CHAPTER 72 Anatomy and Synaptic Connectivity of the Basal Ganglia
Our knowledge of the functional anatomy of the basal ganglia has increased greatly over the past decades as a result of tremendous developments in the field. The use of sensitive tracing methods combined with high-resolution immunocytochemical methods has revealed complex features of the microcircuitry and macrocircuitry of the basal ganglia. The results of these studies led us to reconsider various aspects of the pathophysiology of basal ganglia disorders and the potential role of the basal ganglia in motor and nonmotor functions. In-depth knowledge of the basal ganglia circuitry is a prerequisite for a deeper understanding of the neural systems and functional network changes that underlie the beneficial effects of surgical therapies for movement disorders. This chapter provides an overview of the functional anatomy of the primate basal ganglia with an emphasis on recent findings that led us to reconsider the complex functions of the basal ganglia in normal and diseased states. Because of space constraints, this review does not aim at covering the whole literature on basal ganglia anatomy. Readers are referred to previous comprehensive reviews and compendiums for a survey of the early literature and a more general overview of this field.1–41
Functional Circuitry of the Basal Ganglia
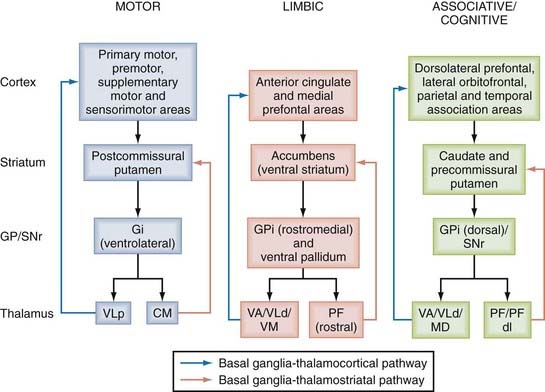
The basal ganglia structures comprise the caudate nucleus and putamen, which make up the dorsal striatum and the nucleus accumbens and olfactory tubercle that form the ventral striatum. In rodents, the dorsal striatum is made up of a single mass of gray matter called the caudate-putamen complex. Other basal ganglia nuclei include the pallidum, which in primates consists of two parts, the internal and external segments of the globus pallidus, commonly referred to as GPi and GPe, respectively. In rodents, the homologue of the GPi is the entopeduncular nucleus and that of the GPe is the globus pallidus. The complex made up of the putamen and globus pallidus is called the lenticular nucleus. The subthalamic nucleus (STN), a small almond-shaped nucleus located laterally just below the thalamus at the junction between the diencephalon and midbrain, is another key basal ganglia structure often referred to as the “pacemaker” or “driving force” of the basal ganglia.10,20,21,30,31 The fact that the STN is the prime target of surgical therapy for Parkinson’s disease (PD) heavily supports its critical role in regulating basal ganglia function in normal and pathologic conditions.42–52 Finally, another major component of the basal ganglia network is the substantia nigra, located at the basis of the mesencephalon. The substantia nigra is divided into two major subnuclei; the substantia nigra pars compacta (SNc) consists of dopaminergic neurons, whereas the substantia nigra pars reticulata (SNr) is made up of GABAergic projection neurons. Other neighboring cellular groups related to the dopaminergic SNc include the ventral tegmental area (VTA) along the midline and the more caudal retrorubral field (RRF) lying along the ventrolateral edge of the upper brainstem. The basic circuit of the basal ganglia involves information originating from the entire cortical mantle and thalamus sent to the striatum, known as the main entrance of the basal ganglia. Once this information has been processed, it is sent to frontal cortical regions or the brainstem via functionally segregated basal ganglia thalamocortical channels of information that flow through the GPi and SNr, commonly known as the basal ganglia output nuclei (Fig. 72-1).
The Striatum: An Entrance to the Basal Ganglia Circuitry
The striatum consists of dorsal and ventral components based on their respective location along the dorsoventral extent of the telencephalon. These striatal regions are functionally different and process segregated information from the cerebral cortex; the main cortical input to the dorsal striatum originates from associative and sensorimotor areas, whereas the ventral striatum is predominantly innervated by limbic cortical regions.1
The striatal neuropil is further compartmentalized into two distinct territories called the patch (or striosomes) and the extrastriosomal matrix. These two striatal compartments receive distinct afferent projections and display a significant degree of neurochemical heterogeneity.2,53 Despite a pretty clear understanding of the anatomic organization of these compartments, their functional significance long remained poorly understood. However, there is recent evidence that imbalanced activity between the patch and matrix compartments may underlie some aspects of repetitive motor behavior known as stereotypies.54–57 The preferential recruitment of patch (or striosome) neurons after chronic exposure to psychostimulants probably represents a neural end point of the transmission from action-outcome associative behavior to conditioned habitual responding.57 The differential regulation of the Ras/Rap/extracellular signal–related kinase (ERK) mitogen-activated protein (MAP) kinase signal transduction cascades between the striosomal and extrastriosomal matrix compartments predicts the severity of the motor side effects induced by chronic dopaminergic antiparkinsonian therapy,58 and preferential loss of striatal patch projection neurons occurs in X-linked progressive dystonia-parkinsonism.59,60 Differential changes in GABAA receptor subunit expression between the patch and matrix compartments may underlie the variation in clinical symptomatology related to changes in mood in patients with Huntington’s disease.61
Cellular Organization of the Striatum
The striatum is largely made up of the so-called GABAergic medium spiny projection neurons, which can be divided into two major phenotypes based on their peptide and relative dopamine receptor expression (see later). There are about 2.8 × 106 medium spiny neurons in the rat striatum, which accounts for 95% to 97% of the total striatal neuronal population.62 These neurons have a small to medium-sized cell body from which emerge smooth proximal dendrites that give rise to a profuse and heavily spiny distal dendritic tree. Two main subtypes of striatal projection neurons have been identified: the “direct pathway” neurons preferentially express D1 dopamine receptors, substance P, and dynorphin and project directly to the GPi and SNr, whereas “indirect pathway” neurons express preferentially D2 dopamine receptors and enkephalin and project mainly to the GPe. Although both neuronal populations look morphologically similar, a recent rodent study has indicated that the dendritic length of D1 projection neurons is significantly greater than that of D2 neurons, an anatomic feature correlated with increased excitability of D2 neurons in adult mice.63–66
The abundance and plasticity of dendritic spines are other important morphologic criteria that could contribute to the physiologic role of medium spiny neurons. On average, single striatal projection neurons of either pathway are covered by about 5000 spines distributed quite homogeneously at about 1 spine/µm of distal dendritic length across species.67–70 The fact that in PD there is severe loss of striatal spines suggests an important regulatory role of dopamine and glutamate in striatal spine plasticity in normal and pathologic conditions.67–70 Although recent data from transgenic mice indicate that the loss of striatal spines in dopamine-depleted animals is specific to D2-containing neurons of the indirect pathway,68 these findings could not be confirmed in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys and humans with parkinsons, both of whom display more homogeneous spine loss between direct and indirect pathway striatofugal neurons.33,70
Medium spiny neurons give rise to a rich plexus of intrinsic axonal arborization within the vicinity of the parent cell bodies and contact neighboring projection neurons.71 Although this local connectivity has long been considered the main substrate for lateral inhibition in the striatum,71 electrophysiologic data have demonstrated that these connections are sparse and distal and consequently are poorly influential on the activity of striatal projection neurons.72,73 However, these connections are specifically organized and unidirectional between pairs of D1– or D2-containing neurons or from D2– to D1-positive projection neurons,74 with connections from D1– to D2-positive neurons being rare. The strength of these intrinsic connections is significantly reduced in dopamine-depleted parkinsonian conditions.74
The striatum also comprises four main populations of interneurons that represent about 2% to 3% of the total striatal neuronal population in rats, whereas in monkeys they account for as much as 23% of all striatal neurons.15,16,75,76 The cholinergic interneurons, which probably correspond to the “tonically active” neurons physiologically identified in the rat and monkey striatum,76–79 display a significant degree of colocalization with calretinin in primates.80 These neurons receive strong synaptic input from GABAergic axon collaterals of substance P–containing striatofugal neurons in rats81 and play a key role in reward-related learning and motivated behavior.77,78,82–89 In rats, they are connected to one another through GABAergic interneurons, thus providing a mechanism for their widespread recurrent inhibition via nicotinic excitation.90 The cholinergic interneurons are key mediators of dopamine-dependent striatal plasticity and learning.24,91–95
The GABA/parvalbumin interneurons are known as the “fast-spiking interneurons.” They form axosomatic synapses on projection neurons, are electrotonically coupled through gap junctions, and control spike timing in projection neurons, thereby providing the substrate for fast-forward intrastriatal inhibition of projection neurons in response to cortical activation (but see the article by Berke96).15,16,76
The GABA/nitric oxide synthase/neuropeptide Y/somatostatin interneurons are categorized physiologically as “persistent and low-threshold spike” neurons.15,16,76 These cells induce large inhibitory currents in projection neurons and release nitric oxide to modulate plasticity at glutamatergic synapses.16 In addition, because somatostatin actions entrain projection neurons into the rhythms generated by some interneurons, they are capable of modifying the processing and output of the striatum.97
The medium-sized GABA/calretinin interneurons represent the largest population of striatal interneurons in humans,80 display physiologic characteristics similar to the persistent and low-threshold spike neurons, and exert powerful monosynaptic inhibition of medium spiny projection neurons.76
Extrinsic Connectivity of the Striatum
Corticostriatal Projections
The cerebral cortex is the main source of glutamatergic input to the striatum. The corticostriatal pathway originates from all cortical areas and displays a highly topographic pattern of distribution in the striatum that imposes functionally segregated maps on it (see Fig. 72-1).98 The anatomic organization of the corticostriatal system has been extensively studied in rats and monkeys and more recently in humans via functional imaging approaches.99–119 These findings led to a basic scheme of functional connectivity between the cerebral cortex and striatum. The somatosensory, motor, and premotor cortices innervate the postcommissural region of the putamen somatotopically in a band-like pattern; the associative cortical areas from the frontal, parietal, and temporal lobes project to the caudate nucleus and the precommissural putamen; and the limbic cortices, the amygdala, and the hippocampus terminate preferentially in the ventral striatum (see Fig. 72-1).1,98 Functionally related associative or sensorimotor cortical inputs from areas connected through corticocortical projections either overlap or remain segregated in the striatum.116–119
In rats, corticostriatal neurons are divided into two distinct subsets: the superficial “intratelencephalic neurons,” which project solely to the striatum and the cerebral cortex, preferentially target “direct pathway” neurons, whereas the deep “pyramidal tract” neurons, which send their main axonal projections to the brainstem and spinal cord with collaterals to the striatum, preferentially target “indirect pathway” neurons.120,121 However, the physiologic significance of these anatomic observations remains unclear because electrophysiologic data suggest that intratelencephalic neurons are the main source of functional excitatory input to both populations of striatal projection neurons.122 There is also significant controversy regarding the existence of pyramidal tract corticostriatal neurons in primates.123–125
The dendritic spines of medium spiny neurons and GABA/parvalbumin-containing (PV) interneurons are the main targets of corticostriatal afferents,126–127 thereby laying the foundation for feedforward inhibition of striatal projection neurons in response to corticostriatal input.15,16,126–128 The cortical drive of feedforward inhibition from GABA/PV interneurons contributes to the imbalance of activity between the two populations of striatal projection neurons in the rat model of PD.129 However, this concept has recently been challenged by electrophysiologic evidence of the lack of correlation between cortical information flowing to GABA/PV neurons versus projection neurons.96
Thalamostriatal Projections
Thalamostriatal Projections from the Caudal Intralaminar Nuclei
The intralaminar thalamic nuclei are a major source of excitatory afferents to the striatum. In primates, the centromedian (CM) and parafascicular (PF) nuclei give rise to projections that largely terminate in different functional regions of the striatum. The medial part of the CM nucleus projects to the sensorimotor postcommissural sensorimotor putamen, whereas the PF nucleus predominantly innervates the associative caudate nucleus and the limbic ventral striatum.14,37 The dorsolateral PF nucleus selectively projects to the precommissural putamen. CM/PF neurons send only sparse projections to the cerebral cortex.130 At the synaptic level, CM and PF input preferentially targets the dendrites of striatal projection neurons.131–133 CM neurons also innervate striatal interneurons immunoreactive for choline acetyltransferase, parvalbumin, and somatostatin, but not calretinin.134 In line with these electron microscopic data, projections from the CM/PF complex tightly regulate the electrophysiologic activity and release of neurotransmitters from cholinergic interneurons135–137 and are required for the sensory responses of the “tonically acive neurons “(probably cholinergic) that are acquired through sensorimotor learning in monkeys.138–140
Thalamostriatal Projections from Other Thalamic Nuclei
The CM/PF complex is not the only source of thalamostriatal projections. Albeit sparse, most thalamic nuclei also contribute to a topographically and functionally organized striatal innervation.14,37,141–145 However, the synaptology of striatal projections from the CM/PF nuclei is strikingly different from that of other thalamic nuclei; that is, CM/PF terminals form synapses predominantly with the dendritic shafts of medium spiny neurons and interneurons, whereas projections from other thalamic nuclei, including the rostral intralaminar, midline, and relay thalamic nuclei, almost exclusively target dendritic spines.14,37,144,145 The degree of axon collateralization to the striatum and cortex of projections from the CM/PF complex versus other thalamic nuclei is significantly different. In contrast to CM/PF projections, which are directed mainly toward the striatum with minimal innervation of the frontal cortical areas, the relay and rostral intralaminar nuclei project predominantly to the cerebral cortex with modest striatal innervation.14,37,131,133,144,146 In general, thalamostriatal projections from single CM/PF neurons are much more focused and give rise to a significantly larger number of terminals than do individual corticostriatal axons.37,125,130 The functional significance of such differences in the pattern of striatal innervation between CM/PF and cortical input remains poorly understood.
Vesicular Glutamate Transporters as Specific Markers of Thalamostriatal versus Corticostriatal Projections
The vesicular glutamate transporters 1 and 2 (vGluT1 and vGluT2) are selective markers of the corticostriatal and thalamostriatal glutamatergic terminals, respectively.14,37,145,147 More than 95% of vGluT1 terminals contact spine heads, whereas only 50% to 60% of vGluT2 terminals do so in monkeys, a pattern that does not change in parkinsonism.145 In rats, there is a significant difference in the microcircuitry of vGluT2 terminals between the patch and matrix striatal compartments such that most axodendritic vGluT2 synapses are found in the matrix, consistent with the idea that the CM/PF complex is the main source of this synaptic input.14,37,133
Functional Roles of the Thalamostriatal Systems
The role or roles of the thalamostriatal systems probably differ between projections that arise from the CM/PF nuclei and those arising from other thalamic nuclei. In nonhuman primates, CM/PF neurons most likely supply striatal neurons with information that has attentional value in that they act as detectors of behaviorally significant events occurring on the contralateral side.138,139 In line with these data, changes in CM/PF activity are induced in response to attention-demanding reaction time tasks in humans.148 Electrical stimulation of the CM nucleus induces complex excitatory and inhibitory electrophysiologic responses in striatal projection neurons and cholinergic interneurons.135–137 In contrast, stimulation of rostral intralaminar nuclei results in complex alterations in cognitive processing, most likely through regulation of cortical and striatal activity.149,150 The function of other thalamostriatal systems is unknown, but they probably act as a positive reinforcer of specific populations of striatal neurons involved in performing a selected cortically driven behavior.14,37,151
Centromedian/Parafascicular Degeneration in Parkinson’s Disease
Postmortem studies of patients’ brains affected with progressive supranuclear palsy, Huntington’s disease, or PD revealed as much as a 50% loss of cells in the CM/PF nuclei.152,153 In patients with PD, the parvalbumin neurons are affected mainly in the PF nucleus, whereas the nonparvalbumin/noncalbindin neurons are specifically targeted in the CM nucleus.153 Asymmetric changes in the shape of the thalamus between patients with PD and healthy controls were recently reported.154 It is not clear whether these thalamic pathologies are also induced in animal models of parkinsonism. In rodents, some studies showed significant loss of PF thalamostriatal neurons in 6-hydroxydopamine–treated rats and MPTP-treated mice, whereas others did not find any cell loss in the PF nucleus under similar conditions.14,37 In nonhuman primates, a significant reduction in the relative abundance of vGluT2-positive terminals forming axodendritic synapses in the putamen of MPTP-treated monkeys was reported, thus suggesting a possible loss of CM-striatal neurons in this animal model.145
The Mesostriatal Dopaminergic Systems
Three main groups of dopaminergic neurons are found in the ventral midbrain: the A8 (RRF), A9 (SNc), and A10 (VTA) regions. Each of these subnuclei consists of predominantly dopaminergic neurons among which are interspersed small populations of GABAergic interneurons, except in the VTA, which has a significant population of GABAergic projection neurons.9 Various neuropeptides, including neurotensin and cholecystokinin, have been identified in subsets of dopaminergic neurons in the medial SNc and VTA.155–158 Ventral tier SNc (SNc-v) neurons are more significantly enriched with dopamine transporter than other ventral midbrain dopaminergic neurons,159 which may account for the preferential vulnerability of SNc-v neurons in response to MPTP.160,161 In addition, the calcium-binding protein calbindin D28k (CB) is strongly expressed in neurons of the VTA and RRF, as well as in dorsal tier neurons of the SNc (SNc-d), but it is not found in SNc-v neurons.9,162 A neuroprotective role of CB in SNc-d and VTA neurons in PD has been suggested, whereas the absence of CB may contribute to the selective vulnerability of SNc-v neurons in parkinsonism.163–165 The pattern of nigrostriatal degeneration at both the striatal and nigral levels is, indeed, correlated with the expression level of CB. At the striatal level, the sensorimotor postcommissural putamen, the most sensitive striatal region to dopaminergic denervation in PD (see later), is devoid of CB-containing neurons.166 In the substantia nigra, the more sensitive SNc-v neurons express a low level of CB immunoreactivity, whereas the relatively spared SNc-d and VTA neurons are enriched in CB.159,167 Finally, SNc neurons targeted by a strong CB-containing innervation from the striatum are more resistant than nigral neurons in CB-poor pockets called nigrosomes.164 Together, these findings highlight the potential neuroprotective role of CB in the pathogenesis of PD.
Based on various tract-tracing studies in monkeys, the following pattern emerged for organization of the nigrostriatal dopaminergic system in primates: (1) the sensorimotor striatum (i.e., postcommissural putamen) receives its main dopaminergic innervation from neuronal columns in the SNc-v, (2) the limbic striatum (i.e., nucleus accumbens) is the main recipient of dopaminergic projections from VTA and SNc-d neurons, and (3) the associative striatum (i.e., caudate nucleus and precommissural putamen) is preferentially innervated by dopaminergic neurons in the densocellular part of the SNc-v.9,33 In rats, the pattern appears to be slightly different in that SNc-d neurons project predominantly to the dorsal striatum.168
Single-cell filling studies have identified two main types of nigrostriatal axons: thin, varicose and widespread fibers that arise from neurons in the SNc-d, VTA, and RRF and preferentially terminate in the matrix striatal compartment, as well as thick varicose fibers that arise from the SNc-v and terminate mostly in the patch striatal compartment.169 A recent study using a more sensitive viral tracing method challenged this concept of dual nigrostriatal fiber systems and instead suggested a much more extensive axonal arborization of individual SNc dopaminergic neurons that do not display any preferential innervation of the patch or matrix compartments.170
It is well established that the pattern of progressive dopaminergic cell loss in PD is not homogeneous but rather displays a complex topographic and regional organization. In PD patients and MPTP-treated monkeys, two main features of nigrostriatal denervation have been well characterized: (1) the dopaminergic projections to the sensorimotor striatum (postcommissural plus dorsolateral precommissural putamen) are affected before those to the associative (caudate nucleus) and limbic (nucleus accumbens) striatal regions,171,172 and (2) VTA projections to the ventral striatum display a much lower degree of degeneration than do other midbrain dopaminergic cell groups.173,174 A preferential dopaminergic denervation of patch over matrix has also been suggested in MPTP-treated monkeys, but these data remain controversial and may be dependent on the animal species used and the method of MPTP administration.165,175
Dopamine has long been known to be a critical neuromodulator of basal ganglia neuronal activity through both presynaptic and postsynaptic mechanisms. Five dopamine receptor subtypes are expressed in striatal projection neurons and interneurons, thus providing multiple targets through which dopamine can mediate its effects. The dopamine-mediated regulation of glutamatergic and cholinergic transmission is severely affected in parkinsonism, thereby contributing to the abnormal changes in basal ganglia network activity described in PD. The morphology and plasticity of dendritic spines are also tightly regulated by dopamine/glutamate interactions, and this provides a substrate for integration and processing of extrinsic information to the basal ganglia circuitry.6,9,33,70
Other important modulatory systems that are not discussed in this review include the serotoninergic system from the raphe nuclei and the noradrenergic ascending projections from the locus coeruleus. Although not as well studied as the dopaminergic system, there is evidence that these two neurotransmitters regulate physiologic activity in various basal ganglia nuclei and possibly contribute to midbrain dopaminergic neuron loss and the development of nonmotor deficits and motor side effects from long-term dopaminergic therapy in patients with PD.176–183
Intrastriatal Dopamine Neurons: A Potential Compensatory Mechanism in Parkinson’s Disease
Dopaminergic interneurons have been described in the striatum of dopamine-depleted rats and monkeys and in the caudate nucleus and putamen of humans with PD.184–188 These aspiny neurons are small and coexpress various markers of dopaminergic neurons—glutamic acid decarboxylase and, in a small subset, calretinin.184,187 They receive scarce synaptic input and show up preferentially in the precommissural putamen and caudate nucleus in MPTP-treated monkeys.187 Their density significantly increases after dopamine depletion and the administration of glial-derived neurotrophic factor in the striatum,189 thus suggesting a potential compensatory role for dopaminergic interneurons in PD.
Extrastriatal Dopaminergic Systems
Although the striatum is well recognized as the main target of midbrain dopaminergic neurons, there is significant evidence that extrastriatal dopamine acting directly at the level of the globus pallidus, STN, and SNr also plays an important role in regulating basal ganglia function in normal and pathologic conditions.9,33 In addition, the existence of a dopaminergic afferent system to the ventral motor thalamus has recently been suggested.189–192 Although the exact origin of this system remains controversial, it could play a critical role in regulating the activity of thalamocortical neurons in basal ganglia–receiving nuclei, thereby contributing to the fine-tuning of information flow through the basal ganglia–thalamocortical loops in normal and parkinsonian conditions. Because of space limitations, this topic cannot be discussed extensively in this chapter, but readers are referred to recent publications and reviews that discuss the anatomic and physiologic evidence for these projections.9,33
The Direct and Indirect Pathways of the Basal Ganglia
Although overly simplistic, the direct and indirect pathway model of the basal ganglia has driven the field of basic and clinical basal ganglia research for the past decades. Obviously, since its introduction, this model has been challenged, revised, and updated as a result of gain in new knowledge of the basal ganglia circuitry, but it remains the most reliable working model of normal and abnormal physiology of the basal ganglia. The “direct pathway” refers to the monosynaptic connection between the striatum and the basal ganglia output nuclei, the GPi and the SNr, whereas the “indirect pathway” refers to the polysynaptic pathway linking the striatum and GPi/SNr via the GPe and STN.193–195 GABAergic striatal neurons give rise to either of these pathways, but they can be segregated into two populations by their peptide content (substance P—direct; enkephalin—indirect) and their preferential expression of dopamine receptor subtypes (D1—direct; D2—indirect).195 A balance between the activity of the two pathways is essential for normal functioning of the basal ganglia. In PD, the loss of striatal dopamine results in increased activity of indirect striatofugal neurons and decreased output from direct striatofugal neurons. Because of the polarity of connections in the direct/indirect pathways, this results in increased GABAergic basal ganglia outflow to the thalamus, which in turn may reduce cortical excitability and decrease motor behavior (Fig. 72-2).
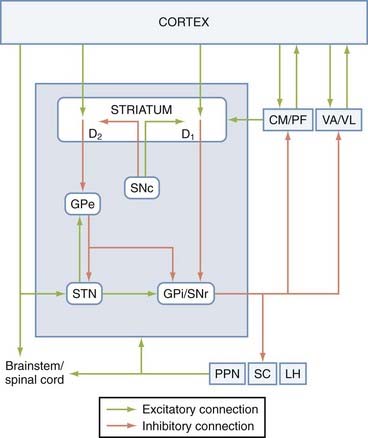
FIGURE 72-2 Direct and indirect pathway model of the basal ganglia. The blue box indicates tightly interconnected basal ganglia nuclei that receive extrinsic input from the cortical, thalamic, and brainstem regions. The extrastriatal substantia nigra pars compacta (SNc) dopaminergic projections to the globus pallidus pars externa (GPe), subthalamic nucleus (STN), and globus pallidus pars interna/substantia nigra pars reticulata (GPi/SNr) have been omitted from this diagram. The connections between the basal ganglia and the pedunculopontine nucleus/superior collicus/lateral habenula (PPN/SC/LH) are depicted in more detail in Figure 72-4. CM, centromedian nucleus of the thalamus; D1 and D2, dopamine D1-type and D2-type receptors; PF, parafascicular nucleus of the thalamus; VA/VL, ventral anterior and ventrolateral nuclei of the thalamus.
This traditional scheme of the basal ganglia circuitry has been challenged over the past decades because of some anatomic and molecular data suggesting that the two pathways may not be as segregated as previously thought. On the one hand, single-cell filling studies have demonstrated that most striatofugal neurons of the direct pathway give off collaterals to the GPe.8,196–198 Although the functional significance of these collateralized projections still remains poorly understood, they surely deserve consideration in the interpretation of functional changes in basal ganglia circuitry in normal and diseased states.
Another challenge to the model comes from molecular studies showing that D1 and D2 receptor mRNA may not be as segregated as originally thought.199–205 Significant controversy about dopamine receptor segregation was also raised in immunocytochemical studies; some reports have shown that D1 and D2 receptor protein immunoreactivity was largely segregated in two distinct populations of striatal spines,121,206 whereas others demonstrated a high level of D1 and D2 receptor immunoreactivity at the single-cell level in the rat striatum.207,208
More recently, bacterial artificial chromosome (BAC) transgenic mice have been developed.209,210 These BAC-D1 and BAC-D2 mice display complete segregation of D1 and D2 receptor mRNA, even when measured with the highly sensitive single-cell mRNA amplification method, an approach that revealed significant D1/D2 colocalization in normal rats.25,68,200,201,205 Whether this strict segregation of the two dopamine receptor subtypes underlies distinct functional dopamine-mediated physiologic effects or different mechanisms of synaptic plasticity between normal animals and these transgenic mice remains to be established. Another important fact to keep in mind while interpreting dopamine-mediated effects in individual striatofugal neurons is the possible coexpression of other D1 or D2 receptor family subtypes (i.e., D3, D4, and D5 receptors) in the two main populations of striatofugal neurons.211–216 To our knowledge, the relative expression level of D3, D4, and D5 receptors in striatofugal neurons of BAC-D1/enhanced green fluorescent protein (EGFP) or BAC-D2/EGFP transgenic mice remains unknown. We believe that such information is absolutely essential to clearly determine the chemical phenotype of striatofugal neurons in these animals and ensure that the functional data gathered from these mice can be translated to normal brains.
The Hyperdirect Corticosubthalamic System: Anatomic and Functional Significance
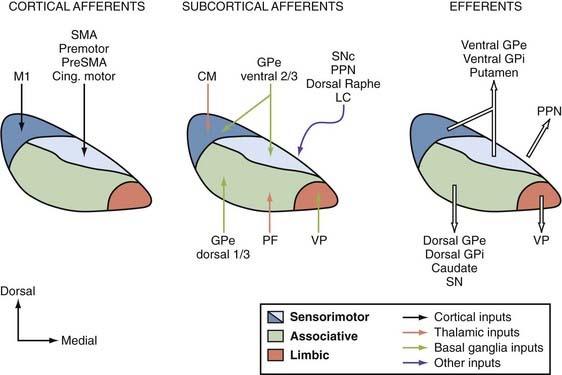
The STN is another major entry station for delivery of extrinsic cortical information to the basal ganglia circuitry. Information flowing along the corticosubthalamic tract is transmitted to the SNr and GPi at a faster pace than information transmitted along the direct and indirect corticostriatofugal systems.217 Basal ganglia output neurons do indeed respond with a fast excitation to electrical stimulation of the motor cortices. Excitotoxic STN lesions abolish these responses. In monkeys, the corticosubthalamic projection originates mainly from the motor cortices. Input from M1 flows along the dorsolateral sector of the nucleus, whereas projections from the supplementary motor area, premotor cortices, and the cingulate motor cortex overlap in the dorsomedial STN. There is a reversed somatotopy between the dorsolateral “M1 domain” and the dorsomedial “supplementary motor area/premotor cortices/cingulate motor cortex domain.”217–219 The ventrolateral half of the STN receives some input from the frontal and supplementary eye field areas, whereas the medial tip of the STN is involved in the processing of limbic-related information (Fig. 72-3). However, the exact sources and pattern of termination of cortical input to the ventral sectors of the STN have been less studied and remain poorly characterized.
An important role for the hyperdirect corticosubthalamic projection in the selection of motor programs has been proposed. According to this hypothesis, the cortical information flowing along the hyperdirect pathway is transmitted to a large and diffuse pool of GPi neurons in a nonspecific manner, thereby exciting a large population of basal ganglia output neurons not related to the selected motor act (i.e., the “surround neurons”). In contrast, the corollary signal transmitted along the corticostriatal system is much more focused and influences a restricted pool of GPi neurons (i.e., the “center neurons”). Via these projections, the cortical input flowing through the striatum and the STN generates a “center-surround” motor act selection model in the GPi that allows proper movements to be performed.217
Although this model generated significant interest, caution must be exercised in its interpretation in light of the anatomic and physiologic observations gathered from monkeys. First, the anatomic relationships between the STN and both pallidal segments are highly specific and topographic, with functionally related neurons in the GPe, GPi, and STN being connected,220,221 which is contrary to the assumption made by the model that the STN provides a diffuse projection to the GPi. Second, a majority of STN neurons increase their activity around the time of movement onset or after the movement during active step tracking movements in monkeys,222 thereby reducing the likelihood that the corticosubthalamic projection is involved in the preparation for movements as suggested by the center-surround hypothesis. However, recent reports have indicated that most STN neurons are active before self-paced movements in the parkinsonian human.223 Thus, the relative importance of the hyperdirect corticosubthalamic system in the basal ganglia circuitry remains a matter of debate that should be addressed in future studies.
Although the functional anatomy of the corticosubthalamic system largely relies on the processing of motor-related input from primary and secondary motor cortices, there is also evidence, gathered largely from rodent studies, that this system may be involved in processing limbic and cognitive information. The recent work of Baunez, Robbins, and colleagues led to the suggestion that the corticosubthalamic projection from the prefrontal cortex to the medial STN plays a role in preparatory processes, attention, perseveration, and other important cognitive or limbic functions in rats.224–233 However, the sources of nonmotor cortical afferents to the STN remain poorly characterized in nonhuman primates. In contrast, the recent use of diffusion-weighted magnetic resonance imaging methods has revealed connections between high-order associative areas of the frontal lobe and the STN in humans.234 It is also important to note that cognitive effects are sometimes induced by bilateral STN-DBS in PD patients.235–242 Although direct connections with the cerebral cortex remain to be established, it is worth noting that the ventromedial STN is tightly linked with the caudate nucleus and related associative regions of the GPe and GPi, thus providing another substrate for STN stimulation–mediated effects on complex cognitive functions.220,221,243,244 The caudal intralaminar nuclei and the brainstem pedunculopontine nucleus (PPN) are two additional sources of glutamatergic input to the STN that may contribute to the increased firing activity of STN neurons in parkinsonian conditions (see Fig. 72-3).221 In turn, the STN sends glutamatergic projections back to the cerebral cortex and PPN.221,243–245
The exact origin of corticosubthalamic neurons remains unknown. Although early data suggested that cortico-STN axons may be collaterals of the descending pyramidal tract in rats246 and cats,247 the recent filling of single pyramidal tract neurons in M1 led to the anterograde labeling of only a few scarcely distributed fibers in the monkey STN,125 thus suggesting that this projection may have a more complex origin than previously thought in primates.
The Pedunculopontine Nucleus as an Integrative Component of the Basal Ganglia
Cellular Organization and Connectivity of the Pedunclulopontine Nucleus
The PPN is made up of a chemically heterogeneous group of neurons in the upper brainstem that lies around the superior cerebellar peduncle. It is surrounded laterally by fibers of the medial lemniscus, medially by the decussation of the superior cerebellar peduncle, dorsally by the RRF, rostrally by the dorsomedial sector of the caudalmost tip of the substantia nigra, and caudally by the cuneiform nuclei. Two major neuronal groups have been identified: the PPN pars compacta (PPNc), which consists of densely packed cholinergic neurons in the caudolateral half of the nucleus, and the PPN pars diffusa (PPNd), which is more medially located and consists of sparsely distributed noncholinergic neurons along the dorsoventral extent of the superior cerebellar peduncle. In humans, the PPN comprises about 10,000 to 15,000 cholinergic neurons,248,249 which make up more than 90% of the PPNc.250 In nonhuman primates, the 40% of cholinergic neurons in the PPN express glutamate immunoreactivity.251 GABAergic, dopaminergic, noradrenergic, and various peptidergic neurons have also been identified within the boundaries of the PPN.252–257
It has long been known that the PPN is tightly linked with the basal ganglia. It receives major projections from the GPi and SNr (see later) and more modest input from the STN. Additional input to the PPN originates from the spinal cord, raphe nuclei, locus coeruleus, deep cerebellar nuclei, superior colliculus, and SNc.255–257 In turn, the PPN sends ascending projections to all basal ganglia nuclei, but most particularly to the SNc and the STN.258,259 Both glutamate and acetylcholine are used as neurotransmitters by these projections. In addition to feedback projections to the basal ganglia, the PPN is also an important source of descending projections to the pontine, medullary, and spinal structures. Through these connections, the PPN can thus be considered a possible relay station where information from the basal ganglia could bypass the thalamocortical loop and be transmitted directly to the reticular formation and spinal cord (see later).
The PPN also sends massive cholinergic and noncholinergic projections to the thalamus. This ascending PPN-thalamus system is critical for mediation of cortical desynchronization during waking and rapid eye movement sleep. Both cholinergic and glutamatergic PPN input to thalamostriatal neurons in the caudal intralaminar thalamic nuclei has been shown,260 thus suggesting that the PPN sends information to the basal ganglia not only directly but also indirectly via thalamostriatal neurons. Therefore, the PPN is strategically located to modulate neuronal activity in functional basal ganglia–thalamocortical and basal ganglia–thalamostriatal loops.253,257,260
Recent studies using diffusion tensor imaging have confirmed and extended our knowledge of PPN connectivity in humans. Despite the obvious limitations of this approach in differentiating afferent from efferent fiber pathways and the likelihood that small fiber tracts may not be detected with tractography, diffusion tensor imaging surely deserves strong interest because of its noninvasive nature and possible use in tracing neural connections in the human brain.261–264
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree