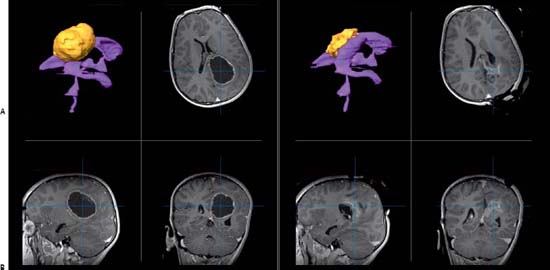
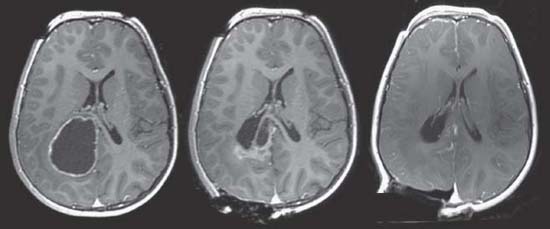
Case 80 Neuronavigation and Intraoperative Imaging Fig. 80.1 (A) Magnetic resonance imaging (MRI) preoperative, three-dimensional (3D) reconstruction (top left) with ventricular system in purple and tumor in yellow. T1-weighted MRIs preoperative with contrast showing tumor in axial (top right), sagittal (bottom left), and coronal (bottom right) images. (B) MRI intraoperative, 3D reconstruction (top left) with ventricular system in purple and tumor in yellow. T1-weighted MRIs intraoperative with contrast showing tumor in axial (top right), sagittal (bottom left), and coronal (bottom right) images. Fig. 80.2 Intraoperative axial T1-weighted magnetic resonance images with contrast showing progression of tumor resection.
 Clinical Presentation
Clinical Presentation
 Questions
Questions
 Answers
Answers
< div class='tao-gold-member'>
80 Neuronavigation and Intraoperative Imaging
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


