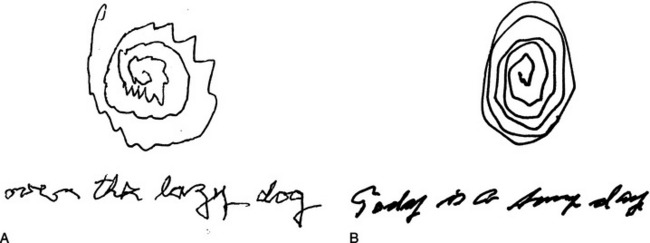CHAPTER 80 Movement Disorders
OVERVIEW
Drugs used to treat movement disorders often have psychiatric side effects, as in the disinhibition syndromes sometimes seen with dopamine agonists. Side effects of psychotropics are frequent and sometimes devastating causes of movement abnormalities. Those caused by neuroleptics are covered in Chapters 42 and 55.
PATHOPHYSIOLOGY
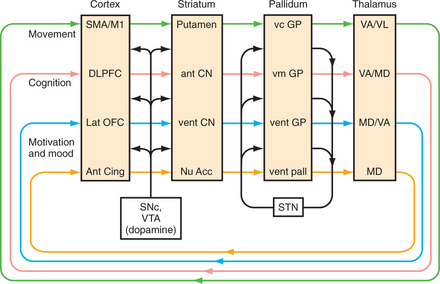
Movement disorders stem from anatomical and pharmacological changes in basal ganglia circuits (Figure 80-1). The overlap between neurological and psychiatric symptoms in movement disorders stems from the fact that circuits controlling cognition, movement, and emotion run in parallel through the basal ganglia, and interact greatly.1 Thus, pathology in Parkinson’s disease may predominantly affect motor areas of the midstriatum (the putamen), but may also spread to affect cognitive circuits in the most dorsal striatum (the head of the caudate), and may affect motivational circuits in the ventral striatum and the nucleus accumbens. Disorders of the basal ganglia broadly affect the motivation to act, both to start and to stop. When damage spreads to affective circuits, it creates disorders of motivation there, too—depression and apathy on the one hand, or excessively goal-directed, manic disinhibition on the other. When a bradykinetic disorder spreads to the basal ganglia’s cognitive circuits, thoughts can be slowed (bradyphrenia) and deficits in executive function may appear without the characteristic aphasia or agnosia of cortical dementias (such as Alzheimer’s disease). Conversely, hyperkinetic disorders can cause wild, unregulated thought manifested by hallucinations and delusions.
Circuitry
In basal ganglia circuitry, all of cerebral cortex projects to the striatum (i.e., the caudate nucleus, putamen, and nucleus accumbens), which then projects to the globus pallidus (external and internal segments), through the thalamus, and finally back to the cerebral cortex. Dopaminergic projections from the substantia nigra pars compacta (motor) and nearby ventral tegmental area (limbic) play an important role. Because this feedback loop ultimately affects movement through pyramidal neurons’ output through the spinal cord, “extrapyramidal symptoms” (EPS) is not an entirely accurate term for basal ganglia problems. However, it is entrenched in clinical usage. The subthalamic nucleus, too, has an important modifying influence. Hypokinetic disorders usually stem from damage to the direct pathway through the internal globus pallidus; hyperkinetic movements result from damage to the indirect pathway through the external globus pallidus and subthalamic nucleus. These pathways control affect and cognition, as well as movement.
Pharmacology
Dopamine is the best-understood neurotransmitter related to basal ganglia function.2 Its receptors fall into two classes, the D1 class, of which D1 is mostly found in the striatum and D5 extrastriatal, and the D2 class, of which D2 is primarily striatal whereas D3 and D4 are mostly extrastriatal. Its release in the motor control areas of the striatum seems to facilitate limb movement via D2 receptors, and to inhibit movement via D1 receptors. Medications most commonly used to affect dopamine neurotransmission are antagonists (such as haloperidol), precursors (such as levodopa), receptor agonists (such as pramipexole), and inhibitors of dopamine metabolism (such as entacapone [a catechol-O-methyltransferase (COMT) inhibitor] and selegiline [a monoamine oxidase-B (MAO-B) inhibitor, at least at low doses]). Acetylcholine is also important in the basal ganglia, particularly in striatal and nucleus accumbens’ control of motivation and memory. To a first approximation cholinergic effects counteract those of dopamine, making anticholinergics (such as benztropine) useful in the treatment of patients with drug-induced parkinsonism. Gamma-aminobutyric acid (GABA) and glutamate are the predominant neurotransmitters in basal ganglia circuitry, but their ubiquity makes them bad targets for pharmacological interventions.
EVALUATION AND SYMPTOMATIC MANAGEMENT
Patient History
Most movement disorders are diagnosed by history and the physical examination, not by laboratory tests. The first priority is to determine the frequency and nature of falling, as falls are the most immediate health risk. The second most immediate health risk is dysphagia causing aspiration, so it is important to ask whether the patient has had trouble swallowing pills, or coughs after drinking liquids. One should ask about what daily tasks are difficult—for example, “Does the tremor interfere with using a spoon (usually an action tremor) or holding a newspaper (usually a rest tremor)?” Drug use, especially use of neuroleptics, but also use of sleeping pills and alcohol, is important.
Physical Observation
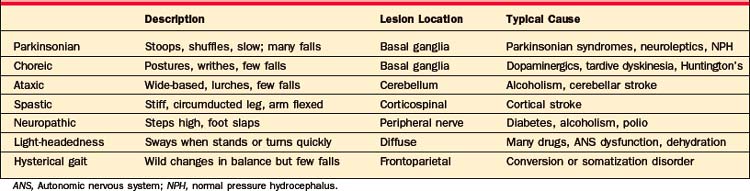
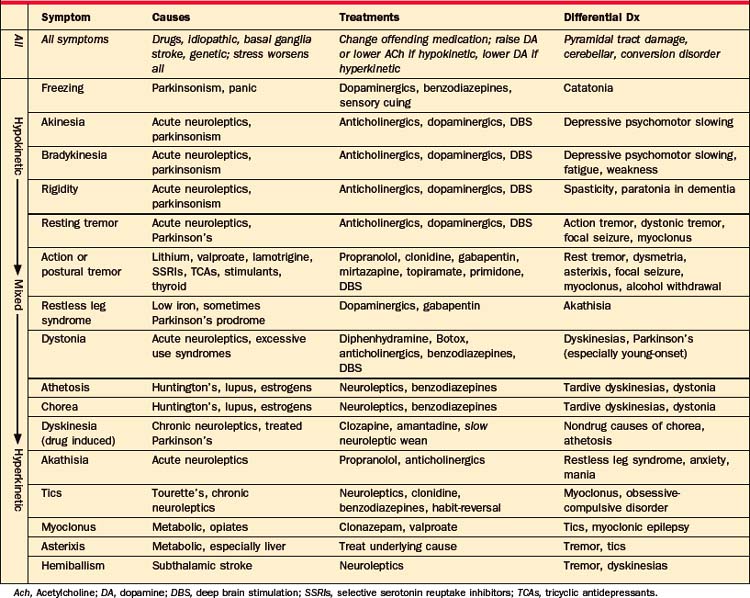
Symptoms of movement disorders are summarized in Table 80-1. They range from hypokinetic to hyperkinetic, and from feeling involuntary to voluntary. Unfortunately, it is not easy to distinguish movement disorders through written descriptions of symptoms, and descriptions in this chapter are no exception. Web-based or published patient videos are the best way to learn this crucial distinction.3 Many are available online (e.g., www.psychiatrist.com/supplenet/v65s09/ovi.pdf).
Table 80-1 Symptoms, Causes, and Treatment of Movement Disorders, with a Focus on Causes Common in Adult Psychiatric Patients
Hypokinetic Signs
Rigidity.
Rigidity is the cardinal hypokinetic sign. While rigidity is characteristic of parkinsonism, it is sometimes present even in hyperkinetic conditions, such as Tourette’s syndrome. Rigidity produces a constant “lead pipe” resistance to movement along the whole range of the joint. It can quickly be assessed without touching the patient, by asking the patient to rapidly rotate the wrist back and forth, as if trying to screw in a lightbulb. Cogwheel rigidity is simply a tremor superimposed on rigidity, although the tremor is not always apparent visually. Patients sometimes complain of an inner tremor not visible to others. Their complaint may be mistaken for somatization or delusion, but it is more commonly evidence for parkinsonism. Rigidity makes movements low-amplitude, and they rapidly decrease in size. Handwriting almost always demonstrates this, and is another simple, noninvasive test (Figure 80-2).
Bradykinesia.
Bradykinesia (slower movements) and akinesia (fewer movements) may culminate in freezing, a sudden inability to move that most often occurs when the patient tries to initiate a movement. Freezing is related to the psychiatric syndrome of catatonia (see Chapter 55). Freezing, like many basal ganglia symptoms, is more a problem with internally motivated behaviors than with ones that are responses to environmental cues. Patients can sometimes break freezes with sensory tricks (such as stepping over a line on the floor, or hearing marching music). This can incorrectly appear to be evidence for a somatoform disorder.
Mixed Signs
Rest Tremor.
Rest tremor hovers in the middle of the spectrum between hypokinetic and hyperkinetic movements. Mixed signs often do not respond well to dopaminergic medications. Rest tremor, for instance, presents when the limb is not in use, looks hyperkinetic but is almost always associated with parkinsonism. Rest tremors are commonly coarse and low-frequency (3 to 5 Hz). Like all basal ganglia movement disorders, they disappear during sleep. Rest tremors often respond more completely to anticholinergics than do dopaminergic drugs. The archetypal rest tremor disappears with voluntary movement of the same limb, but in the real world there is sometimes a co-morbid action tremor. All tremors, rest or action, are rhythmic back-and-forth movements, in distinction to the unidirectional movements of myoclonus and asterixis, or the arrhythmic movements of tics and dyskinesias. Although focal motor seizures are rhythmic, they should not vary with limb movement. A reliable way to tell a rest tremor from an action tremor is to have the patient write a sentence (not the patient’s name) and draw a large spiral (see Figure 80-2).
Hyperkinetic Signs
Chorea and Athetosis.
Apart from the briefest, most ballistic movements, most hyperkinetic movements feel semipurposive, and the patient can usually suppress them for brief periods. For this reason the abnormal movements may mistakenly seem psychogenic. Athetosis, a slow, writhing, nearly dystonic movement, rarely needs to be distinguished from the quicker movements of chorea and dyskinesias, as their causes and treatment are similar. Choreoathetosis is often described as dance-like, but it is rarely so. Rhythmic chorea was common in Charcot’s day, when the most common cause was tertiary syphilis or the hysterical chorea that imitated it (Figure 80-3). Common modern causes include lupus, pregnancy, Huntington’s disease, Wilson’s disease, and use of oral contraceptives or neuroleptics. Typical neuroleptics (such as haloperidol) generally suppress choreoathetosis, but may eventually cause a tardive worsening of the hyperkinesis.
Gait
Apart from neuroleptic malignant syndrome (NMS), the most dangerous movement disorders are those that affect gait and increase the risk of falls. Gait disorders are often multifactorial.4
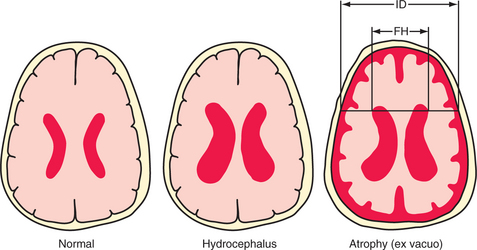
Table 80-1 describes movement disorders that are typically seen in psychiatric patients, and Table 80-2 describes types of gait disorders. Balance is often impaired by hypokinetic movement disorders, in large part because patients cannot correct their posture quickly enough when they make a misstep. This is quite different from ataxia, the poor balance caused by cerebellar lesions or anticonvulsant toxicity. Ataxia can give a wildly swaying gait, yet it paradoxically causes fewer falls. It is often associated with spinning vertigo, distinct from the light-headedness caused by a variety of medications, anxiety, and depression. One cause of gait disorder that surgery may—occasionally—cure is normal pressure hydrocephalus (NPH). Its cardinal symptoms include a parkinsonian gait, incontinence, and, eventually, dementia. Radiographically, NPH causes dilated ventricles with normal sulci, in distinction to the global atrophy more commonly seen with dementia (Figure 80-4). By the time dementia develops, symptoms are hardly ever reversible. In the absence of parkinsonism, incontinence, and the characteristic radiographic findings, dementia is virtually never caused by NPH.