furthermore, the vertebral artery is closely associated with the lateral mass of C7, putting it at risk during placement of lateral mass screws. The distance between the posterior cortex of C7 and the vertebral artery is ~ 1.68 cm.9 Attention to the anatomy of the nerve roots and spinal cord will also help avoid injury to these structures. The midcervical nerve roots exit the spine immediately lateral to the takeoff of the nerve root sleeve of the dural sac and pass through the intervertebral foramina in a gentle, anterior orientation. The coronal angulations of the spinal nerve roots become progressively more perpendicular from the transition of C6 to T1. The C7 and upper thoracic nerve roots tend to course cephalad in the spinal canal lateral to the thecal sac as they course toward their respective intervertebral foramina. The cephalad course and perpendicular orientation of the nerve roots, in particular at T1, place this nerve root at risk, because it is in proximity to the T1 pedicle, during the placement of pedicle screws.10 The spinal cord and spinal canal width at the cervicothoracic junction are greatest at C6 and then decreases from C7 to T2.1 Penetration of either the medial or superior cortex during pedicle screw insertion may carry a higher incidence of neurologic complication. No anterior or anterolateral approach to the cervicothoracic junction region is ideal with respect to avoiding the various neurovascular structures and organs in this region. The approach required to gain access to the spinal pathology of this region determines which of these structures are more at risk in a given surgical procedure; however, it is the nature and location of the spinal pathology that should typically dictate the approach to the junction. The biomechanics of the cervical spine and the biomechanics of the thoracic spine are well documented.11–13 However, the transitional zone between these two segments of the spine has not been well studied. The configuration of the cervicothoracic junction is quite variable from patient to patient. This lack of a clear understanding of the biomechanics of the cervicothoracic junction complicates the decision making regarding the extent of the fixation required to stabilize this spinal segment. The sagittal axis of the spine typically passes through the region of the odontoid process, posterior to the cervical vertebral bodies, through the vertebral bodies at the cervicothoracic junction, and then anterior to the thoracic spine.14 The location of the cervicothoracic junction, along the center of gravity in the sagittal plane, balances flexion and extension forces applied to the anterior and posterior elements. An imbalance of forces or breakdown of the structural elements of the vertebrae at the cervicothoracic junction can alter the configuration of the spine at this level. A deformity of the spine can result, depending on the extent and magnitude of the destruction of the vertebral body, the precise location of the neutral axis of the spine, and the configuration of the patient’s cervicothoracic junction transition, which varies from patient to patient. Although a complete categorization of the various configurations of the cervicothoracic junction has not been established, there appears to be variability in the degree of translation between the cervical lordosis and the thoracic kyphosis. This can range from a relatively neutral or straight segment of the spine through the cervicothoracic junction, or a more typical change from lordosis to kyphosis. The location of the transition from lordosis to kyphosis can be located at the C7-T1 interspace or below but may vary from patient to patient. The premorbid configuration of the cervicothoracic junction probably has bearing on the nature and orientation of forces applied to this segment of the spine in normal and pathologic conditions. A relatively straight transition from the cervical spine to the thoracic spine imparts a preponderance of axial load on the spine. As the acuity of the transition from lordosis to kyphosis increases, so too does the amount of sheer force applied to this segment of the spine. In addition, the rib cage, while helping to stabilize the thoracic spine, potentially acts as a fulcrum about which further sheer stresses can be expected to be transmitted to the lower cervical spine. With such a configuration, incompetence of the osteoligamentous structures, in particular the posterior elements, predisposes to the development of a translational deformity across the involved segment of the spine. The incompetence of the osteoligamentous elements may result from a pathologic process or following surgical decompression. The location of the sagittal neutral axis of the spine lies anterior to the vertebral bodies in the upper thoracic spine. A pathologic process involving the upper thoracic vertebral bodies typically reduces the upper thoracic vertebral bodies’ capacity to resist flexion and predisposes to development of a kyphotic deformity (Fig. 28-2). The magnitude of the flexion forces, and the likelihood of developing kyphosis, increases with the degree of existing kyphosis prior to the onset of the pathologic process. Patients with acute cervicothoracic transitions or acute thoracic kyphoses from other disorders (osteoporosis or Scheuermann’s disease) represent the population at greatest risk. FIGURE 28-2 Magnetic resonance imaging (MRI) of a metastatic lesion involving the cervicothoracic junction that has resulted in a kyphotic deformity. No one surgical approach to the cervicothoracic junction is optimal under all circumstances. The approach selected is determined by the spinal configuration, the nature and location of the pathology, and patient factors. The anatomic features of the thoracic inlet of this segment of the spine have spawned the development of a variety of anterior approaches to the cervicothoracic junction. These various techniques vary with respect to the amount and orientation of access that can be achieved at the cervicothoracic junction and also the extent of the dissection required to achieve this access. The extent of dissection is inversely proportional to the degree of risk and potential complications associated with the procedure. The majority of pathology at the cervicothoracic junction is located in the vertebral body necessitating anterior access. Although direct, an anterior approach must contend with the limited access to this location due to the anatomy at the cervicothoracic junction, as well as any deformity that may be present. Anterior approaches to the cervicothoracic junction include the low cervical approach,15,16 the supraclavicular approach,17,18 the transaxillary approach,17 the sternal-splitting or modified sternalsplitting approaches,17 and the transthoracic approach, as well as several modifications of these approaches.19,20 The multiplicity of anterior approaches to this region suggests the inadequacies that exist in each of these approaches, none of which are ideal in all circumstances. The low anterior approach is simply an anterior approach to the cervical spine extended inferiorly to expose the cervicothoracic junction. A paramedian transverse incision may be used; however, a longitudinal incision made parallel to the medial aspect of the SCM muscle and extended down to the level of the manubrium affords a better exposure and access to the thoracic inlet. The platysma and superficial fascia are sharply divided. Using blunt dissection, a plane is developed between the carotid sheath laterally and the trachea and esophagus medially. The dissection is carried down to the level of the longus colli muscles and anterior longitudinal ligament. A lateral cervical spine x-ray is taken to confirm the level. The needle may need to be placed at the C5–C6 or C6–C7 interspace because the bony anatomy below C7 is often obscured on x-ray by the shoulders. The monopolar cautery is then used to elevate the longus colli, and self-retaining retractors are placed under them transversely and then longitudinally. Decompression, fusion, and placement of anterior instrumentation can then be preformed. This approach can be used to expose down to the T1–T2 interspace with limited morbidity.15 The low anterior approach works well for simple discectomy and fusion; for extension of an anterior decompression, fusion, and instrumentation from the lower cervical spine; or for biopsy; however, this exposure can be severely limited in patients with a short neck or a significant kyphotic deformity. The supraclavicular approach enables access to the lung apex, lower brachial plexus, and the C7 and T1 vertebral bodies. For this approach the patient is placed in the supine position with the head turned away from the side of the incision and the ipsilateral arm pulled down to open the supraclavicular space. A transverse incision is made 1 cm above the clavicle that extends from the midline to slightly beyond the posterior border of the SCM muscle. The dissection is carried through the platysma muscle and fascia, and the SCM muscle is identified and divided with the monopolar cautery. A finger under the SCM muscle can be used to protect the common carotid and internal jugular during this maneuver. The inferior strap muscles consisting of the omohyoid, sternohyoid, and sternothyroid muscles are divided to expose the anterior scalene muscle as it lies lateral to the prevertebral fascia. The phrenic nerve, which lies along the anterior surface of the scalene muscle, the carotid sheath, recurrent laryngeal nerve, and internal jugular vein can be retracted medially. The suprascapular and transverse cervical arteries may then be seen crossing the operative field after they arise from the thyrocervical trunk (Fig. 28-3). These arteries may be sacrificed. The anterior scalene muscle may then be divided. Sibson’s fascia invests the anterior scalene muscle and connects the transverse process of C7 to the first rib. This fascia is continuous with the endothoracic fascia on the inner surface of the first rib. Freeing Sibson’s fascia from the C7 transverse process allows access into the thoracic cavity and retraction of the lung. The vertebral artery can then be visualized as it arises from the subclavian artery to course medially over the anterolateral C7 vertebral body. In a left-sided approach the thoracic duct is at risk as it enters the venous system where the jugular and subclavian veins meet.
Surgical Approaches and Fixation of the Cervicothoracic Junction
 Anatomy of the Cervicothoracic Junction
Anatomy of the Cervicothoracic Junction
 Biomechanics at the Cervicothoracic Junction
Biomechanics at the Cervicothoracic Junction
 Approaches and Techniques of Internal Fixation of the Cervicothoracic Junction
Approaches and Techniques of Internal Fixation of the Cervicothoracic Junction
Surgical Approaches
Anterior Approaches
Low Anterior Approach
Supraclavicular Approach
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree