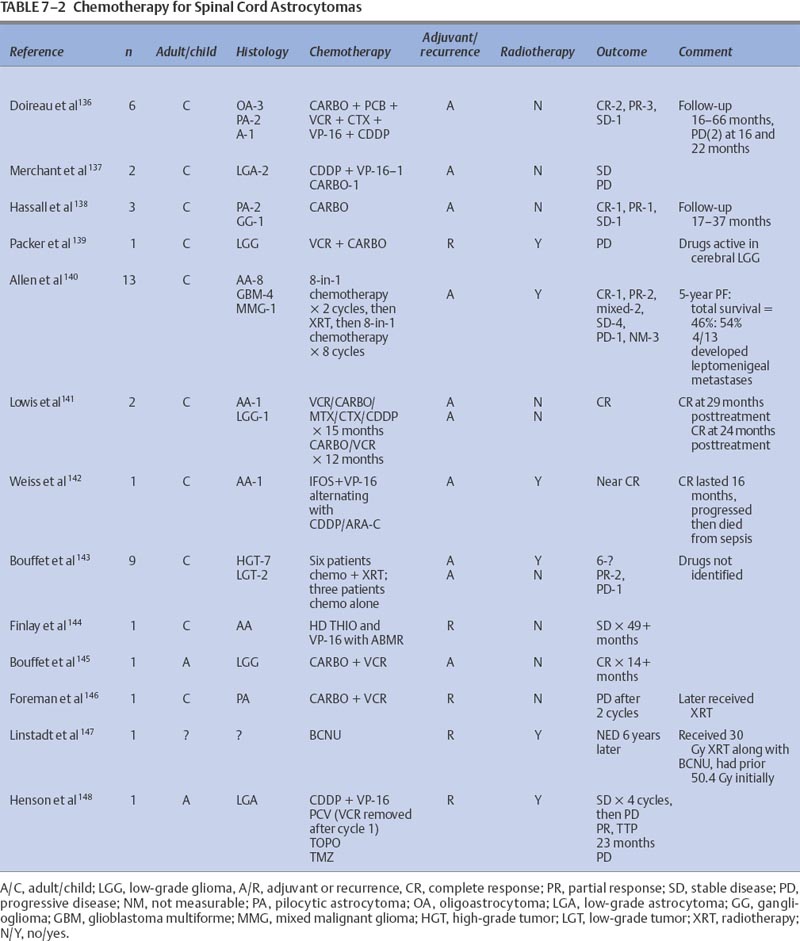
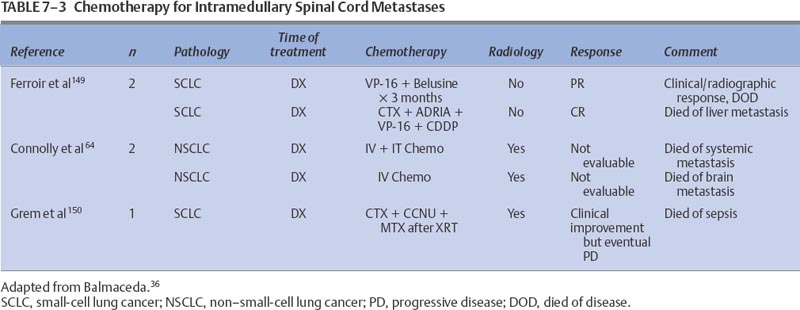
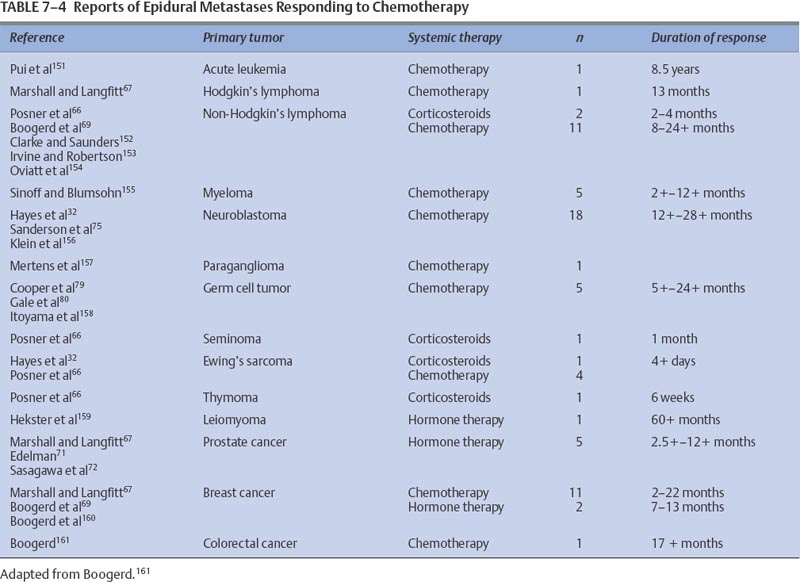
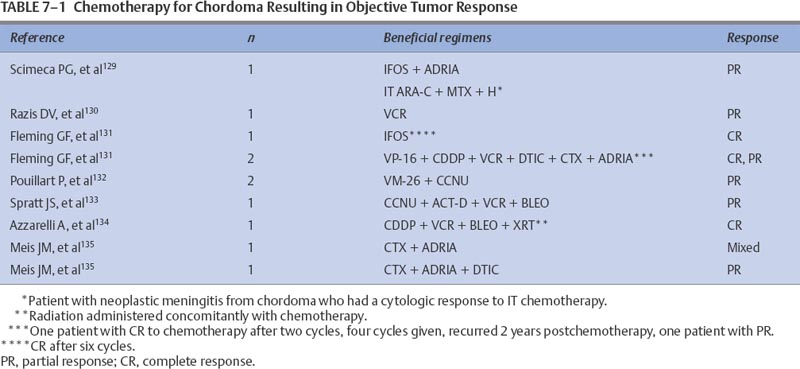
7 Tumors of the spinal cord, bony spine, and peripheral nerves can be grouped by anatomic location and primary histology, and for the purpose of discussing chemotherapeutic options, by whether they are primary or metastatic. Most of these tumor types have not been subjected to extensive experimental investigation in clinical trials. The choice and route of therapy depend on the location of the tumor, its histology, and its chemoresponsiveness. A list of the abbreviations for chemotherapeutic agents discussed in this chapter follows: Chordomas, accounting for 1 to 5% of all primary malignant bone tumors, are rare and slowly growing tumors that arise from remnants of the notochord. Generally located in the midline, ~50% originate in the sacrum, 35% in the clivus, and 15% in the vertebrae. There is a 61% distribution in the lumbar spine, 11% in the thoracic spine, and 28% in the cervical spine. More than one vertebral body is involved in 50% of patients. Occurring predominantly in the fifth decade of life, the male-to-female predominance is 2:1.1 For primary and recurrent chordomas, surgical resection is the mainstay of treatment. Radiotherapy is occasionally used for resistant or residual disease but has little effect on overall outcome. These tumors are not considered amenable to chemotherapy,2–4 although some authors have suggested that it could be worthwhile to further examine the usefulness of alkylating agents for treating chordomas.5–7 Most reports of chemotherapy in chordoma are of single cases or small series of patients. Table 7-1 presents a summary of potentially beneficial chemotherapies for chordoma. Therapy with combinations that include etoposide (VP-16), cisplatinum (CDDP), vincristine (VCR), dacarbazine (DTIC), cyclophosphamide (CTX), adriamycin (ADRIA), ifosfamide (IFOS), or bleomycin (BLEO) are reasonable for patients with recurrent chordoma and for whom treatment other than surgery and radiotherapy is warranted. Osteosarcomas, malignant bone tumors that usually arise in the metaphysis of long bones, can originate as primary spinal tumors or can metastasize to the spine from extraaxial tumor sites. Only ~2% of osteosarcomas are found in the spine. The median age of patients with osteosarcoma of the axial skeleton is 25 years, slightly older than the median age of all osteosarcoma patients, which is 17 years.8 There is also a slight male preponderance. Osteosarcomas of the axial skeleton are more refractory to treatment than limb tumors.9 Preoperative combination chemotherapy and radiotherapy may improve survival and may allow more complete tumor resection,10,11 although the optimal chemotherapy for osteosarcoma is unsettled. Whether complicated multidrug regimens improve outcomes compared with better tolerated and simpler regimens like ADRIA and CDDP is unknown.12 Various chemotherapy regimens have been used to treat osteosarcomas, including combined methotrexate (MTX), BLEO, CTX, actinomycin D (ACT-D), and ADRIA; ADRIA alone; and high-dose (HD) MTX alone.11 MAID chemotherapy (ADRIA, IFOS, DTIC, and Mesna), used as adjuvant therapy only, resulted in a 26% response rate.13 Adding CDDP and ADRIA to HD MTX, BLEO, CTX, and ACT-D preoperatively did not increase event-free survival.14 Anecdotal reports relate dramatic and prolonged remissions with the use of intraarterial ADRIA, followed by systemic combined CTX, VCR, MTX, phenylalanine mustard, and ADRIA (COMPADRI-III),15 with the combination of ADRIA, carboplatin (CARBO), and VP-16,10 as well as with ADRIA and epirubicin (EPI).16 Chondrosarcomas are a heterogeneous group of tumors whose basic neoplastic tissue is cartilage. Approximately 24% of malignant bone tumors and almost 11% of primary spinal tumors are chondrosarcomas. Five percent of chondrosarcomas are found in the spine. Two of the major classifications of osteochondroma are central and peripheral, referring to the tumor’s location within the bone. The median age of diagnosis of peripheral chondrosarcoma is 32 years compared with 44, 55, and 26 years for grade I, II, and III central chondrosarcomas, respectively.17 Treatment is surgical resection and occasionally radiotherapy. No reports of effective adjuvant chemotherapy have been published, and chemotherapy is seldom used to treat chondrosarcomas.18,19 Adjuvant ADRIA, however, has been used to treat chondrosarcoma with at least one reported prolonged remission.20 Malignant fibrous histiocytomas (MFHs) are high-grade bone tumors. Histologically, they resemble soft tissue MFHs. MFHs occur primarily in adults with a peak incidence in the fifth decade and with a slight male predominance.21 Malignant fibrous histiocytoma is treated similarly to osteosarcoma with preoperative chemotherapy, surgery, and often postoperative chemotherapy. Regimens used for osteosarcomas are frequently used for MFHs. The principles of chemotherapeutic management of MFH in the spine are no different from those used for treating nonaxial bone tumors. MFHs of non-axial bone, pre- and postoperatively treated with MTX-and CDDP-based regimens, have resulted in tumor necrosis in 90% of surgical specimens and in disease-free survivals of 67% at an average of 7.5 years follow-up.22 Pre- and postoperative treatments with drug regimens, including MTX, CDDP, and ADRIA, have resulted in 71% of patients being disease-free at a follow-up of 6.3 years.23 Reports of spinal MFHs treated with adjuvant regimens, including HD MTX and VCR, have resulted in remission-free survivals for as long as 48 months.24 Preoperative HD MTX alone resulted in a median progression-free survival of 31.5 months in five spinal tumor patients. The use of chemotherapeutic regimens, which include MTX, CDDP, and ADRIA, seems to be a reasonable approach for this group of patients.25 Ewing’s sarcoma is a small, round, blue cell tumor that usually occurs before the age of 20 years. With a 2:1 ratio favoring a male predominance, this tumor has a predilection for the femur, a location involved in 20 to 25% of all Ewing’s sarcoma patients. This chemosensitive tumor can involve the vertebral body, epidural space, or rib as a primary tumor, or it can directly metastasize to the bony spine, each instance of which can compress the spinal cord. These tumors are treated with surgery, radiotherapy, and chemotherapy.26,27 The benefit of neoadjuvant chemotherapy for adults and children is the same,28 but patients with Ewing’s sarcoma of the spine may not fare as well as patients whose tumors lie outside the spine.26 Standard chemotherapy for nonmetastatic disease currently consists of a combination of VCR, ADRIA, and CTX, alternating with combined IFOS and VP-16 for a total of 48 weeks.29 Other regimens have been studied with some success. Neoadjuvant therapy with VCR, ACTD, ADRIA, and CTX has resulted in 5-year disease-free and overall survivals of 54% and 59%, respectively.30 The addition of IFOS may improve these results.31 MAID adjuvant therapy for adults has resulted in response rates as high as 77%.13 Chemotherapy can also result in decompression of the spinal cord.32 Hayes and colleagues32 reported excellent responses in five Ewing’s sarcoma patients with epidural tumors treated with CTX followed by ADRIA. Also known as eosinophilic granuloma or histiocytosis X, Langerhans cell histiocytosis can manifest with lesions in the vertebral spinal column. The disease is characterized by a proliferation of histiocytes within the reticuloendothelial system. Most patients are between the ages of 1 and 15 years; the median age of presentation is 10 years; and the male-to-female ratio is 2:1. Soft tissue components can accompany bony lesions, and surgery is usually indicated for lesions that require treatment. Radiotherapy has been used to treat troublesome lesions, but treatment with prednisone and vinblastine (VBL) has resolved a spinal lesion in a 33-month-old girl at follow-up 9 months after treatment.33 As many as 74% of former patients with breast cancer had spinal vertebral metastases that were discovered at autopsy.34 Bony metastases in breast cancer are considered incurable, although significant palliation can be achieved with radiotherapy, chemotherapy, and hormonal therapy. Data have recently suggested that these therapies lengthen survival for patients with metastatic breast cancer. Little work has been done to assess the outcomes of patients with bony spine metastases in response to chemotherapy. Using the newest magnetic resonance imaging techniques, Ciray et al35 demonstrated stable disease or partial responses in 15 of 18 breast cancer patients with bone metastases who were treated with chemotherapy using either combined 5-fluorouracil (5-FU), EPI, and CTX or EPI and docetaxel or Taxol alone. Some of these patients had metastases involving the bony spine, and they derived benefit from the intervention. It is reasonable to assume that other chemotherapeutic and hormonal therapies useful for treating metastatic breast cancer will have beneficial effects for breast cancer patients with metastases to the bony spine. Intramedullary tumors account for most intraspinal tumors in children and for ~25% of intraspinal tumors in adults. Most true intramedullary spinal cord tumors are gliomas. Ependymomas occur most frequently in adults, and astrocytomas account for ~60% of pediatric intramedullary tumors.36 Because these tumors are rare, there are no large studies from which to derive meaningful guidance for the use of chemotherapy in their treatment. It is logical to assume, however, that chemotherapies that are useful for patients with intracranial tumors will also be of some benefit for patients with spinal cord tumors with the same histologies. Similar to intracranial astrocytomas, higher-grade spinal cord astrocytomas behave more aggressively than their lower-grade counterparts. When possible, tumors are removed surgically, and high-grade tumors are irradiated. Lower-grade tumors may be monitored without irradiation following total resection.37 Adjuvant and recurrent chemotherapy approaches have been adapted from those used to treat intracranial astrocytomas. Most of the literature consists of case reports or small series. Balmaceda36 reviewed the literature of chemotherapy for spinal cord astrocytomas (Table 7-2). Spinal cord oligodendrogliomas are usually treated with postoperative radiotherapy.38 The use of chemotherapy for spinal cord oligodendroglioma is not well documented, but it is assumed that the benefits from chemotherapy for intracranial oligodendrogliomas would extend to patients with spinal cord tumors. Whether spinal cord oligodendrogliomas have chromosomal deletions (1p, 19q) similar to intracranial tumors that appear to predict chemotherapeutic susceptibility is unknown. Some guidance can be derived from the case of a 2-year-old boy with a spinal cord oligodendroglioma who was treated with cyclohexylchloroethylnitrosourea (CCNU) and procarbazine (PCB) (discontinued after 6 months for unclear reasons), but he later responded to CARBO alone, exhibiting stable disease for almost 3 years. He was later treated with CARBO alone, and the spinal tumor had shrunk at a 6-month follow-up.39 Surgery and radiotherapy are the mainstays of managing ependymomas. Total surgical resection, older age, and a supratentorial tumor location may improve the prognosis for patients with these tumors.40,41 After radiotherapy, a local recurrence is more common than a leptomeningeal recurrence.42 As is true for intracranial ependymomas, the role, if any, of chemotherapy for spinal ependymoma in adults is not settled. Chemotherapy for intracranial ependymomas has been reviewed.43–45 There is evidence that chemotherapy can induce responses in some patients with an intracranial ependymoma, but there is little evidence to indicate that chemotherapy improves the length of survival.43 However, adjuvant chemotherapy, alternating among PCB and CARBO, VP-16 and CDDP, and VCR and CTX, has allowed some children to avoid radiotherapy without compromising their overall survival.46 Although promising, this chemotherapy strategy needs further confirmation with larger numbers of patients. In a review of chemotherapy used for treating intracranial ependymoma, 11% and 26% response rates were determined for single-agent and combination regimens, respectively.43 Single-agent chemotherapies for recurrent ependymoma, resulting in at least one patient response, included CDDP, CARBO, aziridinylben-zoquinone (AZQ), IFOS, 1-(2-chloroethyl)-3(2,6 dioxo1-piperidyl)-1-nitrosourea (PCNU), VP-16, CTX, and interferon-α (IFN-α). Combination regimens, tested predominantly in newly diagnosed patients, that have resulted in responses include 8-in-1 day regimen; VP-16 and CARBO; VP-16 and IFOS; VP-16 and CDDP, MOPP (mechloroethamine, VCR, PCB, PRED); VETOPEC; and CDDP, CTX, and VP-16. If the tumor has not progressed, myeloablative chemotherapy (CARBO, thiotepa [THIO], and VP-16 followed by autologous bone marrow rescue [ABMR]) has been used.43 Overcoming ependymoma resistance due to the multidrug resistance gene (MDR1)47 may improve chemoresponsiveness. There are only two reports of chemotherapy used specifically for recurrent spinal cord ependymoma. One patient was treated with the alkylator, AZQ,,48 and the other was treated with IFN-α.49 Both achieved a partial response lasting 2.75 and 15 months, respectively. Primary lymphoma of the spinal cord is extremely rare. Tumors are usually of B-cell lineage. Chemotherapy has been used to treat some of these cases.50,51 The combination of VCR, ADRIA, CTX, and prednisone (PRED), then radiotherapy, followed by two additional courses of chemotherapy, was unable to prevent intraventricular seeding of tumor 11 months after diagnosis in one patient.52 Radiotherapy followed by chemotherapy with combined VCR, CTX, PRED, and later with combined BLEO, ADRIA, CTX, VCR, and PRED resulted in remission for 23 months in another patient.51 Radiotherapy and adjuvant PCV (PCB, CCNU, VCR) chemotherapy resulted in a complete response in a 75-year-old patient with a spinal cord lymphoplasmacytoid lymphoma (immunocytoma).53 One patient who failed radiotherapy and later developed a cerebrospinal fluid (CSF) recurrence was treated successfully with intrathecal (IT) MTX and was free of disease 4 years later.54 Logically, drugs active against intracranial lymphoma may be assumed to be efficacious for lymphoma of the spinal cord. Intracranially, germ cell tumors usually occur in the pineal region. They make up 3 to 8% of intracranial tumors in children but only 1% of all intracranial tumors, with a peak incidence in the second decade.55 Histologically, tumors are either germinomas or nongerminomatous (nongerminomatous encompasses tumors that have histologic components of endodermal sinus tumors, yolk sac tumors, choriocarcinomas, or immature teratomas). Nongerminomatous tumors tend to behave most aggressively and are least responsive to treatment. In contrast, intracranial germinomas are quite responsive to radiotherapy, with 5-year survival rates between 65 and 95%. Nongerminomatous intracranial germ cell tumors are less susceptible to radiotherapy, demonstrating 5-year survival rates after radiotherapy between 20% and 56%.56,57 There is evidence that a reduced radiation dose and the addition of chemotherapy for patients with nongerminomatous germ cell tumors improves 5-year survival rates from 62 to 93% and from 0 to 27% for groups with an intermediate and poor prognosis, respectively.58 Regimens that included CDDP, combined CARBO and VP-16, or combined CDDP, VBL, and BLEO were used. Patients with intramedullary germ cell tumors are usually treated with radiotherapy, but chemotherapy may be used in their management, particularly when used to treat a nongerminomatous tumor. The use of chemotherapy in treatment of spinal cord germinoma is exemplified by the one patient who received radiotherapy with combined ACT-D, MTX, and VCR. The patient had an initial complete response,59 which lasted 5 months. At recurrence, the patient was treated with CDDP, BLEO, and VBL, along with irradiation, without response. Another patient, 17 months old with an intramedullary yolk sac tumor, was treated with two cycles of CDDP, VBL, and BLEO followed by radiotherapy and had a short-lived radiographic and tumor marker response.60 Although no conclusions can be made from the small number of patients treated, general chemotherapy guidelines can be derived from the literature on treating intracranial germ cell tumors. Histologically, primitive neuroectodermal tumors (PNETs) are composed of small, predominantly undifferentiated, embryonal-like cells with various degrees of glial or neuronal differentiation. These tumors most often occur in the first two decades of life although, interestingly, spinal PNETs may be more common in adults than in children.61 Because of their propensity to disseminate throughout the CSF, patients with PNET must have their entire craniospinal axis evaluated for tumor spread. Chemotherapy may benefit patients with these tumors, and regimens similar to those used in medulloblastoma are frequently employed. Drugs that have shown some benefit in the treatment of medulloblastomas and that may also benefit PNET patients include VCR, the nitrosoureas, platinum-based compounds, MTX, and CTX.62 Spinal cord PNETs are rare and can arise at all levels of the spine, including intramedullary, intra- and extramedullary, and extradurally. In one review that included 14 PNET patients, most were treated with surgery, radiotherapy, and chemotherapy with poor outcomes. Of the 14 patients, eight died within 2 years, two died at 36 months, one died at 42 months, and three were alive at 6, 15, and 36 months. An example of chemotherapy used after recurrence following surgery was two cycles of a combination of IFOS, CARBO, and VP-16 (ICE), followed by radiotherapy and then by another cycle of ICE chemotherapy. The patient was stable 15 months after treatment.61 Intramedullary spinal cord metastases (ISCMs) usually occur in the setting of widespread systemic metastases from lung or breast cancer, melanoma, or lymphoma.63,64 Patients are treated with corticosteroids, surgery, or radiotherapy and, less often, with chemotherapy.63 Of ISCM patients, 50% and 25% will be found to have parenchymal brain and leptomeningeal metastases, respectively.63 Table 7-3 summarizes the literature on systemic chemotherapy for intramedullary spinal cord metastases. Due to the limited spinal cord penetration of intrathecal chemotherapy, this method of delivery does not play a role in the treatment of ISCM. Because of the common coexistence of neoplastic meningitis and ISCM, however, IT chemotherapy occasionally may be indicated. Most metastatic tumors to the extramedullary spaces are treated with corticosteroids and radiotherapy to prevent spinal cord compromise and loss of ambulation. Experimental evidence from an animal model suggests that chemotherapy may have a positive role in treating human disease,65 which could be important for patients unable to receive radiotherapy. Table 7-4 lists additional reports of successful treatment with systemic therapy of spinal epidural metastases. Corticosteroids exert an antitumor activity in lymphoma and are a component of the treatment of epidural lymphoma,66,67 often in combination with radiotherapy or chemotherapy. In a retrospective review of 140 patients with intermediate-grade non-Hodgkin’s lymphoma (NHL), the seven occurrences of epidural spinal cord disease (ESCD) were treated with combined ADRIA, VCR and HD CTX, or ICE chemotherapy. After chemotherapy alone, five of the seven ESCD cases showed radiographic resolution of ESCD and their neurologic deficits improved. One patient received consolidation radiotherapy to the spine after ICE chemotherapy for relapsed ESCD and had a complete response. Another patient had progression of systemic lymphoma and ESCD despite chemotherapy. The study suggested that chemotherapy may be effective as the initial treatment of ESCD in NHL and may reduce potential complications from spinal surgery and radiotherapy.68 The combination of PRED and PCB with VCR resulted in remission of neurologic symptoms in a third NHL patient with spinal cord compromise.67 Theoretically, drugs that are typically effective for NHL should be effective for treating tumor in the epidural space. Epidural metastatic disease may respond to systemic treatment. In a study that evaluated 64 breast cancer patients with spinal epidural metastases treated with either systemic (chemo- or hormonal) therapy (11 patients) or radiotherapy with or without systemic therapy (53 patients), there was no difference in outcomes in the two groups.69 For two patients who refused radiotherapy, the combination of CTX, MTX, and 5-FU was used effectively, allowing them to maintain an ambulatory status.67 It would be expected that breast cancer patients with extramedullary spinal metastases could respond to the wide variety of antineoplastic agents shown to be effective for treating this type of tumor. Prostate cancer metastasis is the second most common cause of spinal cord compression in men, second to lung cancer metastasis. Between 1 and 12% of patients with prostate cancer develop spinal cord compression.70 This metastatic complication is usually treated with steroids and radiotherapy, but it is also responsive to orchiectomy and hormone therapy.71,72 Occasionally, patients with tumor refractory to radiotherapy respond to hormonal therapy.67 Patients without prior hormone therapy exposure have improved functional outcomes after treatment for spinal cord compression compared with patients who have undergone previous hormone therapy.70 This condition is not considered curable, and the goals of treatment in this setting are maintenance of ambulation, control of pain, and prolongation of quality of life. Neuroblastoma is a childhood embryonal tumor of migrating neuroectodermal cells, which are most likely derived from the neural crest. These cells migrate to the adrenal medulla or sympathetic nervous system plexus. Neuroblastoma is the most common malignant intraabdominal tumor in children, occurring more frequently in boys than in girls. The median age at presentation is 2 years.73 Childhood neuroblastoma with intraspinal extension resulting in epidural spinal cord compression is responsive to chemotherapy. Chemotherapy can obviate the need for laminectomy and radiotherapy. A study reported that patients treated with chemotherapy usually did not require additional therapy, whereas patients treated with either radiotherapy or laminectomy often did.74 Early reports of dramatic relief of spinal cord compression with CTX and ADRIA32 or with a combination of VCR, CDDP, teniposide (TEN), and CTX75 led to the development of clinical trials to assess chemotherapy in this setting. One prospective study assessed 42 patients with a dumbbell-shaped neuroblastoma treated with combined CARBO and VP-16, alternating with a combination of CTX, VCR, and ADRIA. Intraspinal mass size was reduced in 58% of the patients, neurologic deficits improved in 92%, and neurosurgical decompression was avoided in 60%.76 Other studies have supported these findings, although patients with long-standing severe spinal cord compression rarely recover significant neurologic function.77,78 Epidural spinal cord metastases from systemic germ cell tumors can respond to various chemotherapy regimens. Agents effective against primary intracranial germ cell tumors should also be effective in this setting. Platinum-based regimens have showed efficacy, and three patients were reported to have complete neurologic recoveries after treatment with this regimen.79 Complete neurologic recovery and resolution of spinal cord compression have also been reported with the use of combined VBL, BLEO, and CDDP.80 There are myriad rare peripheral and cranial nerve tumors. The most common include schwannomas, neurofibromas, perineuriomas, and malignant peripheral nerve sheath tumors (MPNSTs). Plexiform neurofibromas and neurofibromas of major nerves are considered precursors to most MPNSTs. Malignant peripheral nerve sheath tumors are defined as any malignant tumor arising from or differentiating toward cells of the peripheral nerve sheath. MPNSTs account for 5 to 10% of all soft tissue tumors and are often associated with neurofibromatosis type I. Radiation-induced tumors have also been reported. The usual treatment of MPNSTs is surgical resection and sometimes adjuvant irradiation. Chemotherapy is not considered effective for treating these tumors.81 A retrospective study of 134 patients with MPNSTs, 16% of whom received adjuvant chemotherapy (agents used included mitomycin [MIT], DTIC, CDDP, CTX, IFOS, ADRIA, ACT-D, BLEO, VCR, and VP-16), demonstrated no effect of chemotherapy on overall survival and no control of local or distant metastases.82 The lack of impact of adjuvant chemotherapy on overall survival is confirmed in another report of 120 patients with MPNST.83 Most reports emphasize the lack of responsiveness of these tumors to chemotherapy.84,85 Rarely, support for the use of chemotherapy in MPNST is found, as in the case of a long-term responder and survivor (10 years disease-free) who failed surgery and radiotherapy but responded to an ADRIA-based combination chemotherapy.86 Neoplastic meningitis (NM) is treated with radiotherapy and systemic or IT chemotherapy. Due to the poor prognosis of NM and the risks of treatment-related toxicity, some clinicians do not treat this condition with antineoplastic chemotherapy. The current goals of treatment are palliation and prevention of neurologic deficits. Early diagnosis and treatment, before the development of significant neurologic disabilities, may improve quality of life and prevent neurologic death from neurologic deficits. There is evidence that aggressive treatment (radiotherapy and IT chemotherapy) directed at the meningeal disease can improve survival by 3 weeks to 3 months.87 With no therapy, the median survival is 4 to 6 weeks and death most often results from progressive neurologic decline.88 Therapy for NM can provide effective local control so that patients eventually die from systemic rather than neurologic complications.88–90 Treatment of NM should include the entire neuraxis because tumor cells are disseminated throughout the CSF. Both bulky disease and malignant cells floating in the CSF must be addressed. Therefore, focal and systemic treatments are often needed. Proper management of the patient’s underlying systemic malignancy should accompany any treatments directed at the CSF. Patients whose underlying tumors are quiescent or who are known to be responsive to the available treatments will likely derive the greatest benefit from treatment. Guidelines recently published by the National Comprehensive Cancer Network suggest that risk stratification of patients as having a poor or good prognosis should determine whether aggressive treatment is instituted. This report defines poor-risk patients as those with (1) a low Karnofsky performance status (KPS); (2) multiple, serious, fixed neurologic deficits; and (3) extensive systemic disease with few treatment options. The report defines patients with a good risk as those with (1) a high KPS, (2) no fixed neurologic deficits, (3) minimal systemic disease, and (4) reasonable systemic treatment options, if needed. These guidelines suggest that supportive care and radiotherapy to symptomatic sites should be used for poor-risk patients.91 Once NM has been diagnosed, evaluation of CSF flow by injection of radioactive tracer, usually indium-111-diethylenetriamine pentaacetic acid (DTPA), is recommended to determine if there are areas of occult CSF flow obstruction or loculation because as many as 70% of patients with NM can have CSF ventricular outlet obstructions. CSF flow is usually evaluated after an intraventricular catheter has been placed. Focal radiotherapy treatment administered to obstructions of CSF flow has increased the length of survival for as long as 4 to 6 months.92,93 Involved field radiotherapy for CSF blockage allows IT drug passage opposite the obstruction. Intrathecal chemotherapy is given to treat subclinical leptomeningeal deposits and tumor cells suspended in the CSF and to prevent further seeding of the leptomeninges. The use of IT chemotherapy versus systemic chemotherapy has been debated. At least one report has suggested that IT chemotherapy increases toxicity without adding benefit to radiation and systemic chemotherapy.94 Another study showed better survival outcomes for patients treated with systemic rather than IT MTX.95 To provide patient comfort and to distribute the chemotherapeutic drugs better, most patients have a ventricular reservoir placed for IT drug delivery. Standard IT chemotherapy used in the treatment of NM includes MTX, THIO, cytarabine (ARA-C), and liposomal ARA-C. Few randomized trials have been performed to compare the efficacies of these drugs. Methotrexate is the most commonly used IT drug for NM. It is a cell cycle phase-specific antimetabolite that primarily acts during the S phase. Typical dosing schemes deliver MTX (10 to 12 mg) mixed with preservative-free normal saline IT two times a week for eight doses. If malignant cells clear from the CSF, treatment is administered once a week for four doses, then twice monthly for four doses. Monthly treatments are then administered for variable periods. Intraventricular injections result in therapeutic concentrations (>1 μM) that persist 48 hours.89,96 With the use of MTX, along with irradiation as needed, as many as 50% of patients stabilize or improve.88,89,97,98 Some patients respond to THIO, an alkylating agent.99 It is usually administered IT in 10- to 15-mg doses, a similar schedule to that used for MTX. IT THIO was prospectively compared with IT MTX in 59 NM patients with solid tumors. No difference in efficacy was noted for the two drugs, although THIO was more myelosuppressive.100 Cytarabine, a synthetic pyrimidine analogue, is relatively S phase specific. It is used most frequently for patients with meningeal leukemia or lymphoma. The CSF half-life of ARA-C is much longer than in the serum due to low levels of CSF cytidine deaminase. A new method of delivery for ARA-C was recently approved by the Food and Drug Administration for lymphomatous meningitis. The drug, marketed under the name DepoCyt®, is an injectable, sustained-release formulation of cytarabine that extends the terminal half-life of free cytarabine in the CSF from 3.4 to 140 hours. This feature allows treatments twice a month instead of twice a week.101,102 Measurable ARA-C concentrations can be maintained in the lumbar and ventricular CSF for as long as 2 weeks after one dose of DepoCyt®. A randomized trial published by Glantz and colleagues103 demonstrated the superiority of DepoCyt over standard ARA-C for lymphomatous meningitis. This small study of 28 patients illustrated several improvements of DepoCyt over standard ARA-C. Response rate, time to neurologic progression, and survival were 71% versus 53%, 78.5 versus 42 days, and 99.5 versus 63 days, respectively, for DepoCyt compared with standard ARA-C. DepoCyt was also associated with an improved mean change in KPS at the end of induction. Even though combination chemotherapy improves outcomes in many systemic cancers, this same benefit has not been demonstrated when combination IT chemotherapy has been tested for the treatment of NM. Studies that combined MTX and thiotepa (THIO); ARA-C, MTX, and THIO; and ARA-C with hydrocortisone have failed to show improved response or survival rates compared with single-drug regimens.104–106 IT MTX was compared with IT MTX plus IT ARA-C in a study of 44 patients, with no significant differences in outcomes between the groups.97 Several experimental chemotherapy agents are in various stages of investigation for IT use, including temozolomide (TMZ),107 topotecan (TOPO),108 mafos-famide,109,110 AZQ,,111,112 and 3-[(4-amino-2-methyl-5-pyrimidinyl)ethyl]-1-(2-chloroethyl)-1-nitrosourea (ACNU).113–116 Additional IT strategies that are being investigated include immunotoxins, radiolabeled monoclonal antibodies, IT radioactive (3 emitters, immunotherapy, and gene therapy. Systemic chemotherapy may have a role in the treatment of NM.117 Retrospective evidence suggests that systemic chemotherapy with or without IT chemotherapy may increase the likelihood of longer patient survival.118,119 Most studies also indicate that patients receiving IT chemotherapy fare better when they simultaneously receive systemic chemotherapy. Systemic MTX can result in cytotoxic levels (≥1 μM),120 as demonstrated in a study comparing high-dose intravenous (IV) MTX to IT MTX; the former was found to be the most effective.95 The differences in length of survival in this small study were remarkable (13.8 months versus 2.3 months, IV versus IT MTX, respectively). Other groups have suggested that the toxicity of IV systemic therapies directed toward NM is less than that associated with IT chemotherapy, and that there is no loss of efficacy.94 Evidence suggests that hormonal therapy in conjunction with IT therapy may result in better overall survival in NM.118 There are also case reports of hormonally responsive NM from breast cancer.121 Major toxicity can follow the placement of a vernacular reservoir, delivery of IT chemotherapy, or radiation to the CNS.122 Ventricular reservoirs can be positioned improperly or become infected and necessitate catheter removal. Leukoencephalopathy, manifested by dementia, seizures, progressive quadriparesis, and white matter changes, may be more likely to occur in patients receiving IT chemotherapy than in those receiving systemic chemotherapy94,123 Intrathecal MTX can cause acute arachnoiditis with nausea, vomiting, and changes in mental status. With high CSF levels, IT MTX may be associated with seizures.124 IT MTX also can cause mucositis and myelosuppression if not followed by systemic administration of folinic acid. Necrotizing leukoencephalopathy can be associated with IT MTX, especially when MTX is administered after CNS radiotherapy.125 Intrathecal THIO toxicity is similar to IT MTX toxicity but can cause a greater degree of hematologic toxicity.100 Rare case reports show IT ARA-C associated with neuropathy or myeloneuropathy.126,127 The most common adverse events that have been reported from the use of DepoCyt are headache, arachnoiditis, nausea, vomiting, and fever. Radiotherapy may worsen myelosuppression in heavily pretreated patients and can increase the likelihood of neurotoxicity from IT chemotherapy.124 Treatment of NM from solid tumors stabilizes or improves neurologic symptoms in ~45% of patients.88 Without therapy, NM patients have a poor prognosis, with survival in the range of 3 to 6 weeks. Progressive neurologic dysfunction is often the cause of death.87,88 Several factors may be predictive of longer survival or a more robust response, although there is no consensus on the implications of all factors. Table 7-5 lists patient-and treatment-related factors that can affect survival and treatment response. Glantz and colleagues128 found evidence that patients who responded to IT therapy had improvements in quality-of-life measures, providing support for the concept that effective treatment can provide meaningful benefit to some patients with NM. Table 7-6 provides the averages of the median survivals from studies in which specific primary histologies and outcomes for each group could be ascertained.
Systemic and Intrathecal Chemotherapy for Tumors of the Spine, Spinal Cord, and Peripheral Nerves
 Primary Tumors of the Bony Spine
Primary Tumors of the Bony Spine
Chordomas
Osteosarcomas (Osteogenic Sarcomas)
Chondrosarcomas
Malignant Fibrous Histiocytomas
Ewing’s Sarcomas
Langerhans Cell Histiocytosis
 Metastatic Tumors of the Bony Spine
Metastatic Tumors of the Bony Spine
Breast Carcinomas
 Intramedullary Tumors of the Spinal Cord
Intramedullary Tumors of the Spinal Cord
Primary Tumors
Astrocytomas
Oligodendrogliomas
Ependymomas
Lymphomas
Germ Cell Tumors
Primitive Neuroectodermal Tumors
Metastatic Tumors
 Extramedullary Tumors
Extramedullary Tumors
Metastatic Tumors
Lymphomas
Breast Cancer
Prostate Cancer
Neuroblastomas
Germ Cell Tumors
 Primary Tumors of Peripheral Nerves
Primary Tumors of Peripheral Nerves
Malignant Peripheral Nerve Sheath Tumors
 Metastatic Tumor to the Spinal Fluid—Neoplastic Meningitis
Metastatic Tumor to the Spinal Fluid—Neoplastic Meningitis
Intrathecal Chemotherapy
Methotrexate (MTX)
Thiotepa (THIO)
Cytarabine (ARA-C)
Combination Intrathecal Chemotherapy
Experimental Intrathecal Treatments for Neoplastic Meningitis
Systemic Chemotherapy
Hormonal Therapy
Side Effects of Therapy for Neoplastic Meningitis
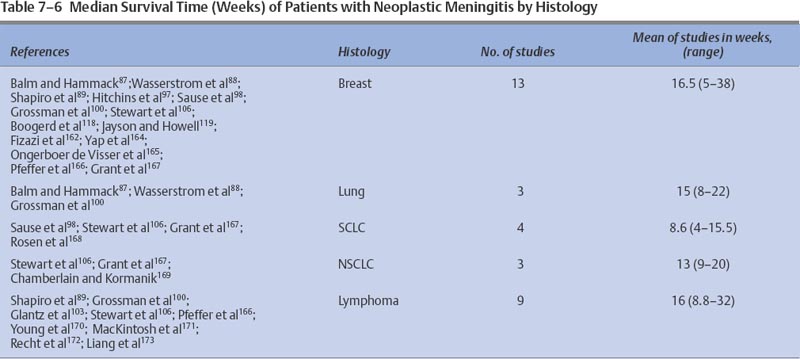
Prognosis
| Reference | Parameter | Impact on survival or response |
| Hitchins et al97; Boogerd et al118; Glantz et al128 | Clear CSF of cells with IT chemo | +,+,+ |
| Chamberlain et al92; Glantz et al93 | No CSF block or CSF block cleared | +,+ |
| Fizazi et al162 | Controlled systemic disease | + |
| Glantz et al128 | History of intraparenchymal tumor | + |
| Jayson and Howell119 | KPS ≥ 70 | + |
| Balm and Hammack87 | Longer duration of neurologic symptoms | + |
| Fizazi et al162 | Concomitant systemic + IT chemotherapy | + |
| Balm and Hammack87 | Treatment with IT chemotherapy | + |
| Balm and Hammack87 | Female sex | + |
| Glantz et al128 | Negative neuroimaging | + |
| Glantz et al128 | Longer pretreatment duration of CSF disease | + |
| Clamon and Doebbeling163 | Spinal involvement | + |
| Clamon and Doebbeling163 | Long delay from diagnosis to neurologic symptoms | + |
| Fizazi et al162 | Low CSF protein | + |
| Balm and Hammack87; Hitchins et al97; | Elevated CSF protein | +, −, 0, − |
| Boogerd et al118; Clamon and Doebbeling163 | ||
| Boogerd et al118; Clamon and Doebbeling163 | Low CSF glucose | −, 0 |
| Balm and Hammack87; Clamon and Doebbeling163 | Cerebral involvement | −, − |
| Grossman et al100 | ECOG >3 | − |
| Grossman et al100; Boogerd et al118 | Cranial nerve deficit | − |
| Grossman et al100 | Progressive systemic disease at study entry | − |
+, positive impact on survival or response; −, negative impact on survival or response; 0, no impact on survival or response; ECOG, Eastern Cooperative Oncology Group scale.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree