Chapter 8 Anatomy and Pathophysiology of Acquired Spinal Disorders
Degenerative Disc Disease and Spondylosis
Degenerative disc disease (with its characteristic clinical syndromes of disc herniation, spondylosis, and radiculopathy) is associated with vascular, biochemical, and anatomic changes in the disc. There is a consistent anatomic pattern of disc degeneration in the spine, with most changes occurring in the midcervical, thoracolumbar, and lower lumbar regions. This pattern is thought to reflect the distribution of the mechanical stresses caused by spine movement and loading, as well as those due to erect posture.1
The intervertebral disc consists of three components: (1) the nucleus pulposus; (2) the annulus fibrosus, which surrounds the nucleus pulposus; and (3) the cartilaginous end plates, which attach these structures to the rostral and caudal vertebrae of the motion segment. The annulus is formed by a series of lamellae that have high collagen content and thereby provide significant resistance to tensile forces. The ventral annulus is usually wider and more organized than the dorsal annulus, with which discontinuous lamellae may be present.2 The nucleus pulposus, derived embyologically from the primitive notochord,3 has a much higher proteoglycan and water content than the annulus fibrosus. The hyaline cartilage end plates are similar in collagen type to the inner annulus fibrosus and the nucleus pulposus.4
The disc acts as a deformable, fluid-like material, whose tendency to bulge is resisted by the tensile stress in the annular lamellae and the end plates. Therefore, a substantial intradiscal surface strength is required to resist a high circumferential annular stress and thus prevent excessive disc deformation (bulging). When disruption of the nucleus pulposus and annulus fibrosus reduces intradiscal pressure, bulging occurs.5
The disc receives its nutrients through small vessels in the cartilage end plates and from the periphery of the annulus.6 With aging, however, the end plates calcify, and vessel loss occurs, until nearly the entire disc becomes avascular.7 The loss of vasculature promotes increased anaerobic metabolism, increasing lactic acid production and cellular necrosis. The water content of the annulus fibrosus decreases from 78% at birth to 70% by the fourth decade, and the nucleus pulposus water content decreases from 90% to less than 70% with maturation.8,9 With this change in vascularity, and with water loss in the region of the inner annulus and nucleus pulposus, there is a relative increase in fibrocytes and chondrocytes, which are more tolerant of a low-pH environment.3
Before the age of 2 years, the nucleus pulposus is translucent and anatomically different from the annulus fibrosus.10 By the second decade, the inner annulus and nucleus grow increasingly fibrous and lose both height and proteoglycans.9 In the third decade, nuclear fragmentation and fibrosis appear. Progressive myxomatous degeneration, swelling, and fissure formation occur in the annulus by the fourth decade.11,12 Eventually the nucleus pulposus may become disorganized, dehydrated, and fragmented with circumferential and radial tears.10,11,13 Grading systems for these patterns of disc degeneration, using plain radiographs or MRI studies, have been published.10,14
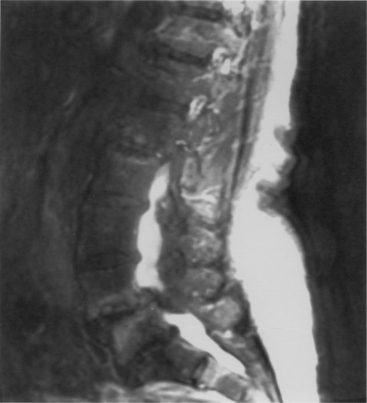
On plain radiographs, the degenerative disc changes range from grade I to grade IV. Grade I represents a normal disc. Grade II demonstrates sclerosis along with disc space narrowing or osteophyte formation. Grade III shows moderate sclerosis, and grade IV is associated with severe sclerosis with disc space narrowing or osteophyte formation.14 Yu et al.10 classified changes in the disc, with reference to the age of the subject and to the stage of degeneration, by comparing the anatomic characteristics with the appropriate MRI findings in cadaveric dissections (Fig. 8-1 and Table 8-1). The primitive notochord is present up to age 10. In the second decade of life, a distinct fibrous band forms in the nucleus and disc height diminishes. In the third decade fragmentation and fibrosis of the nucleus occurs. By the fourth decade there is swelling, separation, and myxomatous degeneration of the annular lamellae with fissure formation.12
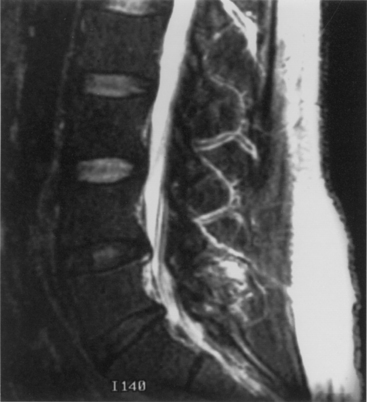
FIGURE 8-1 T2-weighted MRI of lumbar spine demonstrating disc desiccation and herniation at the lower levels.
TABLE 8-1 Classification of Lumbar Discs
| Type of Disc | Anatomic Characteristics | MRI Features |
|---|---|---|
| Immature | Nucleus pulposus and annulus fibrosus differentiated, primitive notochord may be present | High-signal intensity from nucleus and annulus |
| Transitional | Fibrous tissue in equator of annulus | High-signal intensity from nucleus and annulus, low-signal intensity in ventral and/or dorsal region of nucleus pulposus, corresponding to dense fibrous tissue |
| Adult | Annulus and nucleus not differentiated, annulus intact or marked by small concentric or transverse tears | Moderately high-signal intensity from nucleus and annulus, low-signal intensity from Sharpey fibers and fibrous tissue in midportion of disc |
| Early degenerated | Radial tear of annulus, diminishing amount and discoloration of fibrocartilage in nucleus | Diminishing signal intensity from nucleus pulposus, low signal from Sharpey fibers disrupted by region of higher-signal intensity at location of annular tear, slightly diminished disc height |
| Severely degenerated | Replacement of nuclear and annular fibrocartilage with amorphous fiber and cysts | Severely reduced disc height, low (fibrous tissue) or high (fluid) signal intensity from intervertebral disc |
From Yu S, Haughton VM, Sether LA, et al: Criteria for classifying normal and degenerated lumbar intervertebral disks. Radiology 170:523–526, 1989, with permission.
One of the most common disc-related clinical syndromes is a herniated disc with sciatica. With degeneration, fissure formation occurs in a radial distribution. It is likely that the biomechanical cause of disc herniation is related to a combination of complex movements involving compression, lateral flexion, and/or rotation.15–18 With flexion, the nucleus pulposus moves dorsally. The dorsal annulus has fewer and more disorganized lamellae and may be inherently weaker than the thicker ventral annulus. Degeneration of the annulus results in the development of peripheral, circumferential, and, subsequently, radial tears. With complex stresses applied to the dorsally migrating nucleus, herniation may occur along a radial tear.
Disc herniation can cause associated nerve-root impingement. The typical dorsolateral herniated disc affects the nerve root passing to the next lower foramen, but a more laterally herniated disc can affect the nerve root above. Masaryk et al.19 used MRI findings to classify the stages of disc herniation. A bulging disc has an MRI signal similar to the rest of the disc, but the bulge is beyond the adjacent vertebral margins. A prolapsed or protruding disc has nearly breached the outer annular fibers and is barely contained. The disc remains contiguous with the rest of the nucleus pulposus by a pedicle that has a high signal on T2-weighted MRI. The disc is extruded when it completely breaches the outer annular fibers and the posterior longitudinal ligament, but remains in continuity with the disc proper. If the fragment is no longer in continuity with the main part of the disc, it is termed a sequestered, or “free,” disc fragment. The International Society for the Study of the Lumbar Spine20 classified the disc as either contained or noncontained, with the latter group including extruded and sequestered discs. Free fragments may migrate in a rostral or caudal direction. It appears that far-lateral herniated discs are more likely to migrate in a rostral direction, thus affecting the nerve root above the disc space.21
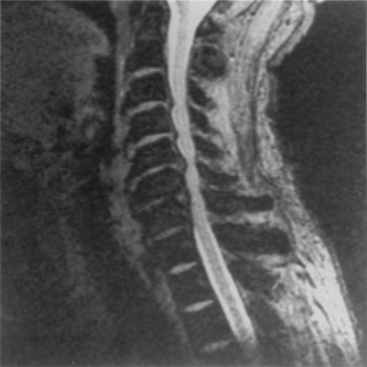
Disc degeneration without herniation may also lead to changes affecting the biomechanical function and stability of both the intervertebral and articular facet joints. Although opinions differ regarding whether facet or disc degeneration is the initial event that causes spondylosis, the three-joint intervertebral-motion–segment concept emphasizes that disease in each component affects the others. This is to say that unilateral or bilateral facet disease or disc degeneration may lead to progressive changes in the other segmental units. Adjacent bone changes are associated with cartilaginous degeneration in these three joints. Spurs and osteophytes form at the site of peripheral annular attachment to the end plates. These osteophytes are thought to be formed in regions of excessive motion. Kirkaldy-Willis22 incorporated this concept into a theory regarding the natural history of spinal degeneration. He believed that facet and disc disease occurred with progressive reciprocal dysfunction. This resulted in ligamentous laxity around the facet joint and increased stresses that lead to internal disc disruption. This condition causes subluxation, disc resorption, and, finally, paradiscal osteophyte formation. Enlargement of the facets also occurs as a result of osteophyte formation. These changes may contribute to lumbar stenosis (Fig. 8-2) or a lateral recess syndrome.23–25
Patients with significant lumbar spinal canal narrowing resulting in stenosis complain primarily of pain, weakness, and leg numbness while walking. This pain can be relieved when the patient flexes the spine by sitting, by leaning forward while walking (shopping cart sign), or by leaning against counters. The symptomatic improvement associated with these maneuvers is related to an increase in lateral recess and spinal canal dimensions. Flexion results in stretching of the protruding ligamentum flavum and posterior longitudinal ligament, as well as reduction of overriding laminae and facets.26 This small amount of change in the circumferential spinal canal, lateral recess, and foraminal region alleviates the pressure on the nerve roots and subsequently relieves the symptoms. Returning to the erect posture leads to repeated compression and a further exacerbation of symptoms. During ambulation, some patients experience the onset of symptoms because of an increased metabolic demand in nerve roots that have become ischemic as a result of stenotic compression. Such “neurogenic claudication” is relieved when the subject sits. Often, bicycling (which is associated with flexion of the lumbar spine) is well tolerated.
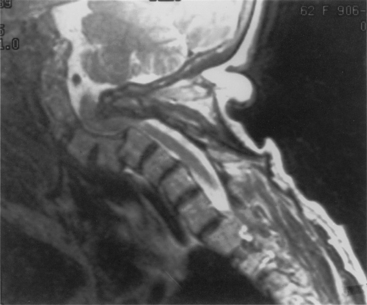
Aging discs in the cervical spine cause characteristic spine alterations that may lead to cervical myelopathy or radicular pain and deficit. In young subjects, the cervical spine assumes a lordotic posture. This results in a greater ventral height of the annulus, compared with the dorsal annular height. With aging, however, intradiscal water loss and disc narrowing occur, thus leading to progressive spine straightening. In young patients, the range of intervertebral motion is greatest at C5-6 and C6-7. Narrowing and degeneration with osteophyte formation is most marked at these levels (Fig. 8-3). With these changes there is progressively less movement. In patients older than age 60, motion at C3-4 and C4-5 increases. Increased degenerative instability in older patients, therefore, is associated with translational subluxation, especially retrolisthesis at C3-4 and C4-5.27 In this scenario the spinal cord of the patient with cervical spondylotic myelopathy may not only be compressed by osteophytes, but may also suffer repeated injuries secondary to intervertebral hypermobility or instability. Dynamic flexion-extension radiographs are necessary to diagnose degenerative spondylolisthesis since static films in neutral position do not demonstrate subluxation, if present. A treatment protocol that does not take these factors into account may be associated with less than optimal success.
Rheumatoid Arthritis of the Spine
Rheumatoid arthritis (RA) affects both the spine and the peripheral joints. It has a prevalence of approximately 1%, with the greatest incidence in the fourth through sixth decades.28 RA is a disease of the synovial joints. The earliest change in the joints is synovitis, followed by an acute inflammatory response as a result of antibody-antigen complex formation. These processes activate the complement cascade and generate biologically active substances, ultimately resulting in complete destruction of the joint. This acute process is followed by a chronic granulomatous process, or pannus formation, which produces collagenase and other enzymes that destroy surrounding cartilage and bone.29 This may lead to instability because of ligamentous incompetence.28,30,31
Considerable controversy regarding the pathogenesis of cervical spine rheumatoid joint disease revolves around whether the initial site of involvement is (1) the apophyseal joint, with resultant facet destruction and progressive secondary instability of the intervertebral disc, or (2) inflammation in the uncovertebral joint, which leads to primary disc destruction with secondary degenerative involvement of the apophyseal joints. Martel32 examined 20 RA patients and found instability associated with apophyseal joint involvement. This leads to vertebral end plate destruction, disc space narrowing, and erosion. At autopsy, the discs showed evidence of necrosis and degeneration, with minimal inflammation. Martel proposed that apophyseal changes caused the instability with secondary disc destruction and end plate microfractures. The relative infrequency of cervical spine disease in juvenile-onset RA was explained by the early bony ankylosis of the apophyseal joints observed in these subjects.
Ball33 reviewed the pathology of 14 RA patients with no radiologic evidence of cervical disease and found that the earliest histologic lesions were in the uncovertebral joints. He suggested that the disc and adjacent bone are then secondarily involved with resultant inflammatory destruction and progressive instability. The fact that uncovertebral joints are not completely developed in the first two decades of life34 might also explain the infrequency with which cervical rheumatoid disease is seen in juvenile-onset RA.35
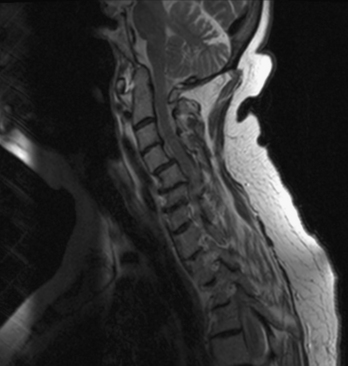
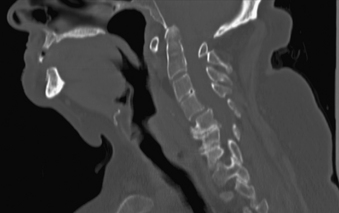
Cervical spine disease is observed in as many as 88% of patients with RA.36 The manifestations include C1-2 instability, occipitocervical (OC) instability (with or without vertical displacement of the dens), and subaxial cervical RA. C1-2 instability is the most common form of cervical rheumatoid involvement and may occur in up to 74% of the patients.37 The dens is surrounded by two synovial joints, one ventrally, between the atlas and dens, and another between the transverse ligament and the dens. With involvement of the synovial joints there is progressive inflammation, destruction, and subsequent transverse ligament laxity, with destruction of the osseous attachments of the ligamentous complex. This loss of ligamentous integrity allows C1 to move ventrally on C2. If there is further significant disruption and osteomalacia of the dens itself, then dorsal C1-2 subluxation can also occur.38 If the synovial apophyseal joints between C1-2 are involved as well, lateral rotation may also be evident in addition to subluxation at C1-2. OC instability results from involvement of the atlanto-occipital articulations. With significant articular facet destruction, there is progressive collapse of the occiput at C1 and vertical displacement of the residual dens (Figs. 8-4 and 8-5). This has also been termed atlantoaxial impaction, vertical subluxation, cranial settling, and basilar invagination.38 Vertical displacement of the dens occurs in 5% to 32% of RA patients.29,39,40 It is believed that vertical displacement of the dens represents a more advanced stage of systemic disease burden; indeed, one 10-year retrospective review of patients with RA cervical instability treated with OC fusion noted significantly worse long-term outcomes in the subset of patients with vertical displacement of the dens.41
In the subaxial region, the levels most commonly involved with rheumatoid synovitis are C2-3 and C3-4. Subluxation (Fig. 8-6) may occur in approximately 7% to 29% of the patients with RA.38 Subaxial region subluxation rates as high as 31% have been noted after rostral surgical fusion; however, there was no increased incidence of myelopathy or pain with fusion-adjacent subluxations.41 These “staircase” subluxations are thought to be caused by significant ligamentous laxity and facet degeneration.36,42 At any of the various sites of rheumatoid involvement, osseous erosion of adjacent bone, caused by osteoclastic resorption, occurs frequently.43
With the significant bony destruction, ligamentous laxity, and the potential for neural compression observed in the rheumatoid cervical spine, the primary emphasis of treatment is reduction of subluxation and fusion/fixation to prevent spinal cord injury. The optimal time to proceed with operative intervention is yet to be determined. Omura et al. stratified their RA population and found that the subset of patients with seropositive disease and systemic evidence of mutilating-type joint involvement are at the highest risk of deterioration of their known cervical lesion.44 Furthermore, retrospective review of RA patients with cervical disease found that best-medical management faired significantly worse when compared with surgical fusion with respect to both morbidity and mortality.44 When substratifying the patient population undergoing surgical fusion, patients operated on earlier in their course and with a better functional preoperative score had a more pronounced overall improvement than those undergoing late surgical management. There is strong evidence for early operative intervention for the stabilization of RA-associated cervical disease.44,45
Surgical fusion yields multiple benefits, including reduction of both pain and neurologic sequelae; retrospective analysis of long-construct dorsal fusion demonstrates significant recovery of these two characteristics, compared with best-medical management, with improvement of an average of one to two grades on the Ranawat scales for pain and neurologic symptoms (Boxes 8-1 and 8-2).41,44,45 These improvements were persistent, even in the setting of failed permanent postoperative reduction of deformity and imbalance.41 The chronic granulomatous pannus decreases in size with the elimination of abnormal movement after successful arthrodesis.46,47 There is no consensus regarding the optimal type of intervention, but one must keep in mind the inherent poor quality of RA bone, the laxity of ligaments, the insidious inflammatory nature of RA itself, and the destructive effects of the myriad pharmacologic interventions, especially with respect to treatment with corticosteroids.45
Scheurmann Disease (Juvenile Kyphosis)
Scheuermann48 first described the progressive dorsal kyphosis of adolescent children in 1920. The deformity is usually evident as a fixed thoracic kyphosis that does not correct with hyperextension, thereby differentiating it from a postural kyphosis. Compensatory hyperlordosis of the lumbar and cervical spine may also be present. A mild scoliosis is noted in 20% to 30% of patients.49 Sorenson50 described the characteristic feature of ventral wedging of 5 degrees or more in at least three adjacent vertebrae. Other characteristics include kyphosis of greater than 40 degrees, vertebral end plate irregularity, and disc space narrowing.51 The prevalence of the disease ranges from 0.4% to 8%.50 It occurs predominantly in males (91% in one series).52 Hereditary patterns of transmission have been identified, though genetic loci have yet to be determined.53
Basic biomechanical factors and forces may play a role in this disorder. The thoracic spine has a natural kyphosis determined primarily by the shape of the vertebrae; in the adolescent thoracic spine 20 to 40 degrees of kyphosis is normal. The dorsal elements, including the ligamentum flavum and the laminae, resist forward flexion of the spine in tension, whereas the ventral bony elements (vertebral bodies) and disc resist compression.54 However, the facet joint capsules in the thoracic region are mechanically “weaker” than those in the lumbar region, so that any factor that increases the torque of the spine can result in greater deformity. The more marked the initial angulation of the spine, the larger the load (subject’s weight), and the longer the duration of load application, the greater the likelihood of the progression of the deformity.
Scheuermann disease must be differentiated from juvenile postural kyphosis, which, as the name attests, is a kyphosis seen during flexion that will correct with improved posture and extension. The apex of the curve is smooth. The condition will improve with therapy that targets improved posture and core strengthening.53
The pathogenesis of the disease remains unclear. Scheuermann believed that aseptic necrosis of the ring apophyses caused interruption of growth, which resulted in ventral vertebral body wedging.55 Subsequent work has refuted this theory by demonstrating that the apophyses do not contribute to longitudinal growth. Such growth is now known to result from endochondral ossification of the end plates.55 Schmorl56 felt that damage to the end plate by herniated disc material was of importance. Schmorl nodes are, however, not limited to the kyphotic region of the spine and are common in otherwise normal patients. It has been postulated that osteoporosis is involved,57,58 but recent investigations have found no differences in the trabecular bone density between patients with Scheuermann disease and controls matched for age, gender, and race.52,59 Other factors such as inflammation,60 hormonal influences,61 genetic factors,62 altered calcium metabolism,63 hypovitaminosis,64 neuromuscular disorders,65 extradural cyst formation,66,67 defective collagen formation of the end plate,68 and a decrease in the collagen-proteoglycan ratio of the end plate61 have been implicated, but their roles in the development of the disease have not been substantiated.
There is a high association (>90%) between ventral osseous extensions from the anterior margin of the vertebral body and the diseased vertebrae, a feature that is absent in normal specimens.52 Histologic examination reveals disorganized endochondral ossification, which may be a result of abnormal stress. Traumatic features of vascular and fibrocartilage proliferation are evident in the ventral end plates in Scheuermann disease.52,68,69 The dorsal vertebral height in cases of Scheuermann disease is not significantly different from that of controls, implying that either the ventral and dorsal stresses are different or that the kyphotic changes occur after dorsal growth is completed (the normal pattern of ring apophysis closure starts dorsolaterally, then works ventrally).52 Possibly, the natural thoracic kyphosis, being exacerbated by a rounded back, results in the development of the abnormal kyphosis.
Back pain is uncommon in the growing child with Scheuermann disease. Low back pain has been reported to be common (up to 50%)51 in adults with progressive, untreated dorsal kyphotic deformities. In other studies pain was not a significant problem.51 Progression of deformity is documented in 80% of patients older than 25 years of age, but the extent of deformity and pain is generally not severe.70 The kyphosis most commonly progresses before skeletal maturity, but can occur in adulthood.71 Disc degeneration is also associated with the deformity. Development of neurologic complications is rare, but is due to thoracic disc hernation, dural tenting, extradural cysts, and vascular compromise.72
Examination of the patient with Scheuermann disease can reveal a hyperpigmented lesion at the apex of the thoracic curve—a result of friction injury from the abnormally protruded spinous process. Patients often have a forward-protruding head, flexion contractures of the shoulders and hips, as well as tight hamstrings.53
Treatment is often indicated to correct the deformity, prevent its progression, and alleviate pain. The extent of the kyphosis and the age of the patient are important criteria for intervention. The nonoperative forms of treatment, such as bracing (Milwaukee brace) or casting, are the first line of treatment for most cases in which the kyphotic deformity is less than 65 degrees. These cases have a high success rate in correcting the deformity, especially if treatment begins before closure of the iliac apophyses (i.e., skeletal maturation).73 Operative treatment with fusion is reserved for cases of progressive deformity, pain not responsive to an adequate trial of casting or bracing, degenerative changes in adults associated with the kyphosis, cardiopulmonary compromise, and for a deformity greater than 65 degrees.71
Dorsal long-construct instrumentation that extends rostrally and caudally well above the thoracic apex is often adequate for stabilization and correction of the deformity. In the event of extreme kyphotic deformity, both a ventral and dorsal surgical approach is necessary for a more definitive correction,53 as well as the maintenance of correction until fusion in the setting of greater tension forces opposing the correction. A large retrospective review comparing 78 patients treated with either dorsal instrumentation alone or combined anterior-posterior instrumentation showed a comparable degree of deformity correction. The rates of proximal junctional kyphosis and surgical complications were clinically and statistically significantly increased in the combined procedure. A decreased rate of postoperative loss of correction was observed with the combined procedure. A higher rate of proximal junctional kyphosis was correlated with a greater degree of postoperative kyphosis, greater pelvic incidence, and less imbalance correction. The authors conclude that dorsal arthrodesis and fixation alone should be the favored procedure whenever possible due to the lower complication rate.74 On the other hand, anterior ligamentous and disc release with video-assisted thoracoscopic surgery (VATS) combined with dorsal spinal fusion may yield lower complication rates and increased sagittal deformity correction, due to the anterior tension band release.75
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree