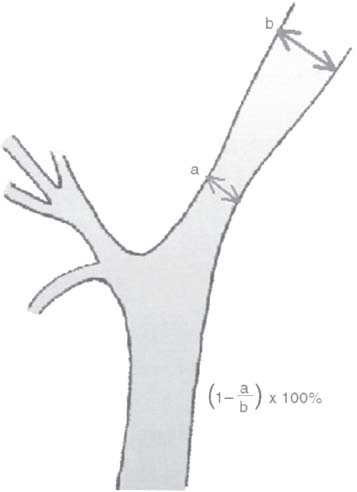
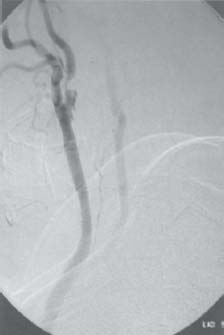
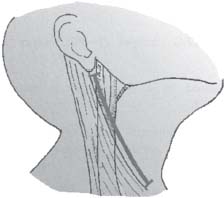
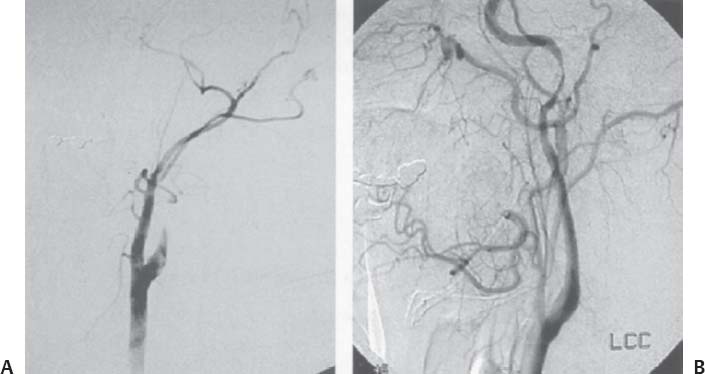
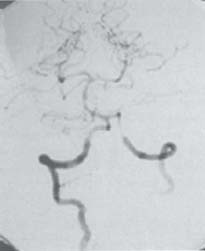
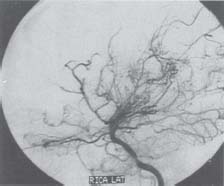
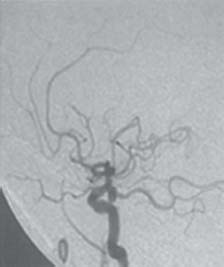
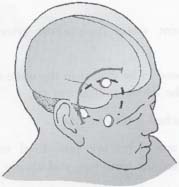
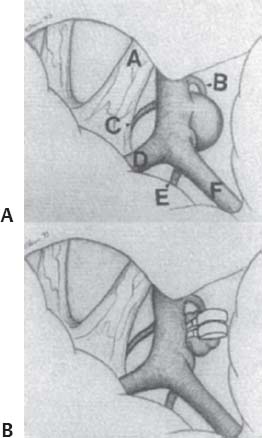
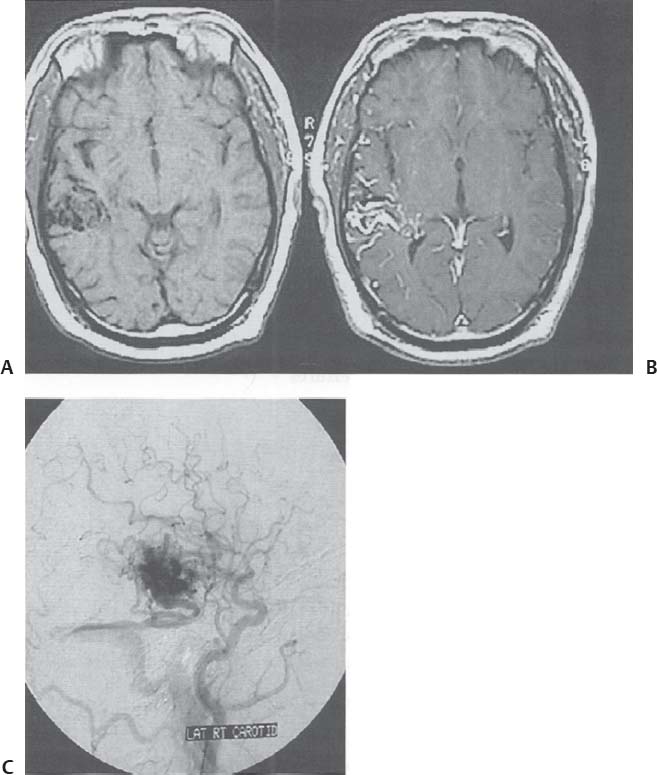
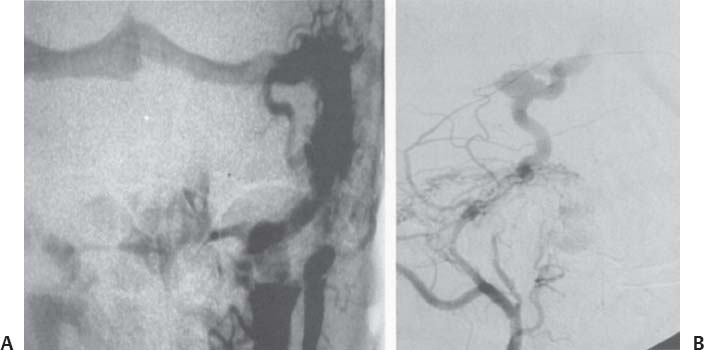
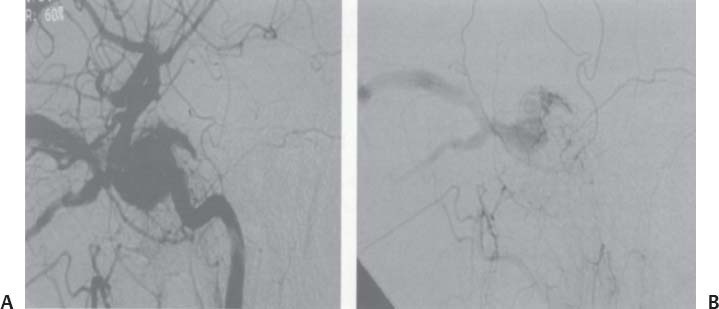
C H A P T E R 9 CEREBROVASCULAR DISEASE I. STROKE A. Transient ischemic attack (TIA)—deficit lasts < 24 hours B. Reversible ischemic neurologic deficit (RIND)—deficit lasts < 1 week C. Cerebrovascular accident (CVA)—stroke, deficit lasts > 1 week D. Amaurosis fugax—transient monocular blindness usually due to a small fibrin embolus E. CVA risk after an ocular TIA—17% in 2 years F. CVA risk after a hemispheric TIA—43% in 2 years (usually within 3 months) G. CVA etiologies 1. 60% from atherosclerosis—possibly from an embolic plaque or ischemia; usually 50% stenosis is necessary to be symptomatic 2. 20% lacunar—small-vessel lipohyalinosis 3. 15% from cardiac embolism 4. 15% of CVAs are hemorrhage; 85% are dry H. Cerebral autoregulation 1. Occurs with cerebral perfusion pressure (CPP) 50–150 mm Hg to keep cerebral blood flow (CBF) normal (around 55 mL/100 g of brain tissue/min) I. CVA symptoms 1. Carotid circulation—unilateral motor, sensory, visual, or speech deficit 2. Vertebral circulation—bilateral motor, sensory, or visual deficits, or syncope J. Evaluation 1. History—tobacco use, elevated cholesterol, hypertension (HTN), DM, angina, and claudication 2. Physical evaluation—funduscopy, assessment of superficial temporal artery (STA) pulses, bruits 3. Laboratory evaluation—complete blood cell count (CBC), platelets, prothrombin time/partial thromboplastin time (PT/PTT), electrolytes, arterial blood gases (ABG), rapid plasma reagin (RPR; syphilis), erythrocyte sedimentation rate (ESR), electrocardiogram (ECG), and 24-hour cardiac monitor 4. Radiologic evaluation a. Computed tomography (CT) i. Initially may be normal or with edema that blurs gray/white junction ii. After one week shows gyral ribbon enhancement iii. Many weeks later reveals atrophy b. Carotid duplex—combines color flow Doppler and β-mode ultrasound i. Doppler effect—change in sound pitch as sound waves hit a moving target; the frequency decreases as it leaves and increases as it approaches c. Carotid and cerebral magnetic resonance angiography (MRA) or angiogram—may demonstrate a string sign or occluded vessels as well as luxury perfusion (increased CBF adjacent to a CVA due to impaired autoregulation from the acidosis). Don’t operate if a tandem cerebral lesion is more stenotic than the carotid lesion. 1. Recombinant tissue-plasminogen activator (t-PA) use does not change the deficit at 24 hours, but improves it at 3 months; 30% of patients are more likely to have little or no deficit. 2. Must be administered within 3 hours of symptoms 3. Exclusion criteria—CT with edema or hemorrhage, systolic blood pressure (SBP) > 185 mm Hg, seizures, anticoagulants, surgery within 14 days, CVA within 3 months, or glucose > 400 4. Increased risk of intraparenchymal hemorrhage 6.4% from 0.6%, though the mortality stays at 20% 5. Dose—0.09 mg/kg intravenously (IV) over 1 minute and then 0.81 mg/kg over 60 minutes 6. Reverse t-PA for surgery—Amicar 5 g IV (acts in 15 minutes), cryoprecipitate, and fresh frozen plasma (FFP) L. CVA treatment 1. IV fluid without dextrose 2. Aspirin (ASA)—325 mg orally daily (PO qd) 3. Maintain SBP < 180 mm Hg (but > 130 mm Hg) 4. The risk of another CVA is 1%/week with or without heparin (not recommended). 5. Coumadin—use only for antiphospholipid antibody, atrial fibrillation, or vertebral insufficiency 6. Hemicraniectomy—may be used for cerebellar or right middle cerebral artery CVAs with excessive mass effect 7. Avoid hyperthermia and hyperglycemia; they make the neurons more vulnerable to ischemia. M. Best medical management 1. Start with ASA (325 mg qd) or Ticlid to produce a 30% decrease in CVA rate after a TIA. 2. Ticlid and Plavix may lower the white blood cell (WBC) count. 3. Control HTN, DM, cholesterol, and tobacco use 4. Anticoagulation—for atrial fibrillation or vertebrobasilar symptoms 5. Heparin (converted to Coumadin)—if there is > 95% stenosis N. Surgical management 1. Degree of stenosis—as per the North American Symptomatic Carotid Endarterectomy Trial (NASCET) (1 – a/b) × 100 is the percent stenosis (Figs. 9.1 and 9.2). 2. Asymptomatic Carotid Atherosclerosis Study (ACAS) a. If there is > 60% stenosis, surgery decreases the CVA rate only if the complication rate is <3%. b. The 5-year CVA rate decreased 66% in males and 17% in females (however, there was no decrease in major CVA rate or death). c. The 5-year CVA rate without surgery was 11% and with surgery was 5%. Fig. 9.1 Carotid stenosis diagram. (With permission from Citow JS. Neurosurgery Oral Board Review. 1st ed. New York, NY: Thieme Medical Publishers; 2003: 88, Fig. 10.1.) Fig. 9.2 Carotid artery stenosis. Angiogram demonstrates proximal internal carotid artery plaque. (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 149, Fig. 194.) 3. NASCET a. In the presence of a hemispheric or retinal TIA and stenosis >70%, surgery decreases the CVA rate 17% and the death rate 7% over 1.5 years. b. A 2-year CVA rate is 40% without ASA, 26% with ASA, and 9% with ASA and carotid endarterectomy (CEA). c. There is a 1%/month risk of CVA while waiting for surgery. 4. Recommendations a. Asymptomatic carotid stenosis—only operate if >60% stenosis b. Symptomatic carotid stenosis—operate if >50% stenosis 5. Carotid endarterectomy a. Perform 2–6 weeks after CVA and perform another ultrasound immediately before surgery to detect any changes. c. Preoperative ASA 325 mg qd × 5 days and resume postop at 48 hours. i. Ticlid reverses in 2 hours with methylprednisolone. ii. Change over to ASA before surgery. d. Selective shunting—based on electroencephalogram (EEG) changes (1–4% require a shunt). i. EEG changes—usually caused by a shower of cholesterol plaques, not blood clots. ii. Treatment—heparin 5000 U, thiopental 3 mg/kg, raise the SBP to 170 mm Hg, shunt, and consider angiography with possible urokinase or embolectomy e. Patch implantation—if small vessel, long plaque, or repeat surgery f. Exposure i. Make the skin incision over the medial border of the sternocleidomastoid (SCM). ii. Watch for the greater auricular nerve over the SCM (section causes numbness in the jaw and ear) iii. Expose the digastric muscle proximally and omohyoid muscle distally iv. Divide the common facial vein v. Follow the descendent hypoglossi to cranial nerve (CN) XII. vi. Place loops around the common carotid artery (CCA), internal carotid artery (ICA), external carotid artery (ECA), and superior thyroid artery (Fig. 9.3) Fig. 9.3 Carotid endarterectomy incision. (With permission from Citow JS. Neurosurgery Oral Board Review. 1st ed. New York, NY: Thieme Medical Publishers; 2003: 90, Fig. 10.3) g. Clamping i. Bolus with heparin 70 U/kg 5 minutes before clamping ii. Clamp first the ICA, the CCA, the superior thyroid artery, and then the ECA (aneurysm clips on all but a vascular clamp on the CCA) iii. After the endarterectomy, suture the vessel closed with 5.0 Prolene iv. Unclamp first the ECA and flush the CCA and ICA v. Open the CCA and finally the ICA h. Emergent CEA i. Only used if there is a sudden deficit with a lost bruit in the presence of known stenosis, during angiography, or after CEA ii. Don’t operate on a totally occluded carotid artery after 2 hours if there is a fixed deficit iii. There is a 3%/year CVA risk from stump embolism with complete occlusion. i. Complications i. Injuries to CN XII—near the facial vein ii. Injuries to recurrent laryngeal nerve—near the trachea; keep the medial retractor at the platysma iv. Reactive cerebral hyperemia v. Recurrence—stenosis recurs in 20% of patients over 10 years. j. Postop evaluation i. Look for pronator drift, dysphasia, pupillary reactivity, STA pulses (to find ECA or CCA occlusion), tongue deviation, hoarseness, and hematoma with dysphagia or tracheal deviation. ii. Beware of vessel leak, pseudoaneurysm, TIA/CVA, cerebral hemorrhage, or seizure iii. CN injuries a. CN X—vocal cord paralysis or hoarseness b. CN XII—tongue deviation c. CN VII—lip depressor weakness from mandibular branch iv. Possible HTN from loss of baroreceptor reflex v. Postop TIA—evaluate with CT and angiogram vi. Fixed postop CVA a. Return to the operating room (OR) and reopen the vessel (clamp the CCA and ECA, open the ICA to see back-flow, try to insert a Fogarty #4 catheter to remove the clot) b. Use aggressive IV fluids to increase the SBP to 180 mm Hg, increase O2, and use pressors if needed c. A neck hematoma may need to be removed to enable intubation (try once, don’t paralyze the patient, open the neck in the OR). d. Consider performing a CT if there is a nonlocalizing deficit vii. Angioplasty b. Risk of embolism and subintimal dissection c. Stent may be placed after angioplasty. O. CVA outcome—25% mortality, 25% disabled, 50% return home P. CVA from cardiac embolism 1. Accounts for 1/6 of CVAs 2. 2% of myocardial infarctions (MIs) develop a CVA within 2 weeks. 3. Without anticoagulation—risk of CVA with atrial fibrillation is 4.5%/ year and with a bioprosthetic heart valve is 3%/year. 4. With anticoagulation—risk of CVA with a mechanical mitral valve is 3%/year and a mechanical aortic valve is 1.5%/year. 5. Evaluation a. Rule out a paradoxical embolism from a patent foramen ovale in young patients that suffer a CVA. b. Rule out cerebrovascular disease and a source for an embolism (echocardiogram and ECG) c. There is frequent hemorrhagic transformation at 48 hours. 6. Treatment a. No proven benefit with early anticoagulation—12% CVA risk in 2 weeks vs risk of hemorrhagic transformation b. Perform CT at 2 days—if no clot, use heparin and Coumadin together for 3 days until the international normalized ration (INR) is 2–3. c. Coumadin use with atrial fibrillation decreases the CVA risk 75% vs 40% with ASA. Q. Young CVA patients—search for deficiencies of protein C and S and antithrombin III, and the presence of factor V Leiden, antiphospholipid antibody, systemic lupus erythematosus (SLE), syphilis, tuberculosis, DM, HTN, tobacco use, and increased cholesterol R. Lacunar CVA—from small-vessel disease by lipohyalinosis of middle cerebral artery (MCA) branches 1. Signs/Symptoms a. No cortical symptoms b. Most common manifestation is pure sensory loss. c. Less common syndromes are pure motor hemiparesis or ataxic hemiparesis. 2. Treatment—carotid endarterectomy does not help. S. Lateral medullary syndrome (Wallenberg syndrome) 1. Cause—vertebral or PICA occlusion 2. Signs/Symptoms—ipsilateral vertigo (CN VIII), facial numbness (CNV), dysphagia (CNs IX, X), Horner syndrome, cerebellar ataxia, loss of light touch in the ipsilateral body (posterior columns), and contralateral loss of pain/temperature (spinothalamic tract) T. Vertebrobasilar insufficiency 1. Cause—usually subclavian steal or vessel stenosis 2. Signs/Symptoms a. 5 Ds—diplopia, dysarthria, defect visual, dizzy, drop attack b. Bilateral motor, sensory, or visual symptoms 3. Treatment—decrease risk factors, medications (Coumadin or ASA), and surgery (endarterectomy, VA– ICA trans position, or bypass) U. ICA dissection 1. Signs/Symptoms—pain, oculosympathetic Horner syndrome (sweat fibers travel with the ECA), CN XII deficit, TIA (due to embolism or occlusion), and subarachnoid hemorrhage (SAH) 2. Evaluation a. Angiogram with string sign or double lumen (dissection occurs 2 cm distal to the CCA bifurcation) Fig. 9.4 Carotid artery dissection. (A) Angiograms demonstrate cervical internal carotid artery (ICA) and (B) petrous ICA tapered narrowings. (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 155, Fig. 200.) b. Associated with cystic medial necrosis, fibromuscular dysplasia, Ehlers-Danlos syndrome, Marfan syndrome, and syphilis (Fig. 9.4A,B) 3. Treatment a. Heparin 2 weeks followed by Coumadin 12 weeks b. If symptoms progress, consider direct surgical repair, ligation, stenting, or bypass (STA–MCA) c. If there is a large CVA or a complete deficit, use ASA and hold the Coumadin for 2 weeks. V. Vertebral dissection—usually occurs between C1 and 2 or at C6 (where the artery enters the foramen transversarium). 1. Signs /Symptoms a. Neck pain, CVA (lateral medullary syndrome), or SAH b. Extradural lesions usually present with stroke from embolism or vessel occlusion, whereas intradural lesions present with SAH. Fig. 9.5 Vertebral artery dissection. Angiogram demonstrates left distal vertebral artery narrowing. (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 156, Fig. 201.) 2. Treatment—heparin and Coumadin for 6 months (don’t anticoagulate in the presence of a large CVA or SAH) or trap the vessel proximal to posterior inferior cerebellar artery (PICA) if possible (open procedure or endovascular with a balloon) (Fig. 9.5) W. Venous sinus thrombosis 1. Causes—related to infection, dehydration, pregnancy, oral contraceptive pill (OCPs), and deficiency of proteins C, S, antithrombin III, and factor V Leiden 2. Evaluation—CT and magnetic resonance venography (MRV) 3. Treatment a. Address the primary disorder, increase hydration, and use heparin and Coumadin b. If symptoms are increasing, use t-PA or urokinase to lyse the clot c. Consider endovascular therapy from the femoral vein to the superior sagittal sinus with 25 mg t-PA as a 2 mg bolus at each end of the sinus; advance the catheter to the anterior sinus and infuse 1 mg/min × 19 minutes d. Anticoagulation is safe in the presence of scattered punctate hemorrhages from venous occlusive disease. Fig. 9.6 Moyamoya disease. Angiogram demonstrates distal internal carotid artery occlusion with prominent leptomeningeal collaterals (“puff of smoke”). (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 154, Fig. 199.) X. Moyamoya disease—bilateral distal ICA occlusion with rete mirabile (trans dural ECA anastomoses causing a puff-of-smoke appearance). 1. Signs/Symptoms—CVA in children; hemorrhage in adults 2. Evaluation—magnetic resonance imaging (MRI) and angiogram (there are frequently aneurysms) (Fig. 9.6) 3. Treatment a. Medical therapy is of unknown benefit. b. Surgical treatment—STA–MCA bypass, encephalomyosynangiosis (temporal muscle to brain), encephaloduroarteriosynangiosis (STA branches placed inside a dural opening), or an omental flap c. Encephaloduralsynangiosis i. Identify the STA and middle meningeal artery branches on the angiogram. ii. Palpate the STA and locate with a Doppler; incise around it with 15-blade knife. iii. Make two burr holes and connect with a craniotomy. iv. Open the dura where avascular and sew the STA adventitia to the pia with 10.0 Prolene v. Lay down the dura and bone vi. Make a few more burr holes II. INTRAPARENCHYMAL HEMORRHAGE A. Hypertensive hemorrhage—most common locations are putamen 60%, thalamus 20%, pons 10%, and cerebellum 5% (near the dentate nucleus). 1. Signs / Symptoms a. Headache, weakness, and dysphasia b. Pontine hemorrhages may produce pinpoint pupils and “locked-in syndrome.” c. Cerebellar hemorrhages present with contralateral eye deviation, focal weakness but not hemiplegia, and a rapid loss of consciousness. d. Most patients have a history of HTN and usually present to the emergency room (ER) with an SBP over 180 mm Hg. 2. Treatment a. Lower the blood pressure 30% with Nipride (overaggressive lowering to a “normal range” may lead to hypoperfusion CVA). b. Anticonvulsants—usually not needed c. Rehemorrhage—relatively rare d. Ventricular extension in up to 50% of cases—may require external ventricular drainage e. Surgery—not proven to improve functional outcome with putamen or thalamic clots, though mortality may be decreased. f. Surgical evacuation—for cerebellar clots > 4 cm or with hydrocephalus g. Functional outcome—best with volumes 10–30 mL B. Amyloid angiopathy 1. Usually occurs in older patients 2. Most common cause of lobar hemorrhage in normotensive elderly patients 3. Clot typically extends from the centrum semiovale to the subarachnoid space. 4. Cause of the hemorrhage is replacement of the contractile elements in the media of the arteries and arterioles of the leptomeningeal and superficial cortical vessels with noncontractile amyloid that is yellow-green with dichromism (birefringence) when stained with Congo red dye and viewed under polarized light. C. Iatrogenic 1. Hemorrhage rate in CVA patients treated with t-PA is 6%; in those with MI treated with t-PA it is 2%. 2. Risk of cerebral hemorrhage with Coumadin therapy for atrial fibrillation is 0.3%/year. 3. Delay for 1 week anticoagulation after intraparenchymal hemorrhage in patients with mechanical heart valves D. Evaluation—consider evaluation of an intraparenchymal hemorrhage with an angiogram (to rule out vascular lesions) in patients <45 years and with no history of HTN E. Multiple intraparenchymal hemorrhages in young patients 1. Causes—trauma, drug abuse (cocaine), vasculitis systemic lupus erythematosus, Wegener granulomatosis, polyarteritis nodosa, infection, or coagulopathy F. Multiple intraparenchymal hemorrhage in older patients 1. Causes—amyloid angiopathy, embolic CVA, vasculitis, syphilis, or metastatic tumor III. CEREBRAL ANEURYSMS A. Causes of spontaneous SAH 1. Aneurysm 80% and vascular malformation 10% 2. By far, the most common cause of SAH overall is trauma. C. Rupture rate—the rupture rate of unruptured aneurysms < 10 mm is 0.05%/y and >10 mm is 1%/year. However, these numbers may be misleading because the most common rupture size is 6–9 mm. 1. Morbidity and mortality per rupture—5% in patients < 30 years and 30% in patients > 60 years 2. The surgical mortality/morbidity for clipping is around 5%. 3. It is probably worthwhile to scan first-degree relatives of aneurysm patients with an MRA given that 20% of patients have a family member with at least one. D. Rerupture rate of ruptured aneurysms 1. 4% in 24 hours, 20% in 2 weeks, 50% in 6 months, and then 3%/y 2. Rerupture rate for a “dog ear” incompletely clipped aneurysm is 0.4%/y E. Outcome—40% mortality, 30% disabled, 30% normal, 10% die at home F. Hunt and Hess grade 0—unruptured aneurysm 1—asymptomatic or mild headache and slight nuchal rigidity 1a—no acute meningeal/brain reaction, but with fixed neuro deficit 2—cranial nerve palsy, moderate to severe headache, nuchal rigidity 3—mild focal deficit, lethargy, or confusion 4—stupor, moderate to severe hemiparesis, early decerebrate 5—deep coma, decerebrate posturing, moribund appearance. Add one grade for serious systemic disease (HTN, diabetes mellitus [DM], etc.) or severe vasospasm on angiogram. G. Evaluation 1. History—sudden onset of worst headache in life 2. CT, lumbar puncture (LP) (if CT negative) 3. Four-vessel angiogram—20% are multiple 4. MRI/MRA—only if angiogram equivocal 5. CT angiography—gaining popularity 6. Ocular examination—may reveal subhyaloid preretinal, retinal, or vitreous hemorrhage (Terson syndrome, due to elevated intracranial pressure (ICP) and venous pressure, usually resolves in 6 months, may need vitrectomy). 7. Alcock test—vertebral angiogram with carotid compression to test competency of the circle of Willis. 8. Determine aneurysm size—compare with the diameter of the ICA (6 mm) and MCA (4 mm). H. Fisher grade (for vasospasm, per CT) 1 = no subarachnoid blood 2 = diffuse blood or vertical layers <1 mm thick 3 = localized clot and/or vertical layer ≥ 1 mm thick 4 = intracerebral or intraventricular clot with diffuse or no SAH Grades 1, 2, and 4 patients usually do not develop vasospasm. Grade 3 patients frequently develop vasospasm. I. Treatments 1. Nipride to keep SBP 120–130 mm Hg to prevent rehemorrhage prior to surgery 2. Studies suggest that surgery should be performed the same day or the following morning. 3. Gold standard treatment—surgical clipping 4. Endovascular coiling a. Growing in popularity, but as of now, >20% need retreatment and the morbidity/mortality is > 5% 5. Muslin wrapping—not proven to be of benefit 6. Trapping with or without bypass may be needed for unclippable aneurysms. 7. During surgery a. Achieve brain relaxation with mannitol (1 g/kg bolus), Lasix, hyperventilation, and ventricular drainage (or lumbar drainage). b. Maintain normotension (but use mild HTN during temporary clipping) and mild hypothermia (to 32–34°C with passive drift). 8. Temporary clipping time > 5 minutes—use Pentothal (4 mg/kg bolus and 2 mg/kg/h titrated to burst suppression if available), propofol (170 μg/kg/min), or etomidate 9. Deep hypothermia to 18°C permits 1 hour of circulatory arrest. 10. Intraop angiography—useful when available to determine if clip placement is adequate 11. Asymptomatic aneurysms > 10 mm should probably be treated unless there are serious medical issues (advanced age, etc.). J. Specific aneurysms 1. Cavernous ICA aneurysm a. May not require surgical intervention because hemorrhage is not intradural and the tendency to rupture is low b. Signs/Symptoms—may be caused by mass effect or carotid– cavernous fistula (CCF) formation after rupture c. Exposure via pterional approach with proximal control by cervical carotid exposure (or endovascular approach) 2. Carotid cave segment ICA aneurysm a. Exposure via pterional approach with proximal control by cervical carotid exposure b. Drilling of the anterior clinoid is usually necessary. 3. Ophthalmic artery aneurysm a. Exposure via pterional approach with proximal control by cervical carotid exposure (perform first) b. Aneurysm usually lies directly under the optic nerve and points up from the ICA. c. May be safe to clip the parent vessel (ophthalmic artery) if necessary d. Anterior clinoid process may need to be drilled to the optic strut, the optic strut removed, and the falciform ligament cut. e. If bilateral, both can be approached from one side. f. Superior hypophyseal artery aneurysms are just distal to the ophthalmic artery, are approached the same way, and usually point down. 4. Posterior communicating (PCOM) artery aneurysm a. Signs/Symptoms—presentation may be with a dilated pupil with spared ocular movements (Figs. 9.7, 9.8, and 9.9). Fig. 9.7 Posterior communicating artery aneurysm; lateral angiogram. (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 164, Fig. 209B.) Fig. 9.8 Pterional craniotomy incision. (With permission from Citow JS. Neurosurgery Oral Board Review. 1st ed. New York, NY: Thieme Medical Publishers; 2003: 102, Fig. 10.8.) b. Exposure via pterional approach c. Aneurysm usually points laterally. d. Sundt encircling clip should be available in case of tearing of the aneurysm from the ICA. e. Avoid retracting the temporal lobe until near the end of the exposure because this may precipitate hemorrhage Fig. 9.9 A,B Posterior communicating (PCom) artery aneurysm exposure. A, optic nerve; B, PCom; C, superior hypophyseal artery; D, anterior cerebral artery; E, anterior choroidal artery; F, middle cerebral artery. (With permission from Citow JS. Comprehensive Neurosurgical Board Review. New York, NY: Thieme Medical Publishers; 2000: 436, Fig. 5–1.) 5. Anterior choroidal artery aneurysm—exposure via pterional approach 6. ICA bifurcation aneurysm—exposure via pterional approach 7. Middle cerebral artery aneurysm—exposure via pterional approach working from proximal to distal or by splitting the sylvian fissure and working distal to proximal 8. Anterior communicating artery aneurysm—exposure via pterional approach with resection of the gyrus rectus 9. Distal anterior cerebral artery aneurysm—exposure via pterional approach if proximal at the orbitofrontal artery, but usually it requires an interhemispheric approach 10. Posterior cerebral artery (PCA) aneurysm a. Exposure via subtemporal approach b. Subtemporal craniotomy—performed by making a horseshoe incision around the superior pinna c. Temporal lobe is retracted superiorly to expose the tentorial edge. d. Tentorium should be divided anterior to the trochlear nerve for basilar artery aneurysms and posterior for PCA aneurysms. e. PCA is identified and followed to the aneurysm (Fig. 9.10). Fig. 9.10 Subtemporal craniotomy incision. (With permission from Citow JS. Neurosurgery Oral Board Review. 1st ed. New York, NY: Thieme Medical Publishers; 2003: 104, Fig. 10.10.) 11. Basilar artery apex aneurysm a. Exposure is via pterional approach (used by Yasargil), sub-temporal approach (used by Drake), or half-and-half approach (used by Heros). b. Frontotemporal-orbito-zygomatic exposure can be added to the pterional approach to improve the exposure. c. Pterional approach usually uses avenue between ICA and CN III either anterior or posterior to PCOM d. Path between optic nerve and ICA is seldom used, although it might be considered for higher aneurysms. e. Aneurysm > 5 mm below the posterior clinoid—consider a path between CNs III and IV or V and VI. f. Subtemporal approach may be preferred if aneurysm is more than a few millimeters below the posterior clinoid process and pointing posteriorly. g. Liliequist membrane is between interpeduncular and chiasmatic cisterns. h. Endovascular coiling is a reasonable option, though the recurrence rate is 20%. i. Giant basilar arteries may be treated with clipping during circulatory arrest, Hunterian ligation, or endovascular coiling (though the mass effect is not reduced). 12. Midbasilar artery (AICA) aneurysms a. Exposure via transpetrosal approach in lateral position with presigmoid supra- and infratentorial exposure with division of superior petrosal sinus. b. Anterior petrosectomy exposes 5–15 mm below the posterior clinoid process. c. Approach on the side contralateral to the aneurysm 13. Vertebrobasilar junction aneurysms a. Exposure via a presigmoid approach with posterior petrosectomy 14. Posterior inferior cerebellar artery (PICA) aneurysms—exposure is via a suboccipital retrosigmoid craniotomy possibly with a C1 hemilaminectomy. 15. Giant aneurysms (> 2.5 cm) a. Signs/Symptoms—by mass effect or emboli b. Treatment—surgical clipping, trapping, or Hunterian proximal ligation possibly with bypass c. Prior to placing a Selverstone clamp, test collateral flow with balloon occlusion and Alcock test. 16. Traumatic aneurysms a. Cause—usually due to penetrating trauma involving the interhemispheric or sylvian fissure b. Treatment—open trapping or balloon occlusion at the skull base 17. Mycotic aneurysms a. Cause—most often due to streptococcus associated with bacterial endocarditis b. Treatment—6 weeks of antibiotics i. If aneurysm still present, surgical clipping/trapping is needed ii. Follow patient with angiograms at 1, 3, 6, and 12 months iii. Avoid anticoagulation K. Complications 1. Vasospasm a. Occurs from days 4 to 14 (peak at day 7) as blood breakdown products irritate vessels near SAH b. Risk is assessed by Fisher grade c. Evaluation—transcranial Doppler (>200 indicates spasm), cerebral blood flow studies, and angiography. d. Prevention—nimodipine 60 mg SL every 4 hours (q4h) i. Some studies suggest using intraventricular t-PA 1 mg/d until the blood is dissolved. e. Treatment—triple-H therapy: hypervolemia (central venous pressure [CVP] 12 and wedge 20), hypertension, and hemo-dilution (if hematocrit [HCT] < 30%, O2 delivery impaired) i. Maintain blood pressure at lowest level where the deficit disappears ii. Refractory cases may be treated with intraarterial papaverine or angioplasty. 2. Hydrocephalus (HCP) a. Many surgeons insert a ventricular catheter at the time of surgical clipping to minimize brain retraction and to monitor ICP after surgery to determine if a shunt will be needed. b. If catheter must be inserted before surgery for massive HCP, it should be set to drain at 15 cm of water to prevent detamponading of the aneurysm. c. 30% of patients with aneurysmal SAH will require a shunt. 3. Hyponatremia—usually due to cerebral salt wasting (CSW) by atrial natriuretic factor (patients are hypovolemic) vs syndrome of inappropriate antidiuretic hormone (SIADH; normo- or hypervolemic) a. Treatment for CSW—fluid replacement with normal saline b. Treatment for SIADH—fluid restriction 4. Postop focal deficit—evaluate with angiography (to check for parent vessel occlusion) and CT (to search for clot) and then possibly surgery for clip adjustment L. Benign perimesencephalic hemorrhage—SAH in prepontine or perimesencephalic cisterns possibly due to rupture of a small vein 1. Evaluation—CT, angiogram, MRA a. Consider repeat angiogram in 10 days if blood not at the perimesencephalic cistern or anterior brainstem. Fig. 9.11 Benign perimesencephalic hemorrhage. Computed tomography demonstrates typical subarachnoid hemorrhage extending into the left ambient cistern (angiogram was normal). (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 171, Fig. 225.) b. Also consider cervical spine MRI to rule out arteriovenous malformation (AVM). 2. Seldom recurs (Fig. 9.11) IV. VASCULAR MALFORMATIONS A. AVM hemorrhage 1. Mortality—1%/y and 10%/bleed 2. Morbidity—2.5%/y and 40%/bleed 3. Initial hemorrhage rate—4%/y 4. Rehemorrhage rate—6%/y × 6 months then 3%/y B. Spetzler—Martin grading scale Size—<3 cm (1), 3–6 cm (2), >6 cm (3) Eloquent location—noneloquent (0), eloquent (1) Venous drainage—superficial (0), deep (1) Total—1 and 2 (95% good outcome), 3 (84%), 4 (73%), 5 (69%) 1. Surgery a. Identify and ligate feeding arteries with care taken to avoid sacrificing vessels just passing through. b. Avoid entering the malformation during dissection and ligate the draining veins as the last step. c. Perform surgery 1 week after embolization. d. To decrease the likelihood of normal perfusion pressure breakthrough, which can cause postop edema and hemorrhage, try 3 days of preop treatment with propranolol 20 mg orally daily (PO q.i.d.) e. Surgical risks i. Consider Spetzler-Martin grading scale ii. Aneurysms (feeding, draining, or intranidal) iii. High- or low-flow AVMs iv. Recent hemorrhage—may make the dissection plane more obvious v. 10% of AVMs have aneurysms. vi. Higher risk of hemorrhage with intranidal aneurysms and venous stenosis (Fig. 9.12A–C) 2. Radiation a. Stereotactic radiosurgery—proven useful for lesions <3 cm b. Complete obliteration rate—80% (only 50% for larger lesions) c. 18-month delay before vessels become occluded and hemorrhage risk decreases d. Risk of increased neurologic deficit from therapy is 3%. e. Incomplete obliteration—radiosurgery can be repeated. f. Consider for deep lesions or around eloquent cortex g. Radiosurgery best performed 30 days after embolization Fig. 9.12 Arteriovenous malformation (AVM). (A) Axial T1-weighted magnetic resonance images of noninfused and (B) infused AVM demonstrating serpentine vessels. (C) Angiogram demonstrating early-draining veins and AVM nidus. (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 172, Fig. 228.) 3. Endovascular embolization a. Only useful as an adjuvant to other therapies b. Risk of complications (i.e., CVA)—5% c. Most useful for dural arteriovenous fistula (AVF), CCF, or vein of Galen malformation D. Angiographically occult AVM (cryptic)—due to either a small feeding vessel (beyond the resolution of angiography) or a vessel compressed by a hemorrhage 1. Not visualized on angiogram 2. Frequently associated with seizures 3. 50% are multiple. 4. Hemorrhage—risk is as low as 0.07%/y. 5. Treatment a. Surgery if symptomatic b. Radiosurgery not shown to be of benefit c. Brainstem cavernous malformations have higher risk of hemorrhage and should be resected if there have been two or more hemorrhages (though some surgeons suggest resection after only one). d. Be sure to open brainstem where malformation is closest to the surface. e. An approach superior and lateral to the facial colliculus has been used. F. Dural AVM—actually an AVF because there is no nidus 1. May arise from carotid or vertebral circulation (especially occipital artery) and drain into dural sinuses (usually the transverse—sigmoid junction). 2. Believed to be acquired by collateral revascularization after sinus thrombosis. 3. Signs/Symptoms—pulsatile tinnitus, bruits, headaches 4. Hemorrhage—risk is 4%/y. 5. Most behave benignly, but hemorrhage risk increases with cortical drainage or lesions in tentorial apex (as high as 80%) or anterior fossa (70%). 6. Treatment a. Initially—endovascular embolization of the fistulous connection Fig. 9.13 Dural arteriovenous malformation (AVM). (A) Anteroposterior and (B) lateral external carotid artery (ECA) angiograms demonstrate supply from the ECA with early filling into the sigmoid sinus. (With permission from Citow JS. Neuropathology and Neuroradiology: A Review. New York, NY: Thieme Medical Publishers; 2001: 175, Fig. 232.) b. Blockage of arterial feeders has relatively low success rate because there is frequently vessel recruitment i. If this fails, consider surgery to skeletonize the sinus (Fig. 9.13A,B). G. Carotid-Cavernous Fistula 1. May either be posttraumatic or spontaneous 2. Connection may be from ICA to cavernous sinus (high flow) or meningeal branches of ICA or ECA to cavernous sinus (low flow) 3. Signs/Symptoms—chemosis, proptosis, bruit, eye pain, visual loss, ophthalmoplegia 4. 50% of low-flow lesions spontaneously thrombose. 5. Low-flow lesions may be observed if vision is normal and intraocular pressure <25 (though frequent visual exams are needed). 6. Treatment a. Initially—balloon occlusion Fig. 9.14 Carotid-cavernous fistula. (A) Lateral angiograms of the common carotid artery demonstrating early filling of the cavernous sinus with dilated superior and inferior ophthalmic veins and (B) of the external carotid artery (ECA) demonstrating filling from the ECA. (With permission from Citow JS. Comprehensive Neurosurgical Board Review. New York, NY: Thieme Medical Publishers; 2000: 441, Fig. 5–2.) i. Through ICA to fistula ii. Two balloons to trap the ICA iii. Or cavernous sinus occlusion via superior ophthalmic vein. b. Before trapping the ICA, test tolerance with a balloon occlusion under controlled hypotension to SBP 80 mm Hg and evaluate for symptoms or evaluate with SPECT scan to determine ischemia. c. If patient cannot tolerate trapping, perform ECA–ICA bypass (Fig. 9.14A,B) V. NEONATAL HEMORRHAGE A. Germinal matrix hemorrhage 1. Located in subependymal region and may extend into ventricle 2. Due to impaired autoregulation before 34 weeks and found in 40% of children born before 35 weeks 3. Frequency increases with asphyxia due to HTN and hypercapnia. 5. 75% of grade II hemorrhages have normal IQ. 6. Full-term children usually develop intraventricular hemorrhages. B. Evaluation—ultrasound, occipital-frontal circumference (OFC; 35% develop hydrocephalus), cerebrospinal fluid (CSF; protein < 100 may resolve spontaneously) C. Treatment 1. Acetazolamide 2. Daily removal of CSF with ventricular taps (<800 g) 3. Serial LPs (> 800 g) 4. Ventricular access device (>1100 g) 5. Amount of CSF to remove is 10 mL/kg/tap or until the manometer lowers to 3 cm water (tap before it exceeds 10 cm water) 6. Consider VP shunt if > 2500 g (earlier may cause necrotizing enterocolitis) Helpful Hints
9: CEREBROVASCULAR DISEASE
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree