CHAPTER 42 Antipsychotic Drugs
HISTORY
Chlorpromazine and the Early Agents
Deniker and Jean Delay, who was the chairman of his department, experimented with chlorpromazine and found remarkable tranquilizing effects in their most agitated and psychotic patients.1,2 By 1954, Delay and Deniker had published six papers on their clinical experience with chlorpromazine. They noted in 1955 that both chlorpromazine and the dopamine-depleting agent, reserpine, shared antipsychotic efficacy and neurological side effects that resembled Parkinson’s disease. They coined the term neuroleptic to describe these effects. In 1956 Frank Ayd3,4 described acute dystonia and fatal hyperthermia with chlorpromazine. The first reports of tardive dyskinesia (TD) were published by Sigwald and colleagues in 1959.5
By 1964, several multicenter trials sponsored by the Veterans Administration and the National Institutes of Mental Health (NIMH) were completed, comparing the rapidly growing list of antipsychotic agents. These landmark studies each enrolled several hundred patients and were the first large clinical trials to be conducted in the new field of psychopharmacology. The phenothiazines were found to be highly effective and superior to placebo, barbiturates, and reserpine. With the exception of promazine and mepazine, the phenothiazines were found to be of equivalent efficacy, although they differed in their side effect profiles. In the NIMH collaborative study of over 400 acutely ill patients, 75% of patients were at least moderately improved with chlorpromazine, thioridazine, or fluphenazine, compared to only 23% with placebo.6 While reports of efficacy were impressive, the goal of identifying differences in efficacy between drugs that might allow matching of specific drugs with subgroups of patients was not realized.
The discovery by Carlsson and Lindqvist7 in 1963 that chlorpromazine increased turnover of dopamine in the brain led to the hypothesis that dopamine receptor blockade was responsible for antipsychotic effects. This was confirmed in 1976 by Creese and colleagues,8 who demonstrated that the antipsychotic potency of a wide range of agents correlated closely with affinity for the D2 receptor, which explained the equivalency of efficacy between agents since all were acting via the same mechanism. The dopamine hypothesis led to a reliance on animal models sensitive to D2 blockade as a screen for discovering potential antipsychotic drugs, with the result that new mechanisms were not intentionally explored.
Clozapine
The discovery of the first antidepressant, imipramine, led to the synthesis of related heterotricylic compounds, among which clozapine, a dibenzodiazepine derivative, was synthesized in 1958 by the Swiss company Wander. Clozapine was initially a disappointment, because it did not produce in animal models the behavioral effects associated with an antidepressant or the neurological side effects associated with an antipsychotic. Clinical trials proceeded in Europe but were halted in 1975 after reports of 17 cases of agranulocytosis in Finland, 8 of which were fatal.9,10 However, the impression among researchers that clozapine possessed unique clinical characteristics led the manufacturer, Sandoz, to sponsor a pivotal multicenter trial comparing clozapine with chlorpromazine in neuroleptic-resistant patients prospectively shown to be refractory to haloperidol. The dramatic results reported by Kane and colleagues in 198811 demonstrated the superiority of clozapine for essentially all domains of symptoms and a relative absence of neurological side effects, prompting a second revolution in the pharmacotherapy of schizophrenia.
Other Atypical Agents
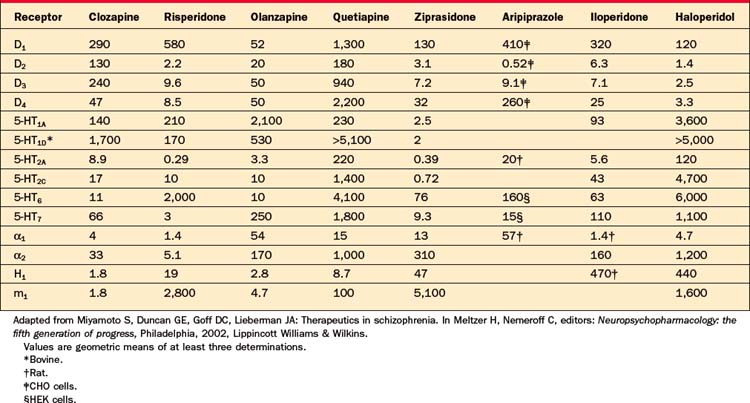
Starting in the mid-1980s, Paul Janssen and colleagues began experimenting with serotonin 5-HT2 antagonism added to D2 blockade after demonstrating that this combination reduced neurological side effects of haloperidol in rats.12 When the 5-HT2 antagonist ritanserin was added to haloperidol in schizophrenia patients, extrapyramidal symptoms (EPS) were diminished and negative symptoms improved.13 This led to development of risperidone, a D2 and 5-HT2a antagonist, the first agent designed to follow clozapine’s example as an “atypical antipsychotic” with the goal of reduced EPS and enhanced efficacy. Multicenter trials comparing multiple fixed doses of risperidone with haloperidol at a single, relatively high dose of 20 mg/day demonstrated reduced EPS, improved negative symptoms, and, in a subset of relatively resistant patients, greater antipsychotic efficacy.14,15 Olanzapine, a chemical derivative of clozapine, similarly demonstrated reduced EPS and superior efficacy compared to haloperidol. Risperidone and olanzapine rapidly replaced the first-generation of neuroleptics, particularly after clinicians became convinced that the risk of TD was substantially lower. However, it soon became apparent that risperidone markedly elevated serum prolactin levels and that olanzapine produced weight gain in some patients to a degree previously seen only with clozapine. Quetiapine, ziprasidone, and aripiprazole were subsequently approved in the United States, based largely on reduced EPS; these agents did not convincingly demonstrate superior efficacy compared to older neuroleptics. Like risperidone and olanzapine, these last three agents acted via D2 and 5-HT2a receptors; aripiprazole differed from the other second-generation antipsychotics by possessing partial agonist activity at the D2 receptor rather than full antagonism. Risperidone became the first atypical agent available as a long-acting injection in the form of “risperidone microspheres” (Tables 42-1 and 42-2).
Table 42-1 Timeline of Antipsychotics in the United States
| First-Generation Antipsychotics (11 Agents) | |
| Chlorpromazine (Thorazine) | 1954 |
| Haloperidol (Haldol) | 1967 |
| Second-Generation Antipsychotics (6 Agents) | |
| Clozapine (Clozaril) | 1989 |
| Risperidone (Risperdal) | 1994 |
| Olanzapine (Zyprexa) | 1996 |
| Quetiapine (Seroquel) | 1997 |
| Ziprasidone (Geodon) | 2001 |
| Aripiprazole (Abilify) | 2002 |
A series of case reports, pharmacoepidemiological studies, and, eventually, direct physiological investigations linked atypical antipsychotics with insulin resistance, a heightened risk for diabetes mellitus (DM), and dyslipidemia (primarily elevation of triglycerides). In response, the FDA issued a class warning for DM, although over time, the evidence most strongly implicated clozapine and olanzapine. At the same time, several meta-analyses looked at comparisons of the atypical antipsychotics with conventional neuroleptics and reached divergent conclusions as to whether the apparent advantages of the newer agents were largely the result of comparisons with excessively high doses of haloperidol or other comparators.16,17 The most comprehensive meta-analysis, published by Davis and colleagues in 2003,17 found a substantial therapeutic benefit of clozapine (effect size of 0.5) compared to conventional agents and an intermediate advantage for olanzapine and risperidone (effect size of 0.25); the therapeutic advantages of these agents were statistically significant and did not appear to depend on the dose of the comparator. In contrast, quetiapine, ziprasidone, and aripiprazole were not found to differ from conventional antipsychotics in efficacy.
The CATIE Study
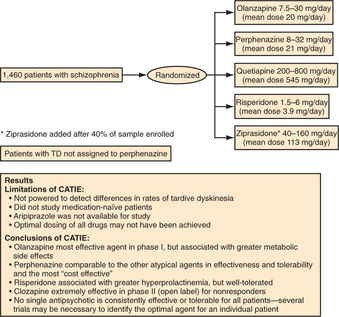
In 1999, the NIMH, recognizing that almost all information regarding the new antipsychotics had come from industry-supported efficacy trials of uncertain generalizability, awarded a competitive contract to Dr. Jeffrey Lieberman to conduct a large, multicenter trial to assess the effectiveness and tolerability of these agents under more representative treatment conditions. Results of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study were first published in 2005 (Figure 42-1).18 The study was conducted at 57 diverse sites across the United States and included 1,493 representative patients with schizophrenia who were randomly assigned to risperidone, olanzapine, quetiapine, ziprasidone, or the conventional comparator, perphenazine, for an 18-month double-blind trial. Dosing was flexible; the range of doses for each drug was selected based on patterns of clinical use (in consultation with the manufacturers). Ziprasidone became available and was added after the study was roughly 40% completed; aripiprazole was not available and was not included. Patients with TD at study entry were not randomized to perphenazine, and their data were excluded from analyses comparing the atypical agents with perphenazine. Patients who failed treatment with their first assigned agent could be randomized again in subsequent phases; one rerandomization pathway featured open-label clozapine for treatment-resistant patients and one pathway featured ziprasidone for treatment-intolerant patients. The secondary randomization pathway did not include perphenazine.
CURRENTLY AVAILABLE ANTIPSYCHOTIC AGENTS
All marketed antipsychotic agents share the common property of dopamine (D2) receptor blockade. The 11 agents approved in the United States between 1953 (chlorpromazine) and 1967 (haloperidol) are commonly referred to as conventional neuroleptics or first-generation antipsychotics (Table 42-3). As a group they possess equivalent efficacy, but differ in their potency and side effects. Reflecting the common property of D2 blockade, these agents all potentially produce EPS, TD, and hyperprolactinemia. Clozapine was the first of the second-generation antipsychotics, also known as atypical antipsychotics. Currently there are six atypical antipsychotics approved in the United States; these agents generally produce fewer EPS, less elevation of prolactin, and less risk of TD than first-generation antipsychotics. Clozapine has clearly demonstrated greater efficacy than the first-generation agents. Of the other second-generation antipsychotics, only olanzapine was found to be more effective than the first-generation comparator in the CATIE study. Risperidone is unique among the atypical agents in producing marked prolactin elevation. Aripiprazole is the only antipsychotic with partial agonist rather than antagonist activity at the D2 receptor; this property is believed to limit neurological side effects. Because aripiprazole was not included in the CATIE study, it is unclear if D2 partial agonism has implications for effectiveness as well. The one characteristic that has most reliably differentiated the atypical agents from the (generic) first-generation agents has been cost.
Table 42-3 First-Generation Antipsychotic Doses
| Equivalent Dose (mg)* | Typical Dose (mg/day) | |
|---|---|---|
| Low Potency | ||
| Chlorpromazine | 100 | 300–800 |
| Thioridazine | 90 | 200–600 |
| Mesoridazine | 50 | 75–300 |
| Medium Potency | ||
| Loxapine | 15 | 60–100 |
| Molindone | 10 | 50–225 |
| Perphenazine | 10 | 8–32 |
| High Potency | ||
| Trifluoperazine | 5 | 6–20 |
| Thiothixene | 5 | 6–30 |
| Fluphenazine | 2 | 5–20 |
| Haloperidol | 2 | 5–20 |
| Pimozide | 1 | 2–10 |
* Equivalent dose (or “chlorpromazine equivalents”) reflects the potency of antipsychotics compared to 100 mg of chlorpromazine as the reference.
Adapted from Baldessarini RJ: Chemotherapy in psychiatry, Boston, 1985, Harvard University Press
GENERAL CLINICAL CONSIDERATIONS
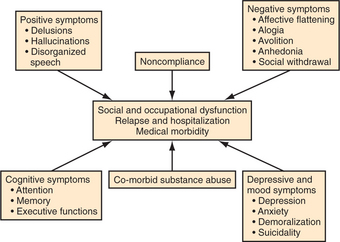
The primary target symptoms for antipsychotic agents fall into three categories: psychotic symptoms (e.g., hallucinations, delusions, and disorganization); agitation (e.g., distractibility, affective lability, tension, and increased motor activity); and negative symptoms (e.g., apathy, diminished affect, social withdrawal, and poverty of speech). Although cognitive deficits are an important contributor to disability in schizophrenia, cognitive deficits usually are not considered a target for antipsychotic agents, in part because they are not very responsive to current agents. Agitation responds to most antipsychotics rapidly and often fully, whereas response of psychotic symptoms is quite variable and negative symptoms rarely exhibit more than modest improvement19 (Figure 42-2).
The time course of antipsychotic response also can be quite variable. In the 1970s, the treatment of psychosis with relatively large doses of intramuscular (IM) haloperidol, known as “rapid neuroleptization,” was advocated, based on the clinical impression that agitation and psychosis responded quickly to this aggressive treatment approach.20 For the next two decades, the consensus shifted to the view that psychotic symptoms require weeks of antipsychotic treatment before responding. Recently, Kapur and colleagues21 analyzed data from studies of IM administration of atypical antipsychotics and documented antipsychotic effects within hours of administration, independent of tranquilization effects. Most likely, tranquilization, or the improvement of agitation and irritability, occurs rapidly with most agents whereas psychotic symptoms may begin to improve quickly in some patients and only after a delay of several weeks in others. Maximal response to antipsychotic treatment can require months. The pharmaco logical treatment of psychotic symptoms is similar to the treatment of infection with antibiotics—the clinician needs to choose a proper dose and then await therapeutic results while monitoring side effects.
Negative symptoms have been found to respond to both conventional and atypical antipsychotics, although a full resolution of negative symptoms is rare.19 It remains controversial whether “primary” negative symptoms improve with pharmacotherapy. Examples of “secondary” negative symptoms include social withdrawal resulting from paranoia, and apathy resulting from depression. The most common etiology of secondary negative symptoms is neuroleptic-induced parkinsonism; the improvement of negative symptoms following a switch to an atypical agent sometimes represents resolution of iatrogenic parkinsonian side effects caused by the previous conventional neuroleptic.
DRUG SELECTION
Selection of an antipsychotic agent usually is guided by the side-effect profile and by available formulations (e.g., tablet, rapidly dissolving wafer, liquid, IM immediate-release or depot preparations). The “atypical” antipsychotics have largely supplanted conventional agents because of their reduced burden of neurological side effects. The potential therapeutic benefits of clozapine are quite broad, including greater efficacy for psychosis, negative symptoms, agitation or tension, suicidal ideation, and relapse.11,22,23 Risperidone and olanzapine were found to offer potential advantages compared to the older conventional agents in early studies, including better efficacy in some patients for psychosis, negative symptoms, and relapse prevention,24–27 although only olanzapine differed in effectiveness compared to the conventional agent, perphenazine, in the CATIE study. Both risperidone and olanzapine fall short of clozapine in overall efficacy. Quetiapine, ziprasidone, and aripiprazole have demonstrated favorable side-effect profiles in premarketing trials and efficacy at least comparable to that of the conventional agents. Whereas it was widely held that the atypical agents as a group are more effective for negative symptoms, in part because of reduced parkinsonian side effects, this has not been found consistently in clinical trials, particularly with low or moderate dosing of the conventional agent. Claims for enhanced efficacy for cognitive deficits with atypical agents have also been challenged recently by the CATIE trial results. Because of the considerable heterogeneity in response, it is not possible to predict the optimal agent for an individual patient; sequential trials may be needed.
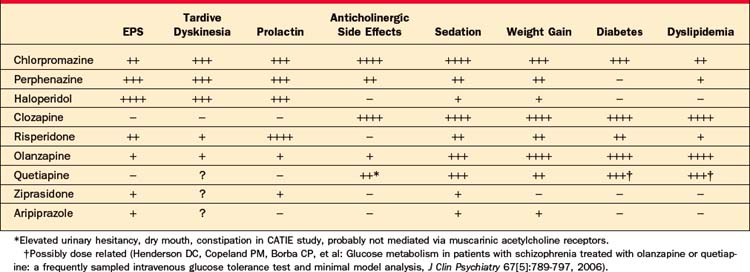
Whereas the relative effectiveness of specific atypical agents cannot be predicted in an individual patient, side effects are more predictable and differ considerably be-tween agents. As listed in Table 42-4, side effects likely to influence tolerability and compliance include sedation or activation, weight gain, neurological side effects, and sexual dysfunction.
CONVENTIONAL ANTIPSYCHOTICS (NEUROLEPTICS)
Examining representative first-generation antipsychotics, it has been found that roughly 65% occupancy of striatal D2 receptors is necessary for antipsychotic efficacy, whereas neurological side effects emerge when D2 occupancy levels exceed approximately 80%.28 Among the conventional antipsychotics, low-potency agents (such as chlorpromazine and thioridazine) have relatively low affinity for the D2 receptor and hence require higher doses (roughly 50-fold greater than haloperidol). In addition, they are less selective for D2 receptors and so are associated with a wider range of side effects, including orthostatic hypotension, anticholinergic side effects, sedation, and weight gain (Table 42-5), and are less readily or safely administered parenterally.29 Thioridazine additionally produces substantial QT-interval prolongation, which may be dangerous in patients with cardiac conduction defects.30 Perphenazine, which was the conventional antipsychotic selected to represent this class in the CATIE study, is a midpotency agent that requires doses roughly threefold greater than are necessary with haloperidol. High-potency conventional agents (such as haloperidol and fluphenazine) are more selective for D2 receptors and more likely to produce EPS, such as acute dystonias, parkinsonism, and akathisia. Because affinity for the D2 receptor is readily measured and is inversely correlated with the typical therapeutic dose for each compound, conversion ratios to calculate equivalent dosing between conventional agents, typically expressed in “chlorpromazine equivalents,” can be calculated (see Table 42-3).
Table 42-5 Side Effects Associated with Low-Potency First-Generation Antipsychotics
Haloperidol is available for parenteral (including intravenous [IV]) administration, and both haloperidol and fluphenazine are available in long-acting injectable depot preparations. In the setting of serious medical illness, particularly if other medications with anticholinergic or hypotensive side effects are administered, haloperidol is often used. Although considerable interindividual variability exists, daily oral doses of haloperidol between 5 and 15 mg are adequate for the large majority of patients; increasing the dose further may only aggravate side effects without improving antipsychotic efficacy. IM and IV administration require roughly half the dose. Great care should be taken with the elderly, in whom 0.5 to 2 mg of haloperidol at bedtime may often be sufficient. If a patient has not previously received antipsychotic medication, it is best to start at a low dose (haloperidol 2 to 5 mg orally) before arriving at a standard therapeutic dose. A large number of studies have indicated that an optimal response with haloperidol generally corresponds with trough serum concentrations between 5 and 15 ng/ml,31 although clinical titration remains the most reliable approach in most situations. The impressive performance of perphenazine at a mean dose of 20 mg/day in the CATIE trial makes it a reasonable option, although, as a first-generation antipsychotic, its potential liability for TD remains a concern. Perphenazine was relatively well tolerated in the CATIE study, although patients dropped out due to EPS at rates two to four times higher than with the atypical agents. The sample size and duration of drug exposure in CATIE were insufficient to compare the risks of TD among antipsychotics.
EXTRAPYRAMIDAL SYMPTOMS AND TARDIVE DYSKINESIA
Dystonias are sustained spasms that can affect any muscle group. Neuroleptic-induced dystonias usually occur within the first 4 days of initiating neuroleptic treatment, or after a dose increase, and often affect the neck, tongue, or back. Dystonia may also manifest as lateral deviation of the eyes (opisthonus) or stridor (laryngeal spasm). Younger patients started on high-potency conventional antipsychotic medication are especially at risk for developing acute dystonic reactions during the first week of exposure to antipsychotic medication.32 The incidence of dystonia decreases by about 4% per year of age until it is almost negligible after age 40.32 Dystonia is a frightening and uncomfortable experience. The occurrence of dystonia early in treatment seriously jeopardizes future compliance with antipsychotic medication, so it is important to anticipate and treat this side effect aggressively. The best method of prevention comes from use of an atypical antipsychotic. When high-potency neuroleptics are started, prophylaxis with anticholinergic agents (such as benztropine 1 to 2 mg twice daily) substantially reduces the likelihood of dystonic reactions in high-risk patients.33 Prophylaxis with anticholinergics is risky in the elderly because of their sensitivity to adverse effects, but is usually unnecessary since the elderly are at very low risk for dystonia. Dystonia is uncommon with atypical agents and probably does not occur with quetiapine or clozapine.
Akathisia is an extremely unpleasant sensation of motor restlessness that is primarily experienced in the lower extremities in patients receiving high-potency conventional neuroleptics.34 Akathisia usually manifests as pacing, although some patients experience akathisia as leg discomfort rather than restlessness. Akathisia increases noncompliance and has been associated with self-injurious behaviors, as well as a worsening of psychosis. Untrained staff frequently mistake akathisia for psychotic agitation, resulting in an unfortunate escalation of the antipsychotic dose. Like other forms of EPS, the most effective remedy is the use of an atypical agent. Because akathisia is dose dependent, lowering the dose may provide relief. Alternatively, propranolol 10 to 20 mg two to four times daily is often helpful.35 A switch to an atypical antipsychotic usually resolves the problem.
Antipsychotic-induced parkinsonism mimics the tremor, stiffness, gait disturbance, and diminished facial expression characteristic of idiopathic Parkinson’s disease. It easily can be mistaken for depression or for negative symptoms of schizophrenia,36 although the presence of tremor and rigidity usually distinguishes this side effect. Parkinsonian side effects are most common with high-potency neuroleptics and occur in a bimodal age distribution with maximal risk in the young and in the elderly. Symptoms frequently improve with a reduction in the dose of neuroleptic or with addition of an antiparkinsonian agent (such as benztropine 1 to 2 mg twice daily or amantadine 100 mg twice or three times daily).37 Because anticholinergic agents can produce an array of troublesome side effects (including constipation, dry mouth, dental caries, blurred vision, urinary retention, and memory impairment), particularly in the elderly, long-term use of these agents should be avoided. The atypical agents as a class produce substantially fewer parkinsonian side effects, and both clozapine and quetiapine are essentially free of EPS, making them the drugs of choice for patients with idiopathic Parkinson’s disease complicated by psychosis.38 Table 42-6 describes EPS.
Table 42-6 Extrapyramidal Symptoms
| Dystonia |
| Acute muscle spasm: can be very distressing Usually occurs within the first 4 days after starting the drug Risk factors: youth and use of high-potency first-generation antipsychotics |
| Parkinsonism |
| Tremor, bradykinesia, rigidity—often confused with negative symptoms Risk factors: use of high-potency first-generation antipsychotics, high doses, youth, and old age |
| Akathisia |
| Restlessness in lower extremities; often results in pacing Risk factors: use of high-potency first-generation agents in high doses |
TD rarely appears after less than 6 months of treatment with an antipsychotic agent, but once present it may be irreversible.39 TD usually takes the form of involuntary, choreiform (quick, nonrhythmic) movements of the mouth, tongue, or upper extremities, although a dystonic form has also been described.40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree