CHAPTER 49 Pharmacotherapy of Attention-Deficit/Hyperactivity Disorder across the Life Span
OVERVIEW
Attention-deficit/hyperactivity disorder (ADHD) is a common psychiatric condition shown to occur in 3% to 10% of school-age children worldwide and up to 4% of adults.1–5 The classic triad of impaired attention, impulsivity, and excessive motor activity characterizes ADHD, although many patients may manifest only inattentive symptoms.6 ADHD usually persists, to a significant degree, from childhood through adolescence and into adulthood.7–9 Most children, adolescents, and adults with ADHD suffer significant functional impairment(s) in multiple domains,10 as well as co-morbid psychiatric or learning disorders.5,11–18
Studies demonstrate that ADHD is frequently co-morbid with oppositional defiant disorder (ODD), conduct disorder (CD), multiple anxiety disorders (panic disorder, obsessive-compulsive disorder [OCD], tic disorders), mood disorders (e.g., depression, dysthymia, and bipolar disorder [BPD]), learning disorders (e.g., auditory processing problems and dyslexia), and substance use disorders (SUDs) and often complicates the development of patients with pervasive developmental disorders (PDDs).4,5,11,14,16–19 Co-morbid psychiatric, learning, and developmental disorders need to be assessed in all patients with ADHD and the relationship of these symptoms with ADHD delineated.20–22
Before the use of medications clinicians should complete a through clinical evaluation that includes a complete history of symptoms, a differential diagnosis, a review of prior assessments/treatments, a medical history, and a description of current physical symptoms (including questions about the physical history, including either a personal or family history of cardiovascular symptoms or problems). Before treatment with medications, it is usually important to measure baseline levels of height, weight, blood pressure, and pulse and to monitor them over the course of treatment (see www.fda.gov/cder/drug/infopage/ADHD/default.htm for the most recent recommendations by the Food and Drug Administration [FDA]). Clinicians and patients/families should select an initial treatment, usually either a stimulant or atomoxetine (ATMX); decide on a target dose (either absolute or weight-based) titration schedule; and decide how to monitor tolerability and response to treatment (rating scales, anchor points, or both).20,21 Patients should be educated about the importance of adherence, safely maintaining medications (e.g., as in college students), and additional types of treatment (e.g., coaching and organizational help) that may be helpful.
STIMULANTS
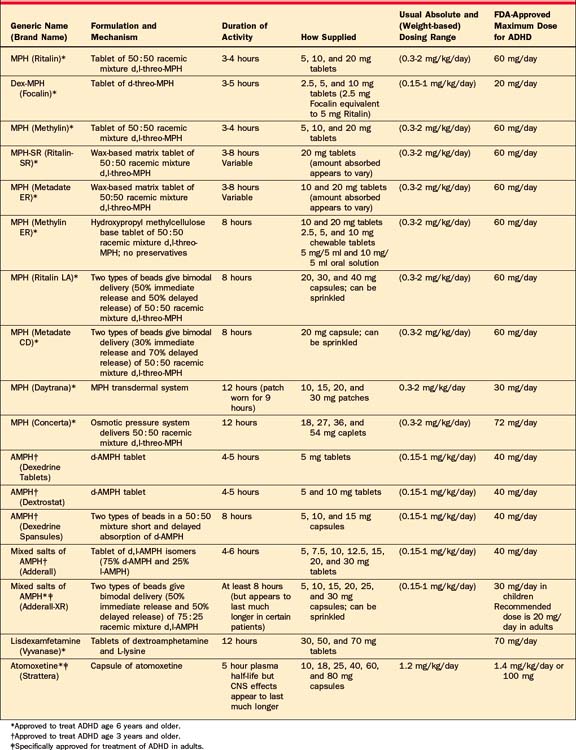
For over 60 years stimulants have been used safely and effectively in the treatment of ADHD,23 and they are among the most well-established treatments in psychiatry.19,20,24 The stimulant most commonly used is methylphenidate (MPH), a mixture of amphetamine salts (MAS) and dextroamphetamine (DEX). The recent development of various novel medication delivery systems has significantly advanced the pharmacotherapy of ADHD (see Table 49-1 for a list of these medications).
Pharmacodynamic Properties of Stimulants
Stimulants increase intrasynaptic concentrations of dopamine (DA) and norepinephrine (NE).25–29 MPH primarily binds to the DA transporter protein (DAT), blocking the reuptake of DA, increasing intrasynaptic DA.26,29 While amphetamines diminish presynaptic reuptake of DA by binding to DAT, these compounds also travel into the DA neuron, promoting release of DA from reserpine-sensitive vesicles in the presynaptic neuron.26,27 In addition, stimulants (amphetamine > MPH) increase levels of NE and serotonin (5-HT) in the interneuronal space.25 Although group studies comparing MPH and amphetamines generally demonstrate similar efficacy,20 their pharmacodynamic differences may explain why a particular patient may respond to, or tolerate, one stimulant preferentially over another. It is necessary to appreciate that while the efficacy of amphetamine and MPH is similar, their potency differs, such that 5 mg of amphetamine is approximately equally potent to 10 mg of MPH.
Methylphenidate (MPH)
As originally formulated, MPH was produced as an equal mixture of d,l-threo-MPH and d,l-erythro-MPH. The ery-thro isomers of MPH appear to produce side effects, and thus MPH is now manufactured as an equal racemic mixture of d,l-threo-MPH.30 Behavioral effects of immediate-release MPH peak 1 to 2 hours after administration, and tend to dissipate within 3 to 5 hours. MPH is primarily metabolized via plasma-based esterases. While generic MPH has a similar pharmacokinetic profile to Ritalin, it is more rapidly absorbed and peaks sooner.31 Due to its wax-matrix preparation, the absorption of the sustained-release MPH preparation (Ritalin-SR) is variable,32 with peak MPH plasma levels in 1 to 4 hours, a half-life of 2 to 6 hours,31,33 and behavioral effects that may last up to 8 hours.31,33 The availability of the various new extended-delivery stimulant formulations has greatly curtailed use of MPH-SR.
Concerta (OROS-MPH) uses the Osmotic Releasing Oral System (OROS) technology to deliver a 50:50 racemic mixture of d,l-threo-MPH.34 OROS-MPH, indicated for the treatment of ADHD in children and adolescents, is available in 18, 27, 36, and 54 mg doses and is indicated in doses up to 72 mg daily. The 18 mg caplet of OROS-MPH provides an initial bolus of 4 mg of MPH, delivering the remaining MPH in an ascending pattern, such that peak concentrations are generally reached around 8 hours after dosing; it is labeled for 12 hours of coverage.30,35 A single morning dose of 18, 27, 36, or 54 mg of OROS-MPH is approximately bioequivalent to 5, 7.5, 10, or 15 mg of immediate-release MPH adminis-tered three times daily, respectively. The effectiveness and tolerability of OROS-MPH have been demonstrated in children,36–38 adolescents,39 and adults40 with ADHD. Recent data support OROS-MPH’s continued efficacy in many ADHD subjects over the course of 24 months of treatment.41
Metadate CD (MPH MR), the first available extended-delivery stimulant preparation to employ beaded technology, is available in capsules of 10, 20, and 30 mg, which may be sprinkled. Using Eurand’s Diffucaps technology, MPH MR contains two types of coated beads, IR-MPH and extended-release-MPH (ER-MPH). Metadate delivers 30% of d,l-threo-MPH initially, and 70% of d,l-threo-MPH several hours later.30 MPH MR is designed to simulate twice-daily (bid) dosing of IR MPH providing approximately 8 hours of coverage. The efficacy of MPH MR capsules has been demonstrated,42 and it is approved for treatment in youth with ADHD in doses of up to 60 mg/day.30 An extended delivery tablet form of Metadate (Metadate ER) is also available in doses of 10 and 20 mg.
Ritalin-LA (MPH-ERC), another beaded-stimulant preparation, is available in capsules of 10, 20, 30, and 40 mg, which may be sprinkled.30 MPH-ERC uses the beaded Spheroidal Oral Drug Absorption System (SODAS) technology to achieve a bimodal release profile that delivers 50% of its d,l-threo-MPH initially and another bolus approximately 3 to 4 hours later, providing around 8 hours of coverage. The efficacy of MPH-ERC has been demonstrated in youth with ADHD.43
The primarily active form of MPH appears to be the d-threo isomer,44–46 which is now available in both immediate-release tablets (Focalin 2.5, 5, and 10 mg) and, employing the SODAS technology, extended-delivery capsules (Focalin XR 5, 10, 15, and 20 mg). The efficacy of D-MPH is well established in children, adolescents, and adults under open- and double-blind conditions.47–50 D-MPH is approved to treat ADHD in children, adolescents, and adults in doses of up to 20 mg per day and has been labeled to provide a 12-hour duration of coverage.30 Although not definitive, 10 mg of MPH appears to be approximately equivalent to 5 mg of d-MPH, and clinicians can reasonably use this estimate in clinical practice.51
The MPH transdermal system (MTS; Daytrana) delivers MPH through the skin via the DOT Matrix transdermal system. The patches are applied once daily and intended to be worn for 9 hours, although in clinical practice they can be worn for shorter and longer periods of time. The MTS usually takes effect within 2 hours and provides coverage for 3 hours after removal. MTS is available in 10, 15, 20, and 30 mg patches.52–54 Since the MPH is absorbed through the skin, it does not undergo first-pass metabolism in the liver; therefore, patients require lower doses with the patch compared to oral preparations (10 mg of MTS = 15 mg of extended-release oral MPH). MTS may be a particularly useful treatment option for patients who have difficulty swallowing or tolerating oral stimulant formulations or for patients who need flexibility in the duration of medication effect.
Amphetamines
Amphetamine is available in two forms, dextroamphetamine (DEX; Dexedrine) and mixed amphetamine salts (MAS; Adderall). DEX tablets achieve peak plasma levels 2 to 3 hours after oral administration, and have a half-life of 4 to 6 hours. Behavioral effects of DEX tablets peak 1 to 2 hours after administration, and last 4 to 5 hours. For DEX spansules, these values are somewhat longer. MAS consist of equal portions of d-amphetamine saccharate, d,l-amphetamine asparate, d-amphetamine sulfate, and d,l-amphetamine sulfate, and a single dose results in a ratio of approximately 3:1 d- to l-amphetamine.30 The two isomers have different pharmacodynamic properties, and some patients with ADHD may preferentially respond to one isomer over another. The efficacy of MAS tablets is well established in ADHD youth55 and adults.56 The extended-delivery preparation of MAS is a capsule containing two types of Micotrol beads (MAS XR; Adderall XR). The beads are present in a 50:50 ratio, with immediate-release beads designed to release MAS in a fashion similar to MAS tablets, and delayed-release beads designed to release MAS 4 hours after dosing.30 The efficacy of MAS XR is well established in children,57,58 adolescents,59 and adults.60,61 Furthermore, open treatment with MAS XR appears to be effective in the treatment of many ADHD youths over a 24-month period.62
Lisdexamfetamine dimesylate (LDX; Vyvanase), previously known as NRP-104, recently received approval from the FDA for treatment of ADHD in 6- to 12-year-old children. Lisdexamfetamine63 is an amphetamine pro-drug in which L-lysine, a naturally occurring amino acid, is covalently linked to d-amphetamine. After oral administration, the pro-drug is metabolically hydrolyzed in the body to release d-amphetamine. Although Lisdexamfetamine appears to have reduced abuse liability and overdose protection, the FDA has recommended a CII schedule. It is available in doses of 30, 50, and 70 mg that appear to be comparable to MAS XR doses of 10, 20, and 30 mg, respectively.
Clinical Use of Stimulants
Guidelines regarding the use of stimulant medications in children, adolescents, and adults in clinical practice have been published.20 Treatment with immediate-release preparations generally starts at 5 mg of MPH or amphetamine once daily and is titrated upward every 3 to 5 days until an effect is noted or adverse effects emerge. Typically, the half-life of the short-acting stimulants necessitates at least twice-daily dosing, with the addition of similar or reduced afternoon doses dependent on breakthrough symptoms. In a typical adult, dosing of immediate-release MPH is generally up to 30 mg three to four times daily or amphetamine 15 to 20 mg three to four times a day. Currently, most adults with ADHD will be treated with a stimulant that has an extended delivery.20
Side Effects of Stimulants
Although generally well tolerated, stimulants can cause clinically significant side effects (including anorexia, nausea, difficulty falling asleep, obsessiveness, headaches, dry mouth, rebound phenomena, anxiety, nightmares, dizziness, irritability, dysphoria, and weight loss).20,64,65 Rates and types of stimulant side effects appear to be similar in ADHD patients, regardless of age. In patients with a current co-morbid mood/anxiety disorder, clinicians should consider whether an adverse effect reflects the co-morbid disorder, a side effect of the treatment, or an exacerbation of the co-morbidity. Moreover, while stimulants can cause these side effects, many ADHD patients experience these problems before treatment; therefore, it is important for clinicians to document these symptoms at baseline.64 Recommendations about management of common side effects are listed in Table 49-2.
Table 49-2 Strategies in Challenging ADHD Cases
| Symptoms | Interventions |
|---|---|
| Worsening or unchanged ADHD symptoms (inattention, impulsivity, hyperactivity) | Change medication dose (increase or decrease) |
| Change timing of dose | |
| Change preparation, substitute stimulant | |
| Evaluate for possible tolerance | |
| Consider adjunctive treatment (antidepressant, alpha-adrenergic agent, cognitive enhancer) | |
| Consider adjusting nonpharmacological treatment (cognitive-behavioral therapies or coaching or reevaluating neuropsychological profile for executive function capacities) | |
| Intolerable side effects | Evaluate if side effect is drug-induced |
| Assess medication response versus tolerability of side effect | |
| Aggressive management of side effect (change timing of dose; change preparation of stimulant; adjunctive or alternative treatment) | |
| Symptoms of rebound | Change timing of dose |
| Supplement with small dose of short-acting stimulant or alpha-adrenergic agent 1 hour before symptom onset | |
| Change preparation | |
| Increase frequency of dosage | |
| Development of tics or Tourette’s Syndrome (TS) or use with co-morbid tics or TS | Assess persistence of tics or TS |
| If tics abate, rechallenge | |
| If tics are clearly worsened with stimulant treatment, discontinue | |
| Consider stimulant use with adjunctive anti-tic treatment (haldol, pimozide) or use of alternative treatment (antidepressants, alpha-adrenergic agents) | |
| Emergence of dysphoria, irritability, acceleration, agitation | Assess for toxicity or rebound |
| Evaluate development or exacerbation of co-morbidity (mood, anxiety, and substance use | |
| [including nicotine and caffeine]) | |
| Reduce dose | |
| Change stimulant preparation | |
| Assess sleep and mood | |
| Consider alternative treatment | |
| Emergence of major depression, mood lability, or marked anxiety symptoms | Assess for toxicity or rebound |
| Evaluate development or exacerbation of co-morbidity | |
| Reduce or discontinue stimulant | |
| Consider use of antidepressant or antimanic agent | |
| Assess substance use | |
| Consider nonpharmacological interventions | |
| Emergence of psychosis or mania | Discontinue stimulant |
| Assess co-morbidity | |
| Assess substance use | |
| Treat psychosis or mania |
Growth
The impact of stimulant treatment on growth remains a concern, and the data are conflicting. For instance, in the MTA study, ADHD youth, treated with a stimulant medication continuously over a 24-month period, experienced a deceleration of about 1 cm per year. Despite this slowing, except for those subjects in the lowest percentile for height, these children remained within the normal curves. Recently, Biederman and colleagues reported on growth deficit in girls with ADHD. Although statistically significant differences were observed between ADHD girls and controls, these deficits were modest, only evident in early adolescence, unrelated to weight deficits or stimulant treatment, and not significant after correcting for age and parental height. The finding of small height differences in preadolescent girls is consistent with Spencer and associates’ earlier work in boys,66 and results from additional long-term studies.41,67 Although stimulants may have a mild negative impact on growth velocity, perhaps more related to ADHD than its treatment,66 height and weight should be monitored but will not pose significant clinical problems for most patients.
Sleep
Parents often report sleep disturbances in their children with ADHD before68–71 and during treatment.72,73 Various strategies (including improving sleep hygiene, making behavioral modifications, adjusting timing or type of stimulant, and switching to an alternative ADHD treatment) have been suggested to help make it easier for patients with ADHD to fall asleep.74 Complementary pharmacological treatments to consider include the following: melatonin (1 to 3 mg), clonidine (0.1 to 0.3 mg), diphenhydramine (25 to 50 mg), trazodone (25 to 50 mg), and mirtazapine (3.75 to 15 mg).20,75 Recently, interest in the use of melatonin, a hormone secreted by the pineal gland that helps regulate circadian rhythms,76 to address sleep problems in children has been growing.77 Melatonin used alone78 and in conjunction with sleep hygiene techniques79 appears to improve sleep in youths with ADHD. In these two well-designed but small studies, the most concerning adverse events included migraine (n = 1), nightmares (n = 1), and aggression (n = 1). Although not yet studied, another consideration is ramelteon, a synthetic melatonin receptor agonist.80
Appetite Suppression
Patients treated with stimulants often experience a dose-related reduction in appetite, and in some cases weight loss.81 Although appetite suppression has been observed to decrease over time,41 clinicians should give guidance on improving the patient’s nutritional options with higher caloric intake to balance the consequences of decreased food intake.20 While appetite suppression is a common side effect of stimulants, little research has been done studying remedies. Cyproheptadine, in doses of 4 to 8 mg, has recently been reported to improve appetite in ADHD patients with stimulant-associated appetite suppression.82
Medication Interactions with Stimulants
The interactions of stimulants with other prescription and nonprescription medications are generally mild and not a major source of concern.83,84 Concomitant use of sympathomimetic agents (e.g., pseudoephedrine) may potentiate the effects of both medications. Likewise, excessive intake of caffeine may potentially compromise the effectiveness of the stimulants and exacerbate sleep difficulties. Although data on the co-administration of stimulants with tricyclic antidepressants (TCAs) suggest little interaction between these compounds,85 careful monitoring is warranted when prescribing stimulants with either TCAs or anticonvulsants. Although administering stimulants with ATMX is common clinical practice, and appears to be safe, well tolerated, and effective based on clinical experience, to date only small samples have been studied; therefore, patients taking this combination should be monitored closely.86 In fact, co-administration of stimulants with MAOIs is the only true contraindication.
Despite the increasing use of stimulants for patients with ADHD, many of them may not respond, experience untoward side effects, or manifest co-morbidity, which stimulants may exacerbate or be ineffective in treating.18,87,88 Over the last 10 years ATMX has been systematically evaluated and is FDA-approved for the treatment of ADHD in children, adolescents, and adults.30
ATOMOXETINE
Unlike the stimulants, atomoxetine (ATMX; Strattera) is unscheduled; therefore, clinicians can prescribe refills. ATMX acts by blocking the NE reuptake pump on the presynaptic membrane, thus increasing the availability of intrasynaptic NE, with little affinity for other monoamine transporters or neurotransmitter receptors.30
ATMX should be initiated at 0.5 mg/kg/day and after a few days increased to a target dose of 1.2 mg/kg/day. Although ATMX has been studied in doses of up to 2 mg/kg/day, current dosing guidelines recommend a maximum dosage of 1.4 mg/kg/day. Although some patients have an early response, it may take up to 10 weeks to see the full benefits of ATMX treatment.89–91 In the initial trials, ATMX was dosed bid (typ ically after breakfast and after dinner); however, recent studies have demonstrated its efficacy and tolerability in many patients dosed once a day.92–96 Although the effects of ATMX dosed once daily in the morning or at bedtime appear to be similar (with a mean dose of 1.25 mg/kg/morning or 1.26 mg/kg/night), once-daily ATMX appears to be best tolerated when dosed in the evening,94 although for many patients ATMX dosed once daily is effective.93 To date, plasma levels of ATMX have not been used to guide dosing. However, Dunn and colleagues97 found that patients with a plasma level of ATMX greater than 800 ng/ml had more robust responses, although patients treated with higher doses also experienced more side effects.
Although generally well tolerated, the most common side effects in children and adolescents taking ATMX include reduced appetite, dyspepsia, and dizziness,30 although height and weight in long-term use appear to be on target.90 In adults, ATMX treatment may be associated with dry mouth, insomnia, nausea, decreased appetite, constipation, decreased libido, dizziness, and sweating.98 Furthermore, some men taking ATMX may have difficulty attaining or maintaining erections. Several easy strategies can be used to manage ATMX’s side effects. When patients experience nausea, the dose of ATMX should be divided and administered with food. Sedation is often transient, but may be helped by either administering the dose at night or by dividing the dose. If mood swings occur, patients should be evaluated and their diagnosis reassessed.
Similarly, the impact of ATMX on the cardiovascular system appears to be minimal.99 ATMX was associated with mean increases in heart rate of 6 beats per minute, and increases in systolic and diastolic blood pressure of 1.5 mm Hg. Extensive electrocardiogram (ECG) monitoring indicates that ATMX has no apparent effect on QTc intervals, and ECG monitoring outside of routine medical care does not appear to be necessary.
Recently, concerns have been raised that treatment with ATMX may increase the risk of hepatitis. During postmarketing surveillance, two patients (out of 3 million exposures) developed hepatitis during treatment with ATMX.100 Patients and families should contact their doctors if they develop pruritus, jaundice, dark urine, right upper quadrant tenderness, or unexplained “flu-like” symptoms.30
Recently, the FDA issued a public health advisory, and the manufacturer later added a black box warning regarding the development of suicidal ideation in patients treated with ATMX.30 Similar to the selective serotonin reuptake inhibitors (SSRIs), there was a slight increase in suicidal thinking in controlled trials. Parents and caregivers should be made aware of any such occurrences and should monitor unexpected changes in mood or behavior.
ATMX is rapidly absorbed following oral administration; food does not appear to affect absorption, and Cmax occurs 1 to 2 hours after dosing.101 While the plasma half-life appears to be around 5 hours, the central nervous system (CNS) effects appear to last over 24 hours.102 ATMX is metabolized primarily in the liver to 4-hydroxyatomoxetine by the cytochrome (CYP) P450 2D6 enzyme.101,103,104 Although patients identified as “poor metabolizers” (i.e., with low 2D6 activity) appear to generally tolerate ATMX, these patients do seem to have more side effects, and a reduction in dose may be necessary.105,106 Therefore, in patients who are taking medications that are strong 2D6 inhibitors (e.g., fluoxetine, paroxetine, and quinidine), it may be necessary to reduce the dose of ATMX. Clinically, ATMX is often prescribed in conjunction with stimulants. Although the safety, tolerability, and efficacy of this combination have not been fully studied, reports suggest that this combination is well tolerated and effective.30,86 Therefore, although the full safety of administering stimulants and ATMX together has not been fully established, there are good data from which to extrapolate, and clinicians must balance the risks and benefits in each patient.
RECENT MEETINGS ABOUT SAFETY OF MEDICATIONS USED TO TREAT ADHD
Cardiovascular Safety of ADHD Treatments
The PDAC cited the baseline rate of sudden unexplained death in the pediatric population to range from 0.6 to 6 occurrences per 100,000 patient-years.107 From the FDA’s research, the PDAC presented data indicating that the rates of sudden unexplained death in the pediatric population between 1992 and February 2005, treated with MPH, amphetamine, or ATMX, were 0.2, 0.3, and 0.5 cases per 100,000 patient-years, respectively. Based on these data, the PDAC rejected adding a black box warning, but recommended that current labeling language for amphetamine drugs on CV risks in patients with structural cardiac abnormalities should be extended to all medications approved for the treatment of ADHD. Details regarding these issues are provided in two recent reviews.108,109
The American Heart Association has previously commented on CV monitoring of youths taking psychotropic medications.110 Despite the generally benign CV effects of these medications, caution is warranted in the presence of a compromised CV system (e.g., untreated hypertension, arrhythmias, and known structural heart defects). It remains prudent to monitor symptoms referable to the CV system (e.g., syncope, palpitations, and chest pain) and vital signs at baseline and with treatment in all patients with ADHD. For most pediatric patients it is not necessary to check an ECG at baseline or with treatment. In patients at risk for CV symptoms, it is important to collaborate with the patient’s primary care physician and to ensure that hypertension is not an issue. Recent data from an open-label study of ADHD treatment in adults suggest that if hypertension is well controlled, stimulants may be safely used in the short-term.111 Safety remains the paramount concern; thus, in each case the physician and patient must weigh the risks and benefits of treatment.
Aggression during Treatment with ADHD Medications
From the FDA’s research, the PDAC presented data reporting episodes of aggression with all ADHD medications during clinical trials and in postmarketing surveillance. However, aggression in patients with ADHD usually responds to stimulant treatment.112 During clinical trials, rates of aggression were observed to be similar with active and placebo treatment. The PDAC recommended that the decision about whether to continue therapy following an aggression event is complex and that the physician and parent should evaluate whether the risks outweigh the benefits that the child obtains from the treatment.
Psychotic or Manic Symptoms during Treatment with ADHD Medications
The FDA has received hundreds of reports of psychotic or manic symptoms, particularly hallucinations, associated with ADHD medication use in children and adolescents. FDA drug-safety analysts recommended adding warnings to ADHD medication-labeling advising that ADHD medications should be stopped if a patient experiences signs and symptoms of psychosis or mania. A recent review of stimulant trials revealed that psychotic-like or manic-like symptoms might occur in approximately 0.25% of children (or 1 in 400) treated with stimulant medications.113
Currently the only black box warning for stimulants warns about the potential for substance abuse.
ALTERNATIVE (NOT FDA-APPROVED) TREATMENTS FOR ADHD
Bupropion
Bupropion hydrochloride (Wellbutrin), a unicyclic aminoketone, approved for treatment of depression and as an aid for smoking cessation in adults,30 has been reported to be moderately helpful in reducing ADHD symptoms in children, adolescents,114–116 and adults.117–119 Although helpful, the magnitude of effect of bupropion is less than that seen with either stimulants or ATMX. Bupropion is often used in patients with ADHD and co-morbidities and has been studied in small groups of adolescents with ADHD and nicotine dependence,120 substance use and mood disorders,117 substance abuse and conduct disorder,121 and depression.122 In light of the high rates of marijuana use in patients with ADHD,123 it is important for clinicians to note that adolescents treated with bupropion may experience increased irritability during marijuana withdrawal.124 In addition, bupropion has been helpful in ADHD adults with bipolar disorder125 or with coexisting cardiac abnormalities.126
Bupropion modulates both norepinephrine and DA. It appears to be more stimulating than other antidepressants, may cause irritability, has been reported to exacerbate tics,127 and is associated with higher rates of drug-induced seizures than other antidepressants.30 These seizures appear to be dose-related (> 450 mg/day) and more likely to occur in patients with bulimia or seizure disorder, and thus should be avoided in patients with these problems. In ADHD adults, bupropion IR and SR should be given in divided doses, with no single dose of the IR exceeding 150 mg, or SR 200 mg. Dosing for ADHD appears to be similar to that for depression.128
Tricyclic Antidepressants
Although controlled trials in ADHD youths129 and adults130 demonstrate TCAs’ efficacy, the effects are less robust than with stimulants. Compared to the stimulants, TCAs have negligible abuse liability, have once-daily dosing, and may be useful in patients with co-morbid anxiety, ODD,131 tics,132 and, theoretically, depression (adults). However, given concerns about potential cardiotoxicity and the available of ATMX, use of the TCAs has been significantly curtailed.
Before treatment with a TCA, a baseline ECG should be obtained (as well as inquiry into any family history of early-onset or sudden cardiac arrhythmias).133 Dosing for ADHD appears to be similar to that for depression. ECGs should be obtained as the dose is increased. Monitoring serum levels of TCAs is more helpful in avoiding toxicity than it is in determining optimal levels for response.
Common short-term adverse consequences of the TCAs include dry mouth, blurred vision, orthostasis, and constipation. Since the anticholinergic effects of TCAs limit salivary flow, they may cause dental problems. Following the sudden death of a number of children receiving desipramine (DMI), concerns were raised regarding the possible cardiac toxicity of TCAs in children.134 However, epidemiological evaluation of the association between DMI and sudden death in children has not supported a causal relationship.135 TCAs predictably increase heart rate and are associated with conduction delays, usually affecting the right bundle, thus requiring ECG monitoring.136 However, these effects, when small, rarely seem to be pathophysiologically significant in noncardiac patients with normal baseline ECGs. In patients with documented congenital or acquired cardiac disease, pathological rhythm disturbances (e.g., atrioventricular block, supraventricular tachycardia, ventricular tachycardia, and Wolff-Parkinson-White syndrome), family history of sudden cardiac death or cardiomyopathy, diastolic hypertension (> 90 mm Hg), or when in doubt about the CV state of the patient, a complete (noninvasive) cardiac evaluation is indicated before initiation of treatment with a TCA to help determine the risk-benefit ratio of such an intervention. A serious adverse event associated with use of TCAs is overdose. Hence, close supervision of the administration and storage of TCAs is necessary.
Alpha-Adrenergic Agonists
Clonidine, an imidazoline derivative with alpha-adrenergic agonist properties, has been primarily used in the treatment of hypertension.137 At low doses, it appears to stimulate inhibitory, presynaptic autoreceptors in the CNS.138 Although clonidine reduces symptoms of ADHD,139 its overall effect is less than the stimulants,140 and likely smaller than ATMX, TCAs, and bupropion. Clonidine appears to be particularly helpful in patients with ADHD and co-morbid CD or ODD,141–143 tic disorders,144,145 ADHD-associated sleep disturbances,75,146 and may reduce anxiety and hypervigilance in traumatized children.147
Clonidine is a relatively short-acting compound with a plasma half-life ranging from approximately 5.5 hours (in children) to 8.5 hours (in adults). Therapy is usually initiated at the lowest manufactured dose of a half or quarter tablet of 0.1 mg. Usual daily doses ranges from 3 to 10 mcg/kg given generally in divided doses, bid, three times daily (tid), or four times daily (qid), and there is a transdermal preparation.148 The most common short-term adverse effect of clonidine is sedation, which tends to subside with continued treatment. It can also produce, in some cases, hypotension, dry mouth, vivid dreams, depression, and confusion. Overdoses of clonidine in children under 5 years of age may have life-threatening consequences.149 Since abrupt withdrawal of clonidine has been associated with rebound hypertension, slow tapering is advised.150,151 In addition, extreme caution should be exercised with the co-administration of clonidine with beta-blockers or calcium channel blockers.152 Concerns about the safety of co-administration of clonidine with stimulants have been debated.153 Current guidelines are to monitor blood pressure when initiating and tapering clonidine, but ECG monitoring is not usually necessary.110
Guanfacine, a more selective, alpha2-adrenergic agonist compound, has been used as an alternative to clonidine for the same indications.154,155 Possible advantages of guanfacine over clonidine include less sedation and a longer duration of action. Anecdotal information suggests that guanfacine may be more useful in improving the cognitive deficits of ADHD. School-age children with ADHD and co-morbid tic disorder, treated with guanfacine in doses ranging from 0.5 mg bid to 1 mg tid, showed reduction in both tics and ADHD.156,157 Guanfacine treatment is associated with minor, clinically insignificant decreases in blood pressure and pulse rate. The adverse effects of guanfacine include sedation, irritability, and depression. Several cases of apparent guanfacine-induced mania have been described, but the impact of guanfacine on mood disorders remains unclear.158 An extended formulation of guanfacine is currently being studied.63
NOVEL TREATMENTS FOR ADHD
Modafinil
Modafinil, a novel stimulant that is distinct from amphetamine, is approved for the treatment of narcolepsy.159 Unlike the broad activation observed with amphetamine, modafinil appears to activate specific hypothalamic regions.160,161 Although initial trials in adults were negative, in three recent trials in children modafinil demonstrated efficacy in the treatment of ADHD.162–164 The FDA PDAC voted that modafinil is “not approvable” for pediatric ADHD due to possible Stevens-Johnson syndrome (SJS) in pediatric patients (see www.fda.gov). It may be reasonable to consider combining modafinil with stimulants,165,166 and clinicians should be aware of the potential to exacerbate mania.167 Given the cognitive enhancing properties of nicotine,168 Wilens and colleagues have studied a novel cholinergic activating agent, ABT-418,169 and more recently a nicotine receptor partial agonist, ABT-089.170 At this time the role of nicotinic agents remains investigational.
Although compelling based on efficacy in Alzheimer’s disease, positive initial experience in ADHD patients171
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree