CHAPTER 78 Pain
OVERVIEW
Pain, as determined by the International Association for the Study of Pain (IASP), is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”1 This chapter will describe the physiological aspects of pain transmission, pain terminology, and pain assessment; discuss the major classes of medications used to relieve pain; and outline the diagnosis and treatment of psychiatric conditions that often affect patients with chronic pain.
EPIDEMIOLOGY
Psychiatric co-morbidity (e.g., anxiety, depression, personality disorders, and substance use disorders [SUDs]) afflicts those with both non–cancer-related and cancer-related pain. Epidemiological studies indicate that roughly 30% of those in the general population with chronic musculoskeletal pain also have depression or an anxiety disorder.2 Similar rates exist in those with cancer pain. In clinic populations, 50% to 80% of pain patients have co-morbid psychopathology, including problematic personality traits. The personality (i.e., the characterological or temperamental) component of negative affect has been termed neuroticism, which may be best described as “a general personality maladjustment in which patients experience anger, disgust, sadness, anxiety, and a variety of other negative emotions.”3 Frequently, in pain clinics, maladaptive expressions of depression, anxiety, and anger are grouped together as disorders of negative affect, which have an adverse impact on the response to pain.4
Rates of substance dependence in chronic pain patients are also elevated relative to the general population, and several studies have found that 15% to 26% of chronic pain patients have a co-morbid substance (e.g., illegal drugs or prescription medications) dependence disorder.5 Prescription opiate addiction is a growing problem that affects approximately 5% of those who have been prescribed opiates for chronic pain (although good epidemiology studies are lacking). Other chapters in this textbook focus more specifically on SUDs. This chapter will concentrate on those with affective disorders and somatoform disorders in the setting of chronic pain.
PATHOPHYSIOLOGY OF PAIN TRANSMISSION
Tissue injury stimulates the nociceptors by the liberation of adenosine triphosphate (ATP), protons, kinins, and arachidonic acid from the injured cells; histamine, serotonin, prostaglandins, and bradykinin from the mast cells; and cytokines and nerve growth factor from the macrophages. These substances and decreased pH cause a decrease in the threshold for activation of the nociceptors, a process called peripheral sensitization. Subsequently, axons transmit the pain signal to the spinal cord, and to cell bodies in the dorsal root ganglia (Figure 78-1). Three different types of axons are involved in the transmission of pain from the skin to the dorsal horn. A-β fibers are the largest and most heavily myelinated fibers that transmit awareness of light touch. A-Δ fibers and C fibers are the primary nociceptive afferents. A-Δ fibers are 2 to 5 mcm in diameter and are thinly myelinated. They conduct “first pain,” which is immediate, rapid, and sharp, with a velocity of 20 m/sec. C fibers are 0.2 to 1.5 mcm in diameter and are unmyelinated. They conduct “second pain,” which is prolonged, burning, and unpleasant, at a speed of 0.5 m/sec.
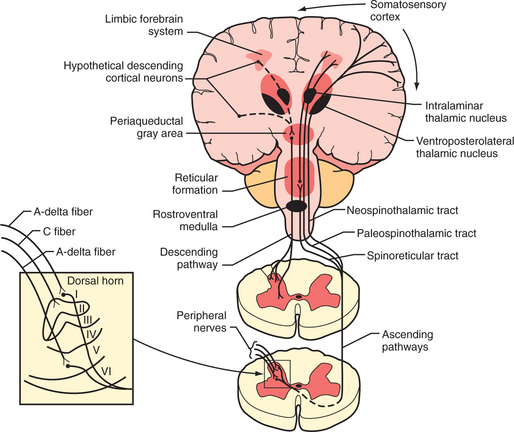
Figure 78-1 Schematic diagram of neurological pathways for pain perception.
(From Hyman SH, Cassem NH: Pain. In Rubenstein E, Fedeman DD, editors: Scientific American medicine: current topics in medicine, subsection II, New York, 1989, Scientific American. Originally from Stern TA, Herman JB, editors: Psychiatry update and board preparation, 2004, McGraw-Hill.)
A-Δ and C fibers enter the dorsal root and ascend or descend one to three segments before synapsing with neurons in the lateral spinothalamic tract (in the substantia gelatinosa in the gray matter) (see Figure 78-1). Second pain transmitted with C-fibers is integrally related to chronic pain states. Repetitive C-fiber stimulation can result in a progressive increase of electrical discharges from second-order neurons in the spinal cord. NMDA receptors play a role when prolonged activation occurs. This pain amplification is related to a temporal summation of second pain or “wind-up.” This hyperexcitability of neurons in the dorsal horn contributes to central sensitization, which can occur as an immediate or as a delayed phenomenon. In addition to wind-up, central sensitization involves several factors: activation of A-beta fibers and lowered firing thresholds for spinal cord cells that modulate pain (i.e., they trigger pain more easily); neuroplasticity (a result of functional changes, including recruitment of a wide range of cells in the spinal cord so that touch or movement causes pain); convergence of cutaneous, vascular, muscle, and joint inputs (where one tissue refers pain to another); or aberrant connections (electrical short-circuits between the sympathetic and sensory nerves that produce causalgia). Inhibition of nociception in the dorsal horn is functionally quite important. Stimulation of the A-Δ fibers not only excites some neurons, but it also inhibits others. This inhibition of nociception through A-Δ fiber stimulation may explain the effects of acupuncture and transcutaneous electrical nerve stimulation (TENS).
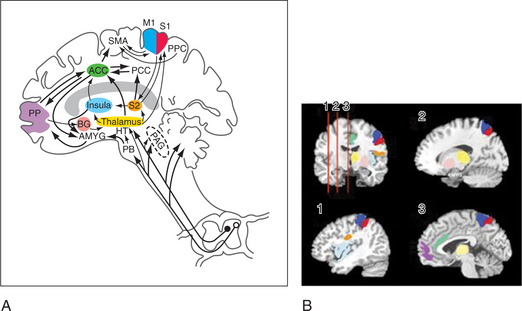
The lateral spinothalamic tract crosses the midline and ascends toward the thalamus. At the level of the brainstem more than half of this tract synapses in the reticular activating system (in an area called the spinoreticular tract), in the limbic system, and in other brainstem regions (including centers of the autonomic nervous system). Another site of projections at this level is the periaqueductal gray (PAG) (Figure 78-2), which plays an important role in the brain’s system of endogenous analgesia. After synapsing in the thalamic nuclei, pain fibers project to the somatosensory cortex, located posterior to the Sylvian fissure in the parietal lobe, in Brodmann’s areas 1, 2, and 3. Endogenous analgesic systems involve endogenous peptides with opioid-like activity in the central nervous system (CNS) (e.g., endorphins, enkephalins, and dynorphins). Different opioid receptors (mu, kappa, and delta receptors) are involved in different effects of opiates. The centers involved in endogenous analgesia include the PAG, the anterior cingulate cortex (ACC), the amygdala, the parabrachial plexus (in the pons), and the rostral ventromedial medulla.
The descending analgesic pain pathway starts in the PAG (which is rich in endogenous opiates), projects to the rostral ventral medulla, and from there descends through the dorsolateral funiculus of the spinal cord to the dorsal horn. The neurons in the rostral ventral medulla use serotonin to activate endogenous analgesics (enkephalins) in the dorsal horn. This effect inhibits nociception at the level of the dorsal horn since neurons that contain enkephalins synapse with spinothalamic neurons. Additionally, there are noradrenergic neurons that project from the locus coeruleus (the main noradrenergic center in the CNS) to the dorsal horn and inhibit the response of dorsal horn neurons to nociceptive stimuli. The analgesic effect of tricyclic antidepressants (TCAs) and the serotonin-norepinephrine reuptake inhibitors (SNRIs) is thought to be related to an increase in serotonin and norepinephrine that inhibits nociception at the level of the dorsal horn, through their effects on enhancing descending pain inhibition from above.
CORTICAL SUBSTRATES FOR PAIN AND AFFECT
Advances in neuroimaging have linked the function of multiple areas in the brain with pain and affect. These areas (e.g., the ACC, the insula, and the dorsolateral prefrontal cortex [DLPFC]) form functional units through which psychiatric co-morbidity may amplify pain and disability (see Figure 78-2). These areas are part of the spinolimbic (also known as the medial) pain pathway,6 which runs parallel to the spinothalamic tract and receives direct input from the dorsal horn of the spinal cord. The interactions among the function of these areas, pain perception, and psychiatric illness are still being investigated. The spinolimbic pathway is involved in descending pain inhibition (which includes cortical and subcortical structures), whose function may be negatively affected by the presence of psychopathology. This, in turn, could lead to heightened pain perception. Coghill and colleagues7 have shown that differences in pain sensitivity between patients can be correlated with differences in activation patterns in the ACC, the insula, and the DLPFC. The anticipation of pain is also modulated by these areas, suggesting a mechanism by which anxiety about pain can amplify pain perception. The disruption or alteration of descending pain inhibition is a mechanism of neuropathic pain, which can be described as central sensitization that occurs at the level of the brain, a concept supported by recent neuroimaging studies of pain processing in the brains of patients with fibromyalgia.8 The ACC, the insula, and the DLPFC are also laden with opioid receptors, which are less responsive to endogenous opioids in pain-free subjects with high negative affect.9 Thus, negative affect may diminish the effectiveness of endogenous and exogenous opioids through direct effects on supraspinal opioid binding.
INTERACTIONS BETWEEN PAIN AND PSYCHOPATHOLOGY
The majority of patients with chronic pain and a psychiatric condition have an organic or physical basis for their pain. However, the perception of pain is amplified by co-morbid psychiatric disorders, which predispose patients to develop a chronic pain syndrome. This is commonly referred to as the diathesis-stress model, in which the combination of physical, social, and psychological stresses associated with a pain syndrome induces significant psychiatric co-morbidity.4 This can occur in patients with or without a pre-existing vulnerability to psychiatric illness (e.g., a genetic or temperamental risk factor). Regardless of the order of onset of psychopathology, patients with chronic pain and psychopathology report greater pain intensity, more pain-related disability, and a larger affective component to their pain than those without psychopathology. As a whole, studies indicate that it is not the specific qualities or symptomatology of depression, anxiety, or neuroticism, but the overall levels of psychiatric symptoms that are predictive of poor outcome.10 Depression, anxiety, and neuroticism are the psychiatric conditions that most often co-occur in patients with chronic pain, and those with a combination of pathologies are predisposed to the worst outcomes.
PAIN TERMINOLOGY
Chronic pain (i.e., pain that persists beyond the normal time of healing or lasts longer than 6 months) involves different mechanisms in local, spinal, and supraspinal levels. Characteristic features include vague descriptions of pain and an inability to describe the pain’s timing and localization. It is usually helpful to determine the presence of a dermatomal pattern (Figure 78-3), to determine the presence of neuropathic pain, and to assess pain behavior.
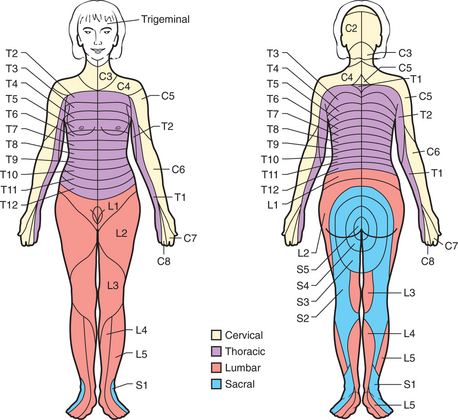
Figure 78-3 Schematic diagram of segmental neuronal innervation by dermatomes.
(From Hyman SH, Cassem NH: Pain. In Rubenstein E, Fedeman DD, editors: Scientific American medicine: current topics in medicine, subsection II, New York, 1989, Scientific American. Originally from Stern TA, Herman JB, editors: Psychiatry update and board preparation, 2004, McGraw-Hill.)
Myofascial pain can arise from one or several of the following problems: hypertonic muscles, myofascial trigger points, arthralgias, and fatigue with muscle weakness. Myofascial pain is generally used to describe pain from muscles and connective tissue. Myofascial pain results from a primary diagnosis (e.g., fibromyalgia) or, as more often is the case, a co-morbid diagnosis (e.g., with vascular headache or with a psychiatric diagnosis).
ASSESSMENT OF PAIN
The evaluation of pain focuses first on five questions: (1) Is the pain intractable because of nociceptive stimuli (e.g., from the skin, bones, muscles, or blood vessels)? (2) Is the pain maintained by non-nociceptive mechanisms (i.e., have the spinal cord, brainstem, limbic system, and cortex been recruited as reverberating pain circuits)? (3) Is the complaint of pain primary (as occurs in disorders such as major depression or delusional disorder)? (4) Is there a more efficacious pharmacological treatment? (5) Have pain behavior and disability become more important than the pain itself? Answering these questions allows the mechanism(s) of the pain and suffering to be pursued. A psychiatrist’s physical examination of the pain patient typically includes examination of the painful area, muscles, and response to pinprick and light touch (Table 78-1).
Table 78-1 General Physical Examination of Pain by the Psychiatrist
| Physical Finding | Purpose of Examination |
|---|---|
| Motor deficits | |
| Trigger points in head, neck, shoulder, and back muscles | |
| Evanescent, changeable pain, weakness, and numbness | Does the psychological complaint preempt the physical? |
| Abnormal sensory findings | Is there a nondermatomal distribution of pain and sensation that suggests either a somatoform or CNS pain disorder? |
| Sympathetic or vascular dysfunction | Is there swelling, skin discoloration, or changes in sweating or temperature that suggest a vascular or sympathetic element to the pain? |
| Uncooperativeness, erratic responses to the physical examination | Is there an interpersonal aspect to the pain, causing abnormal pain behavior, as in somatoform disease? |