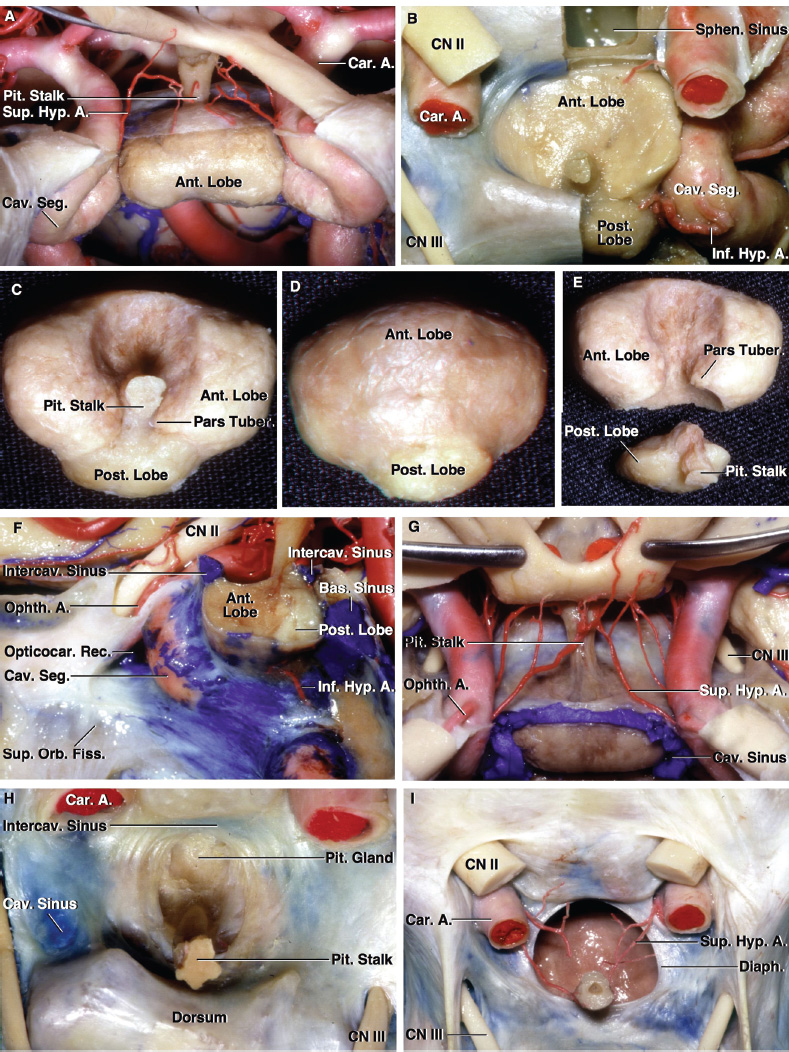
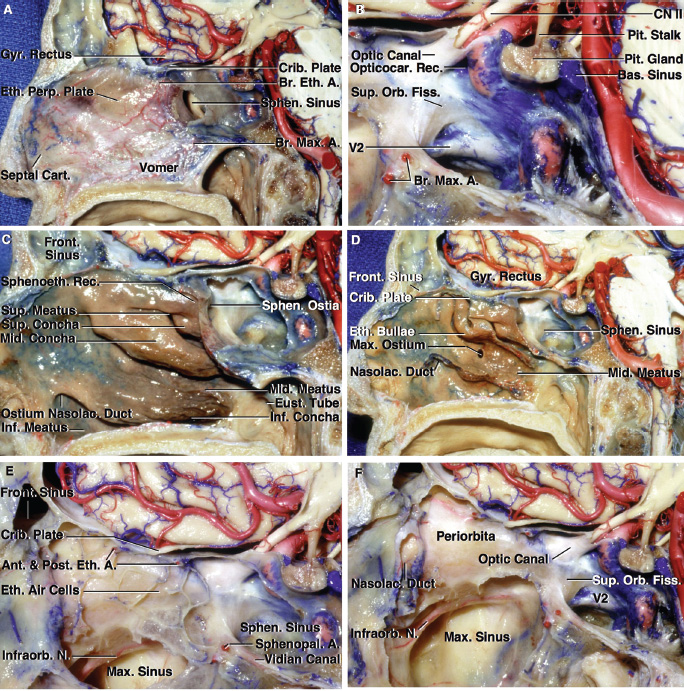
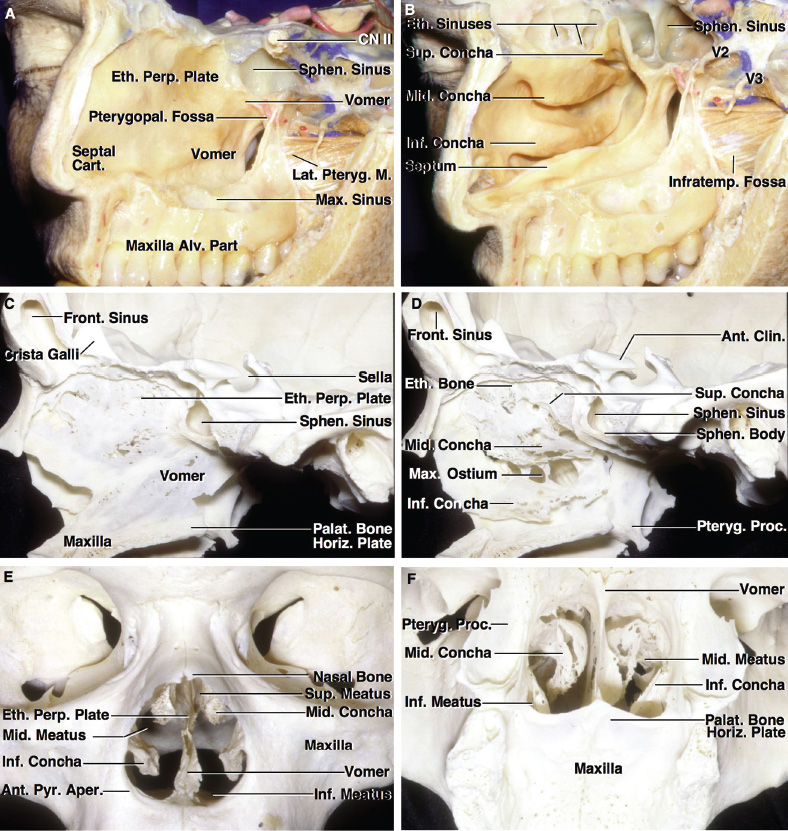
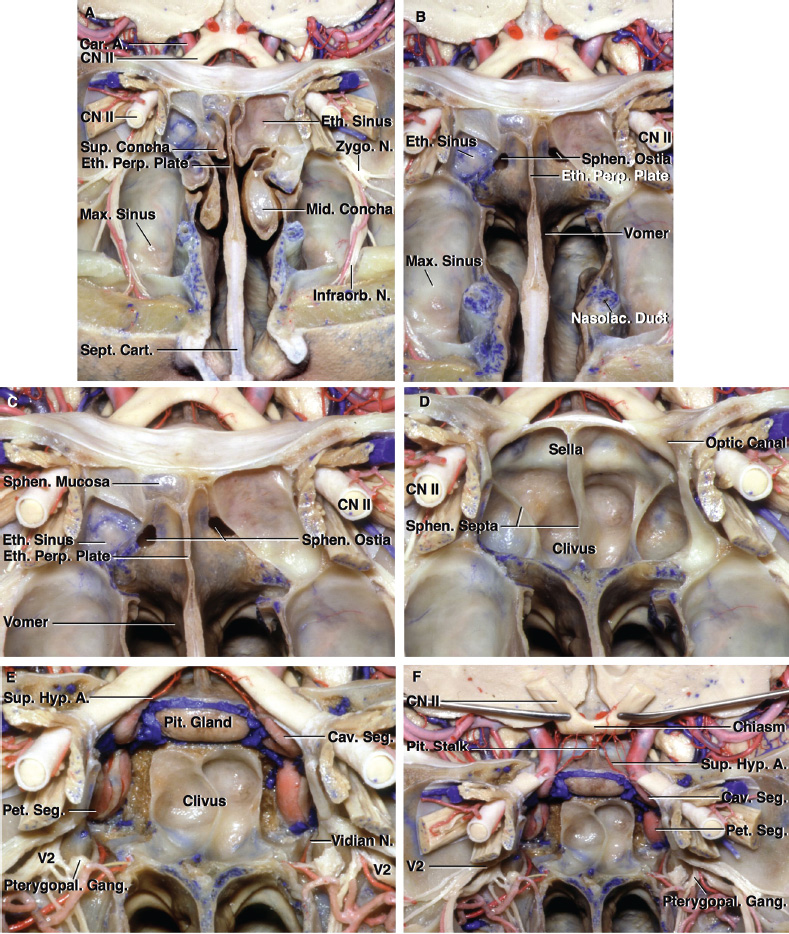
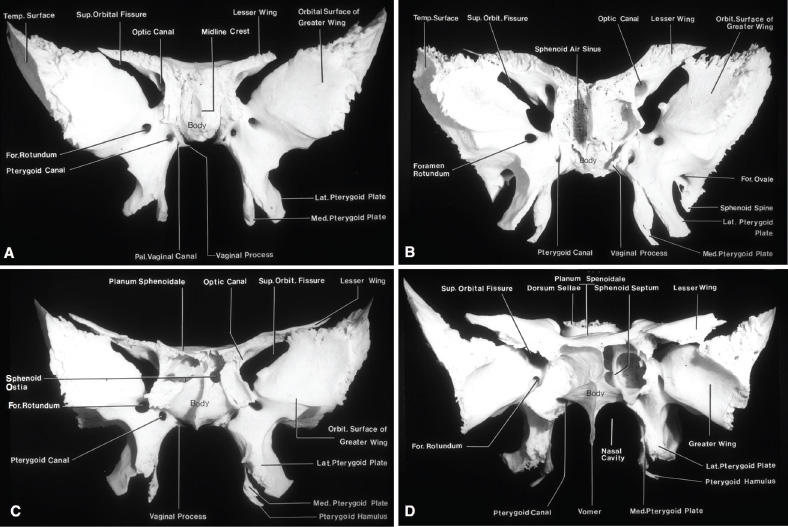
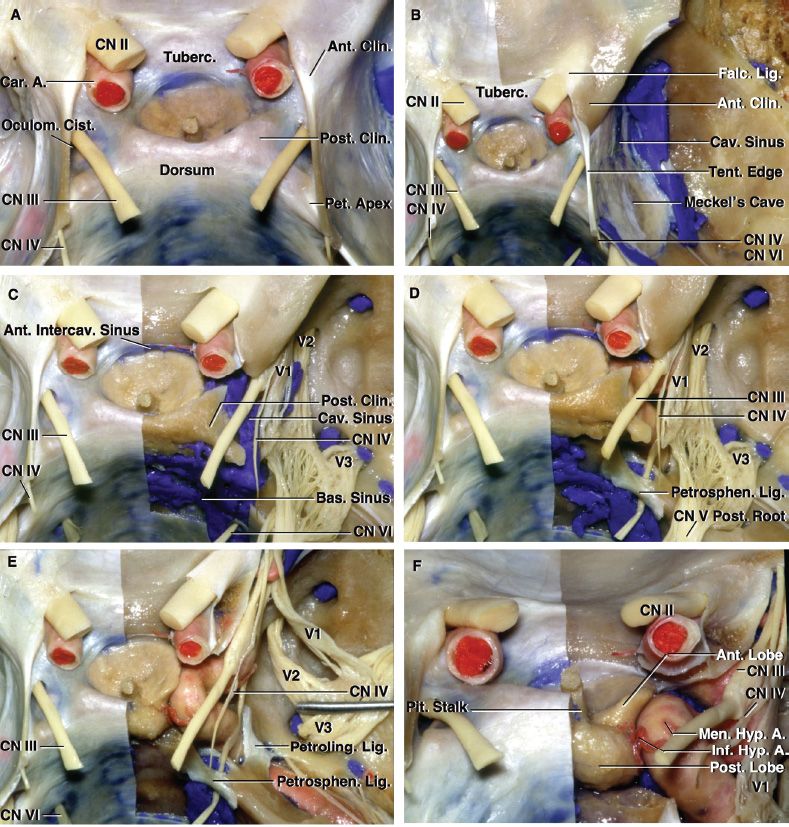
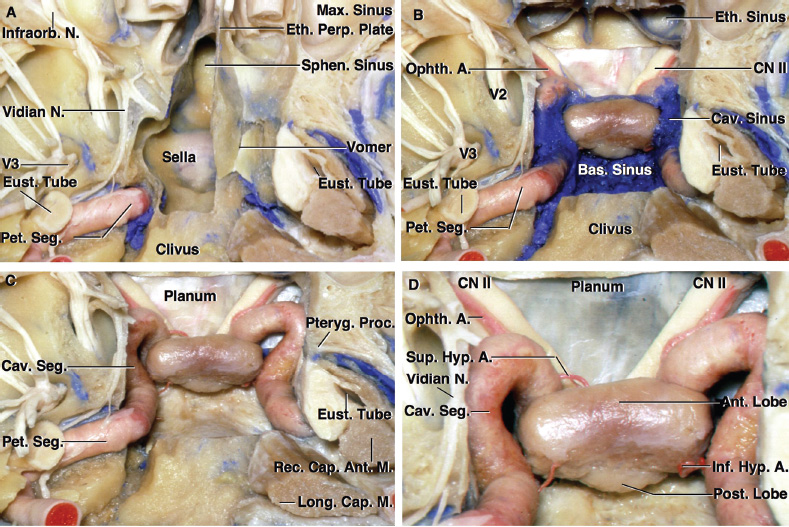
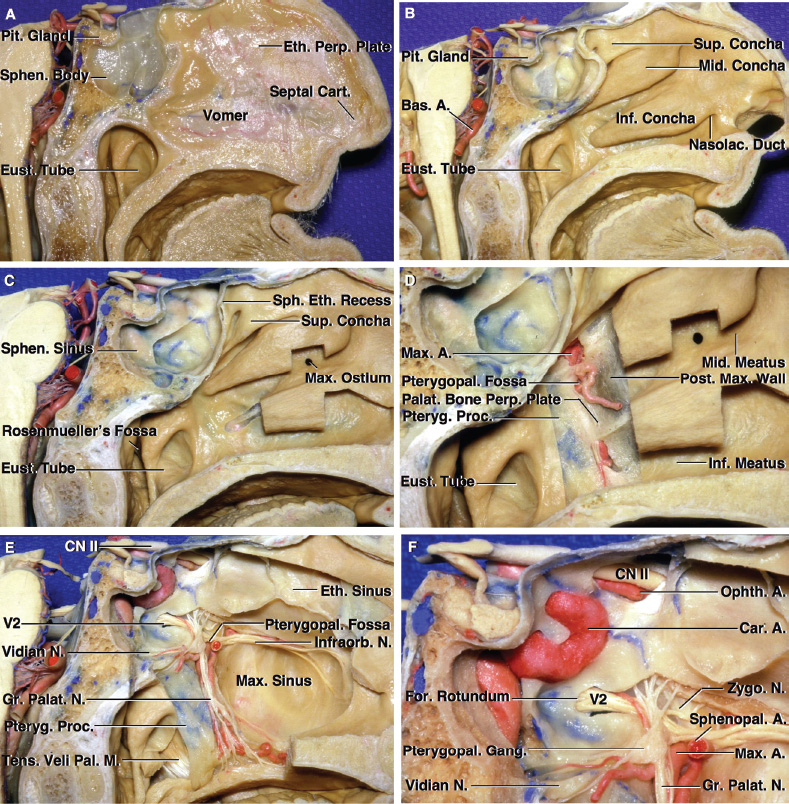
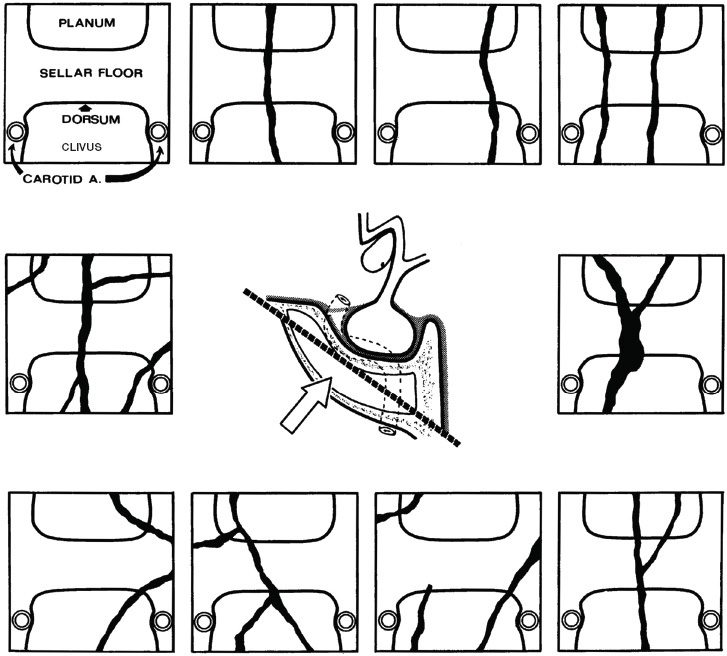
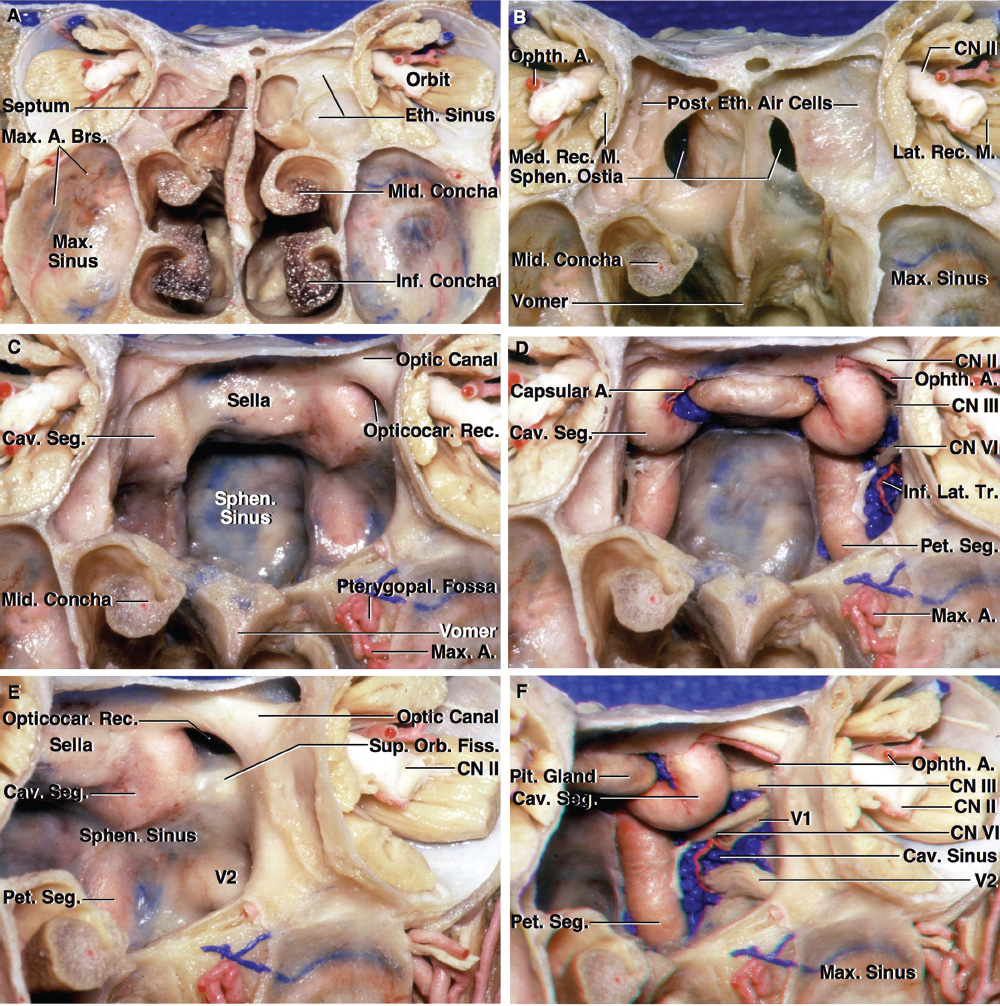
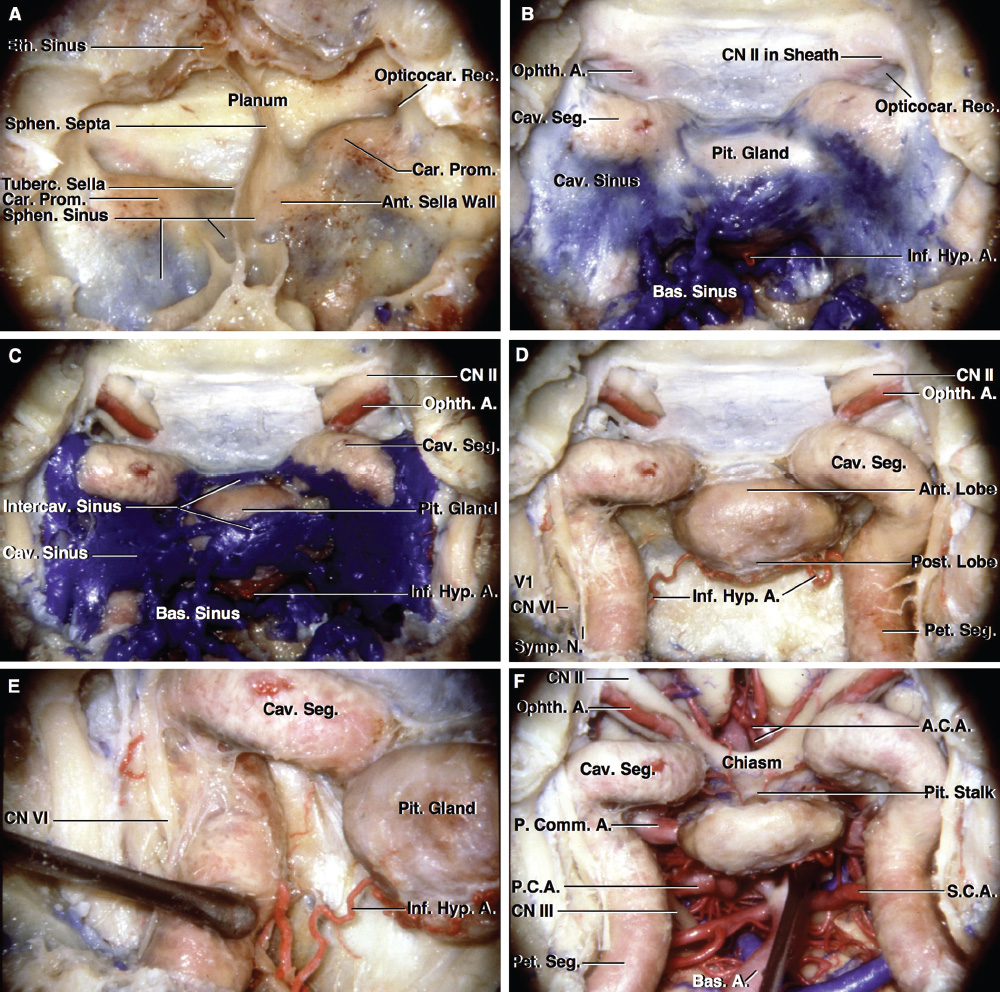
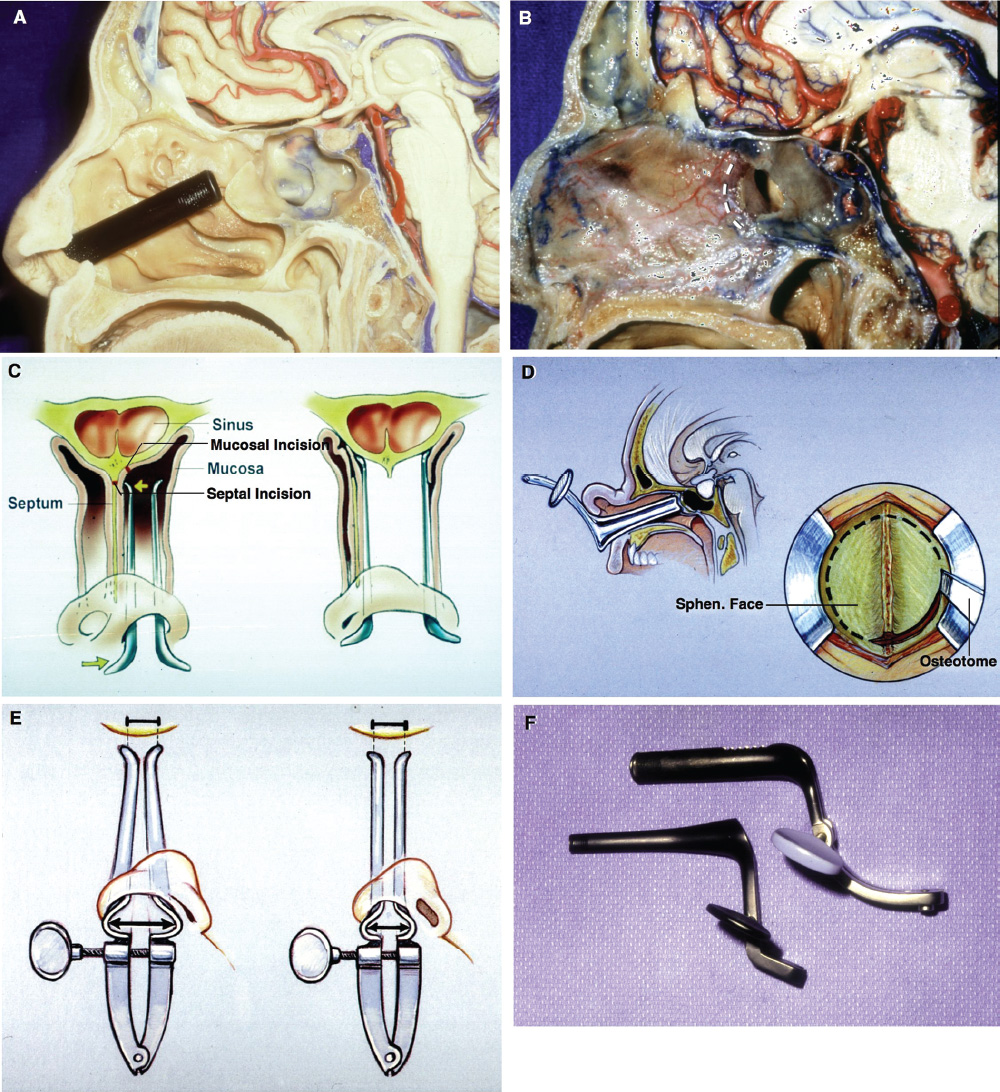
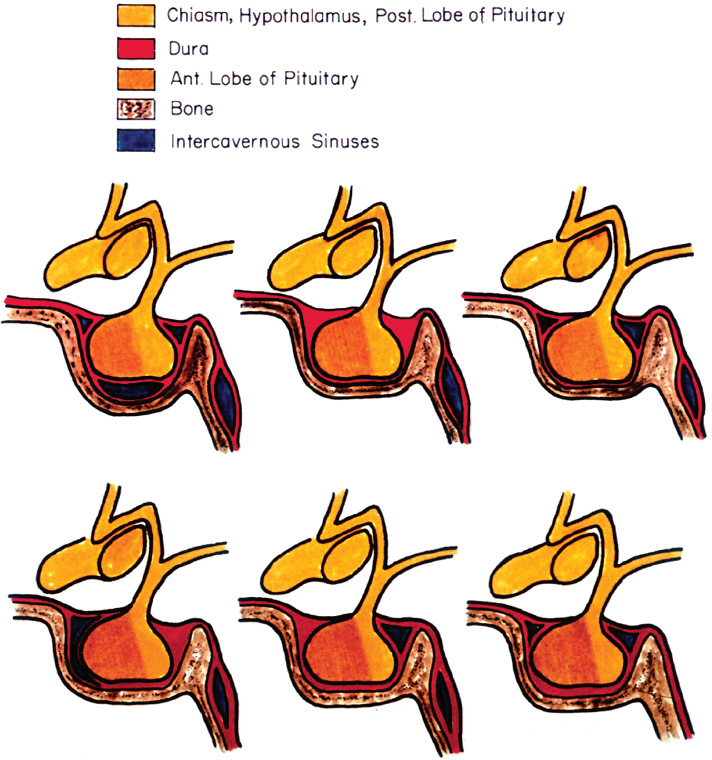
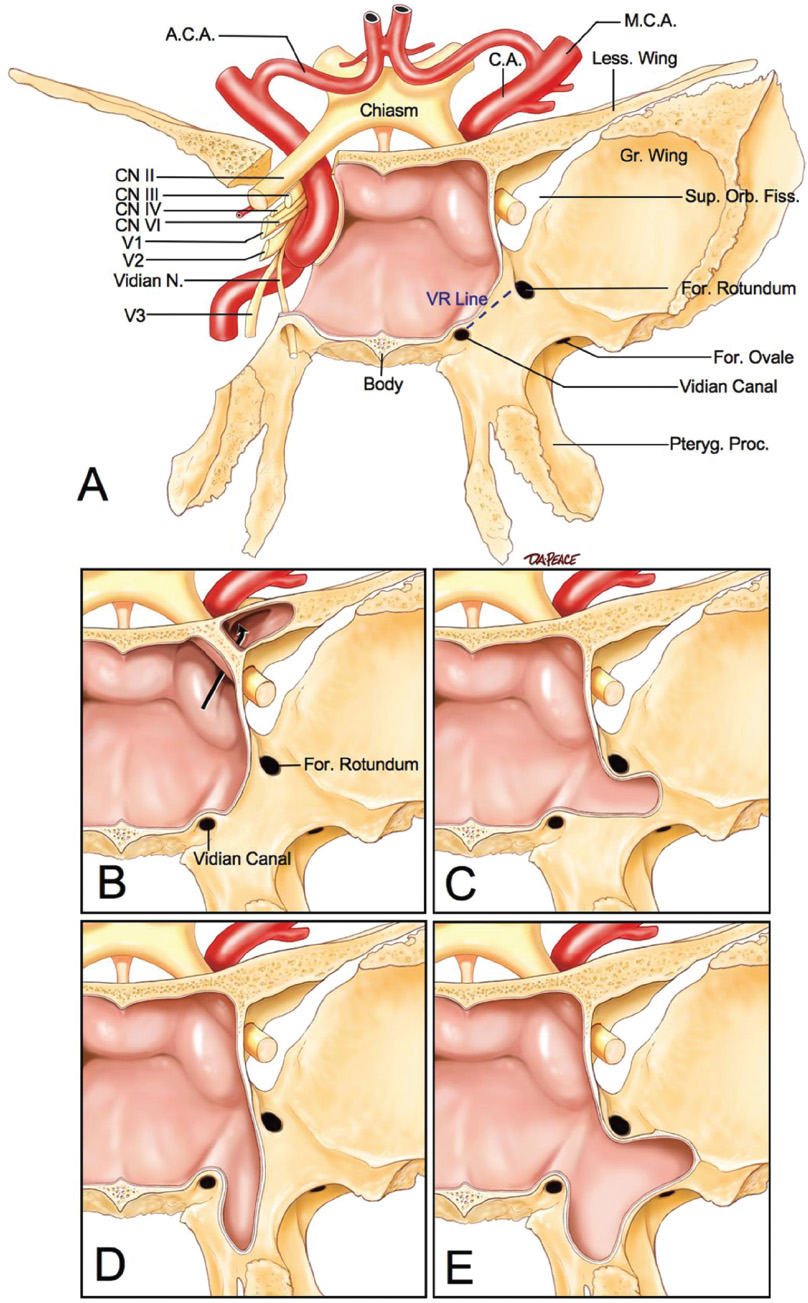
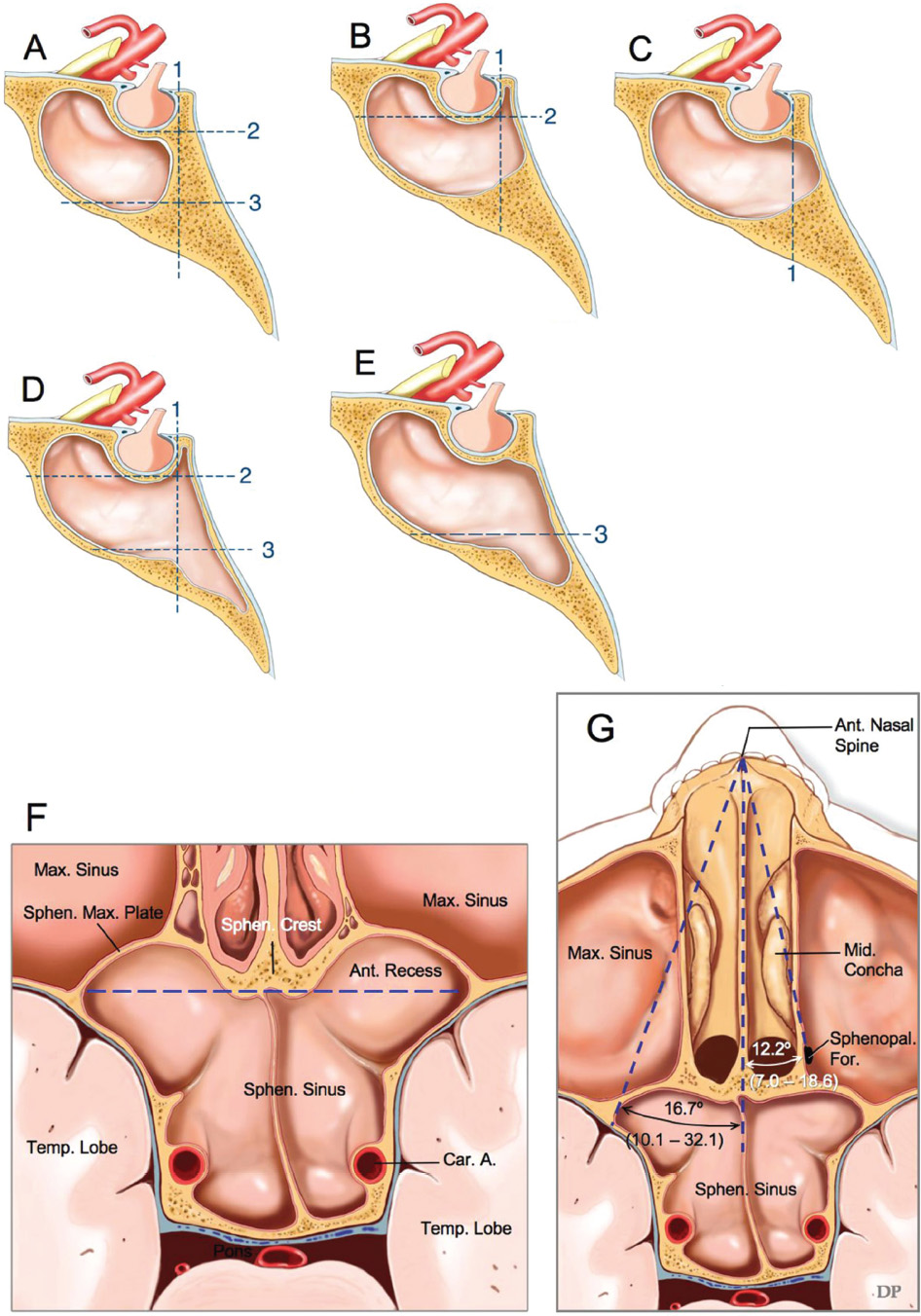
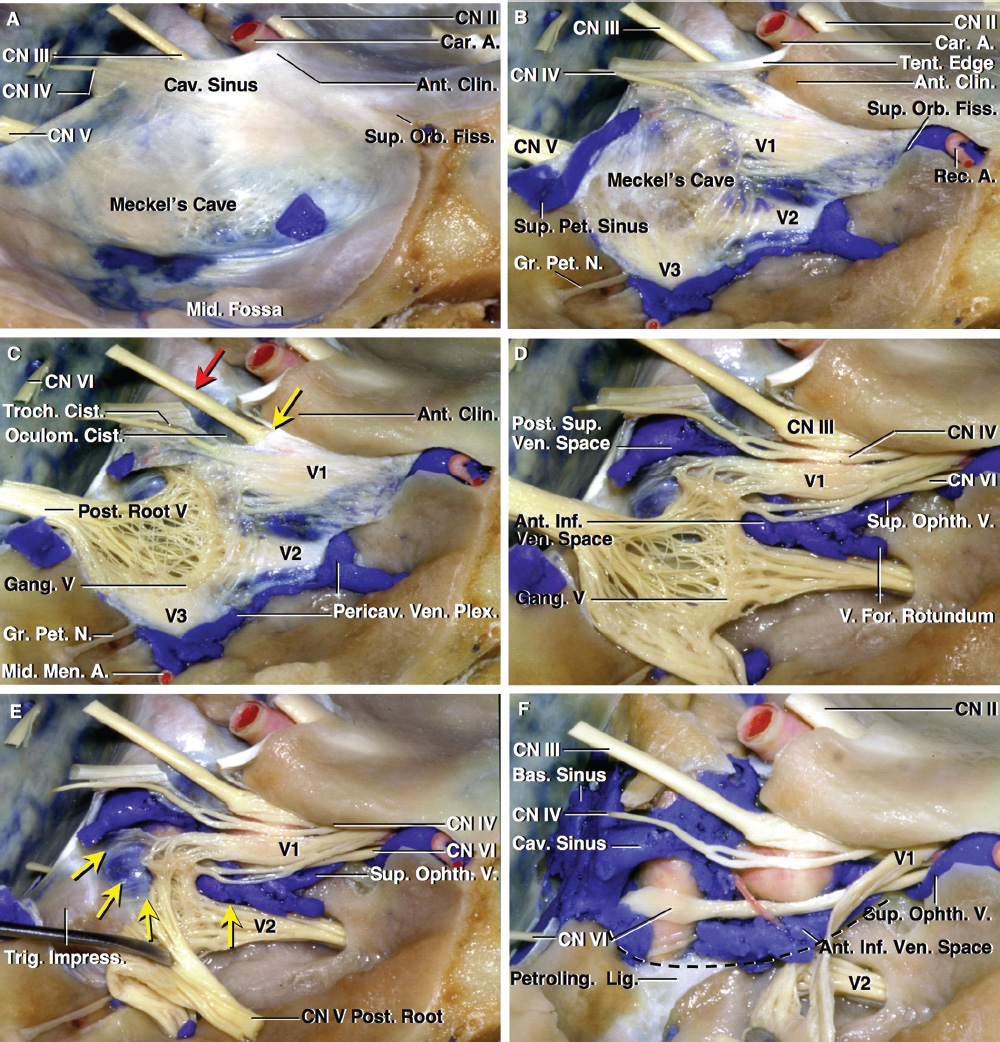
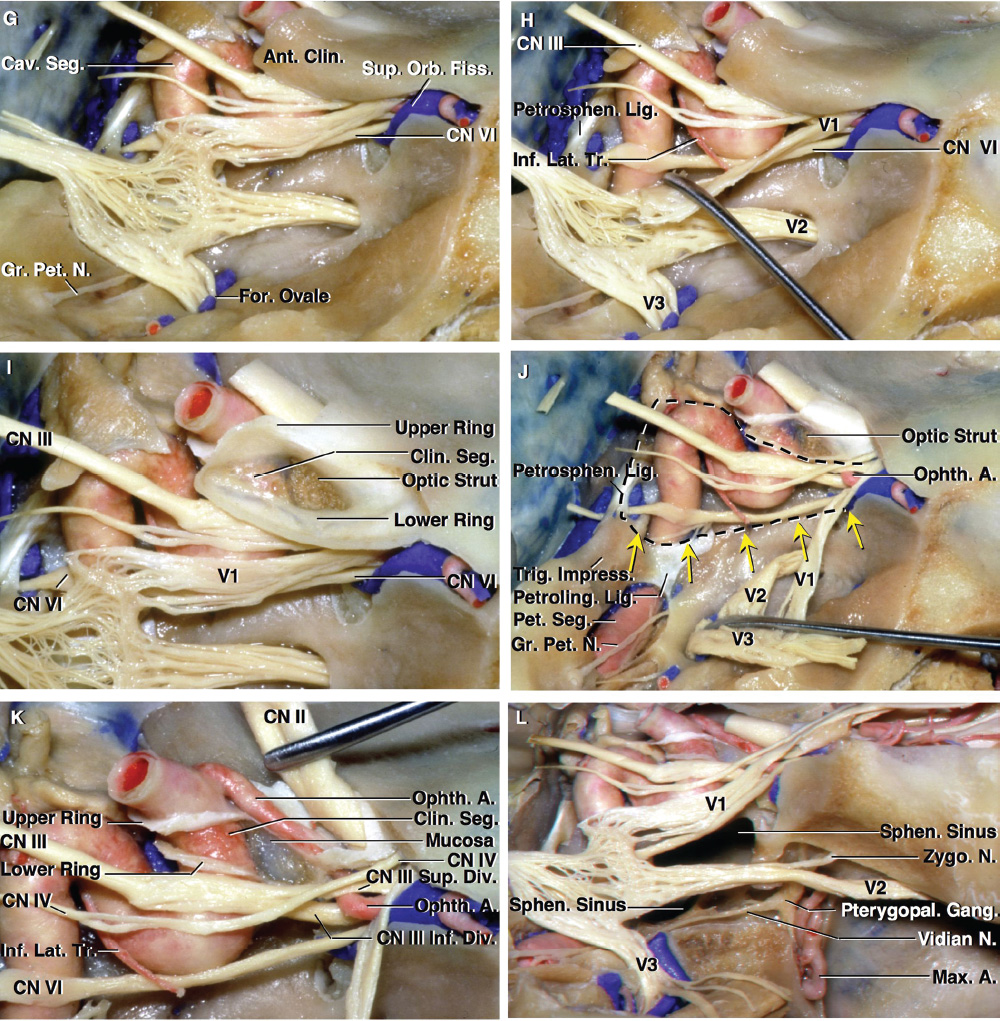
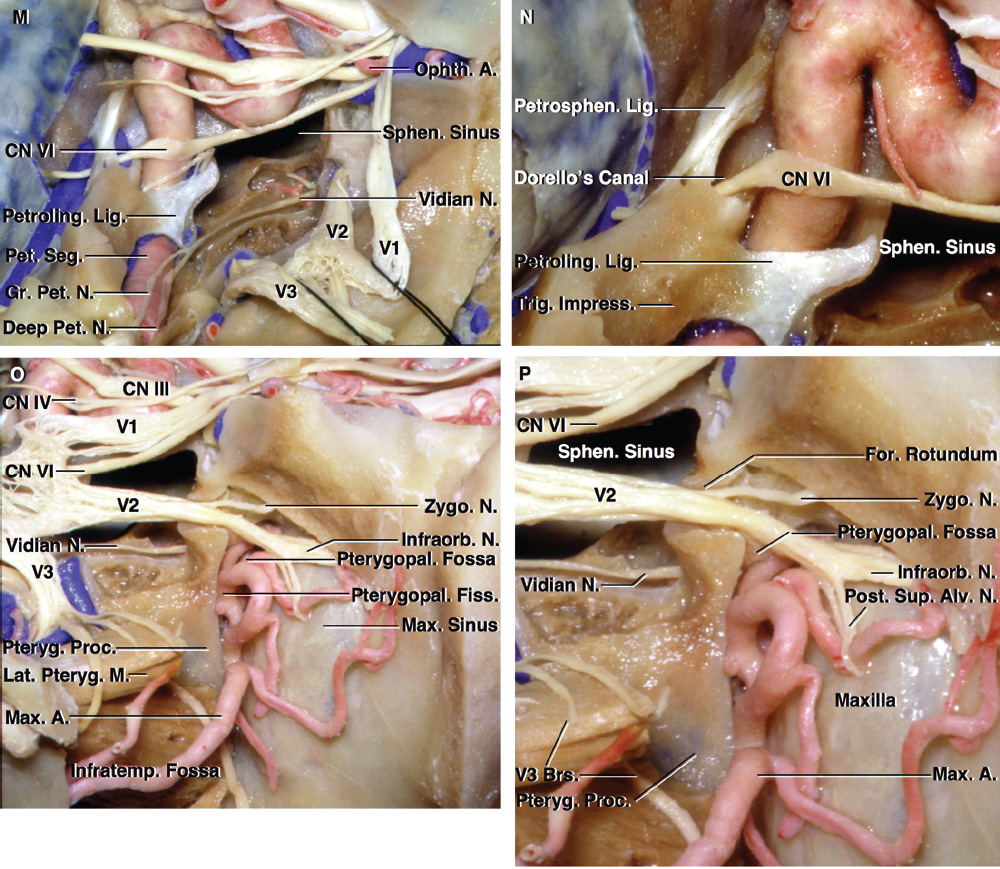
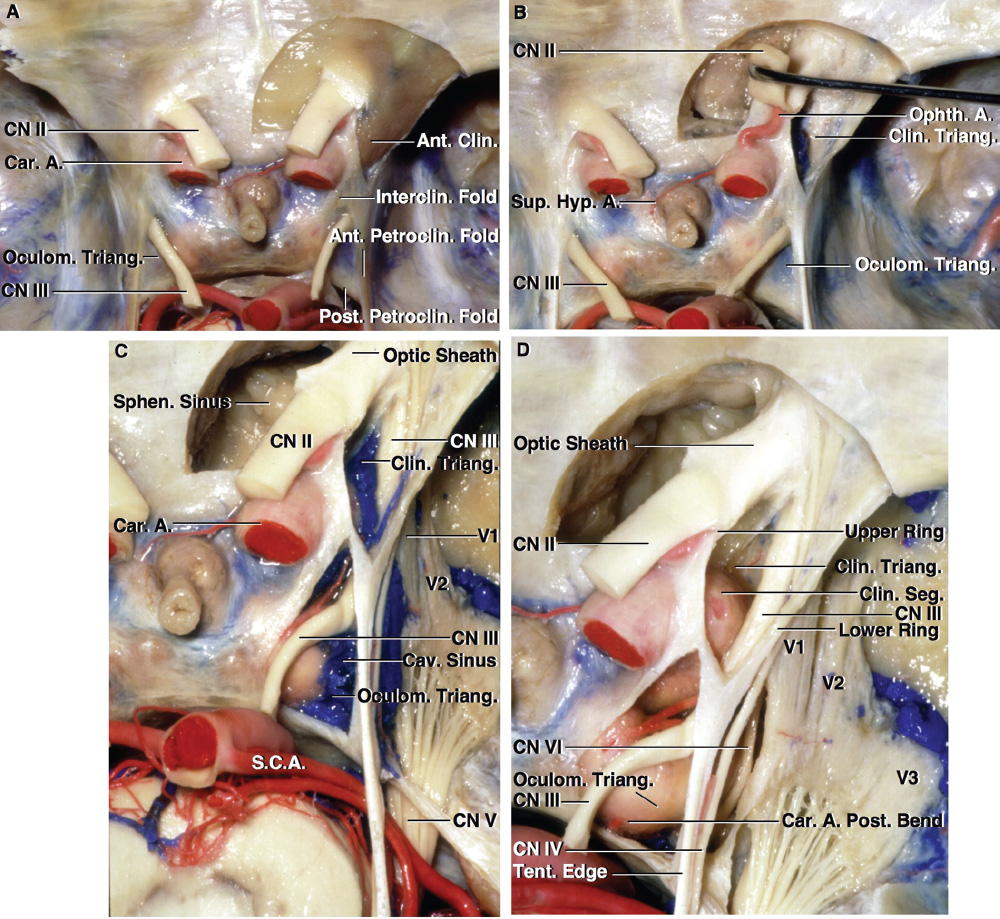
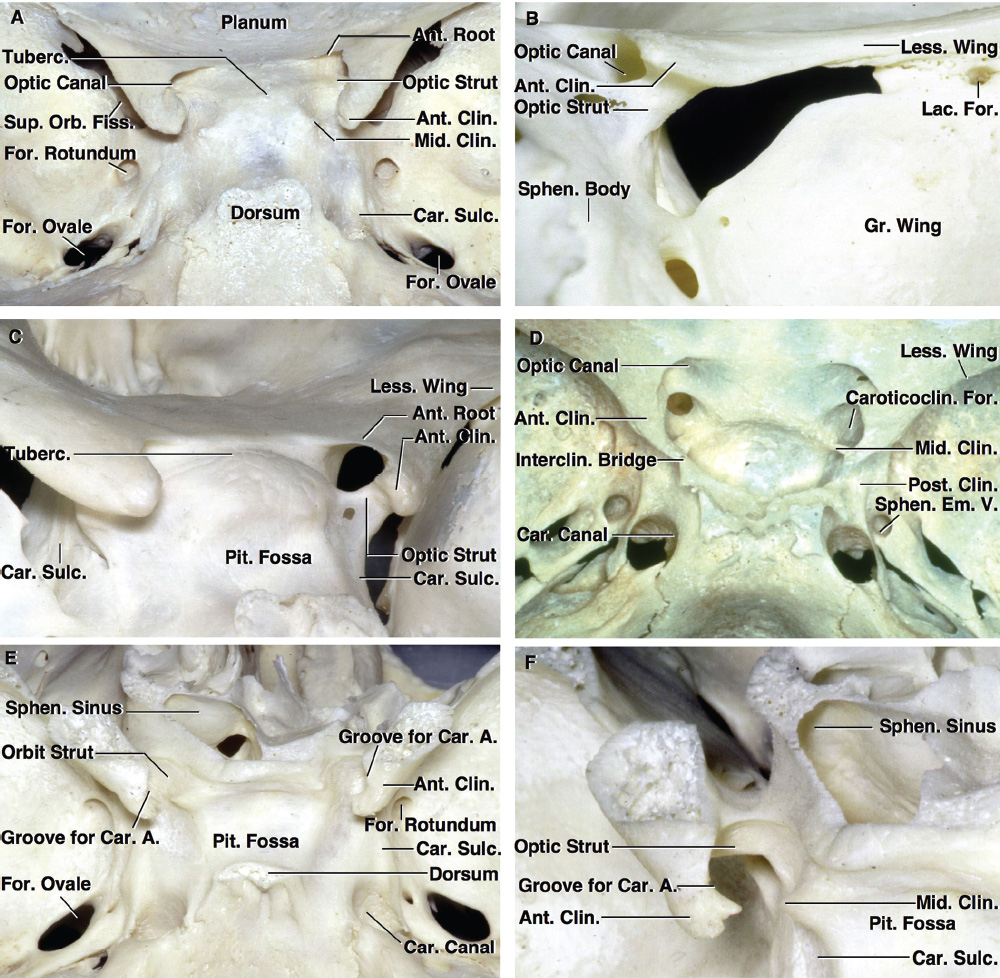
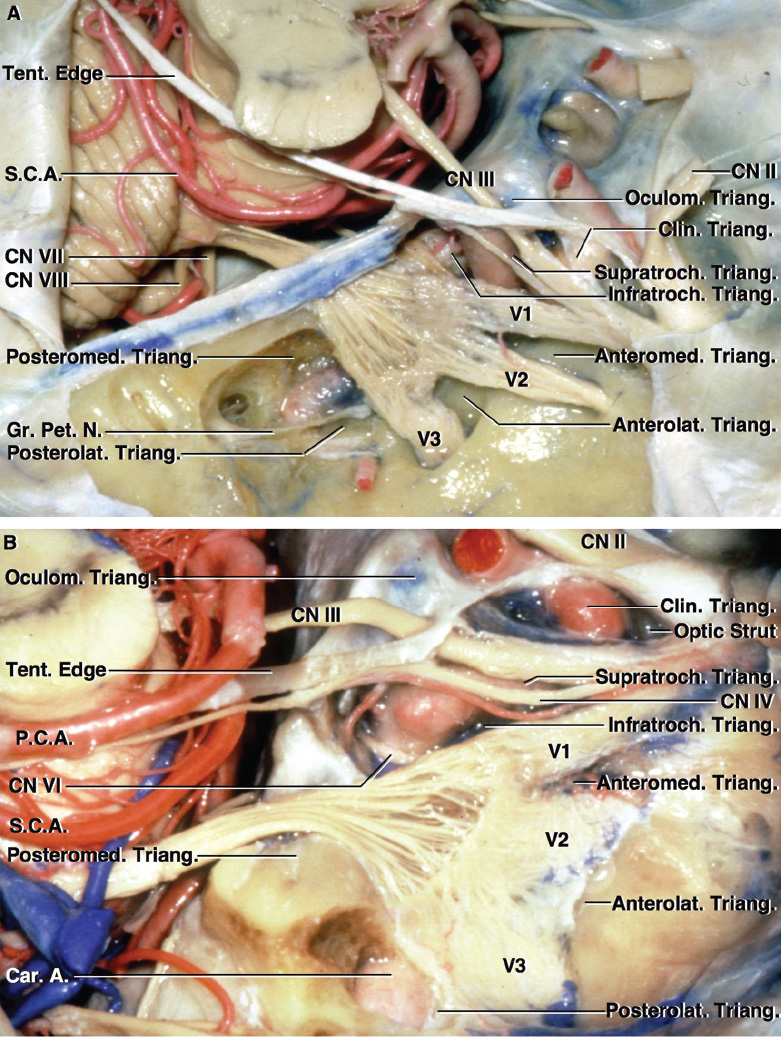
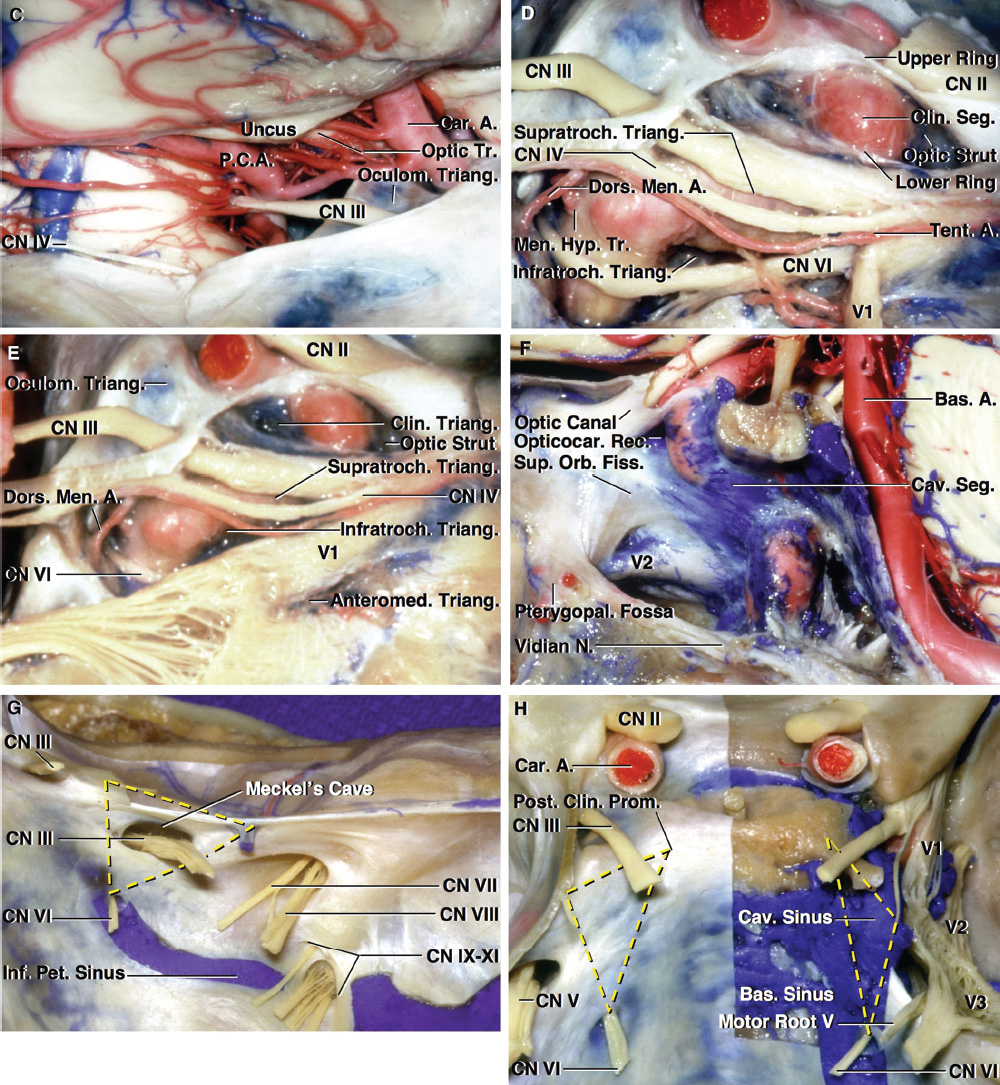
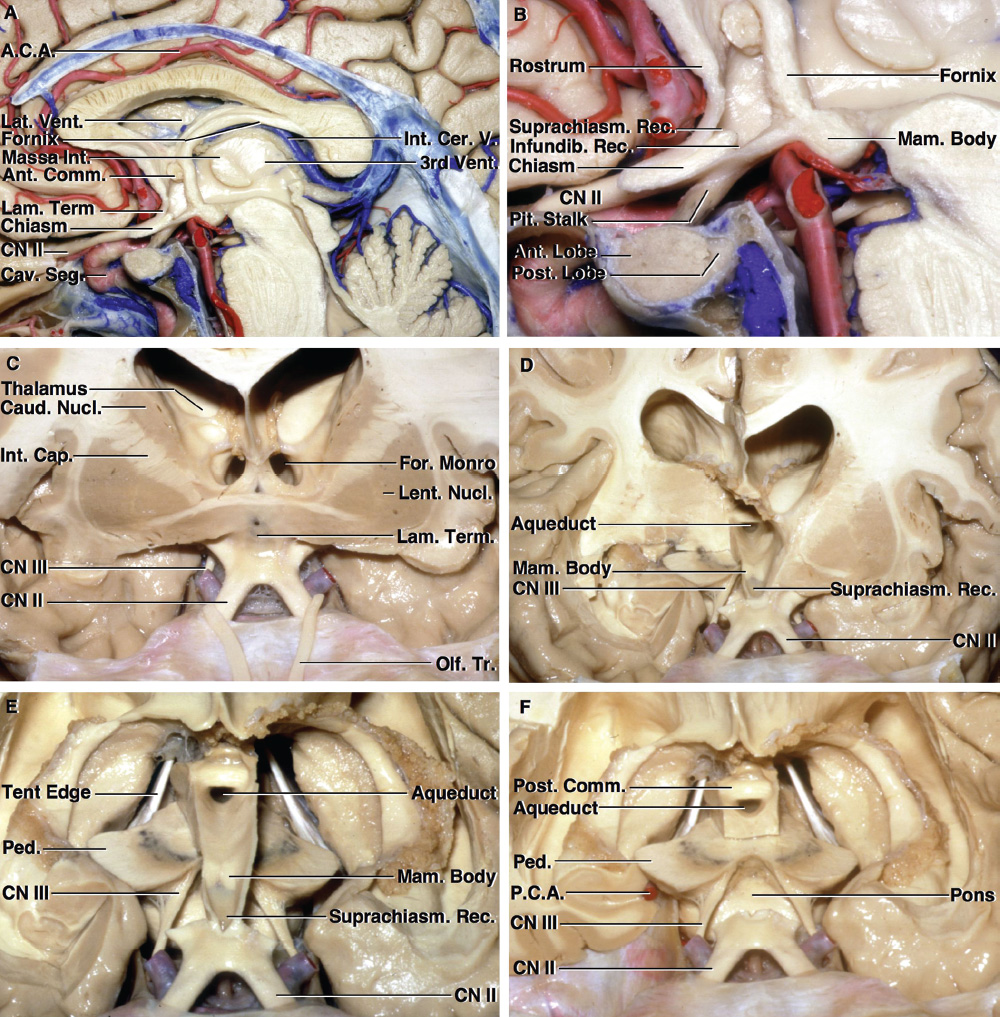
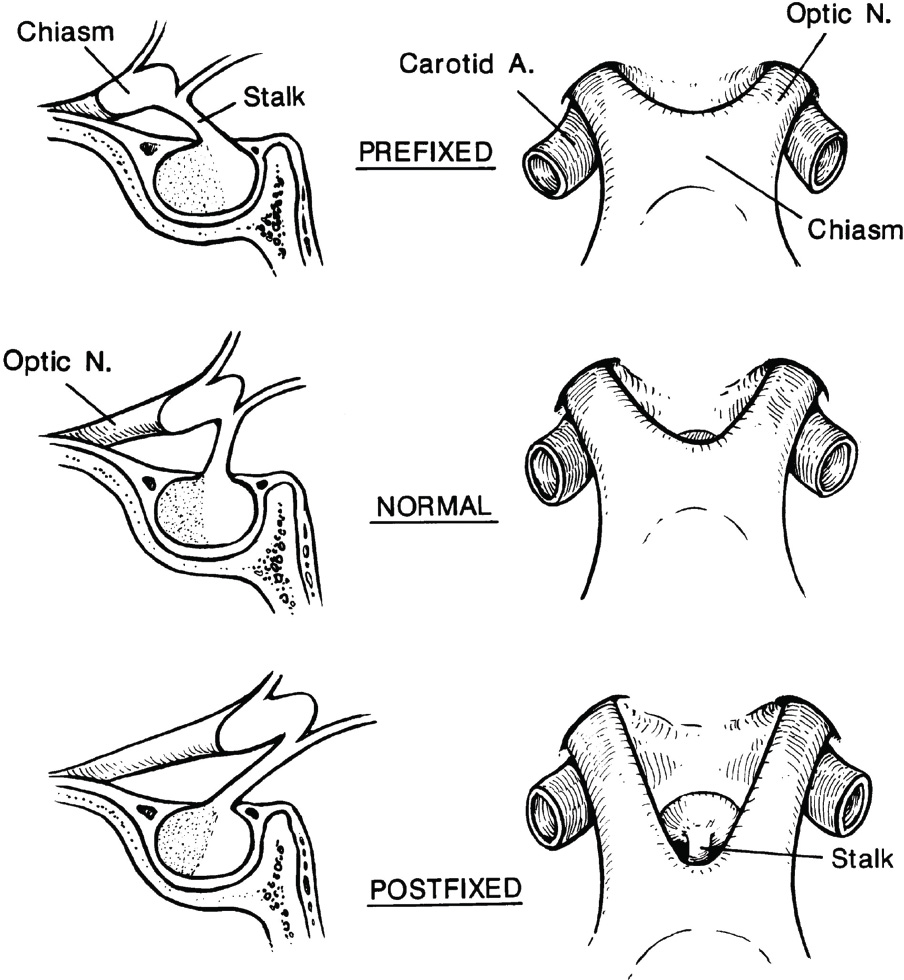
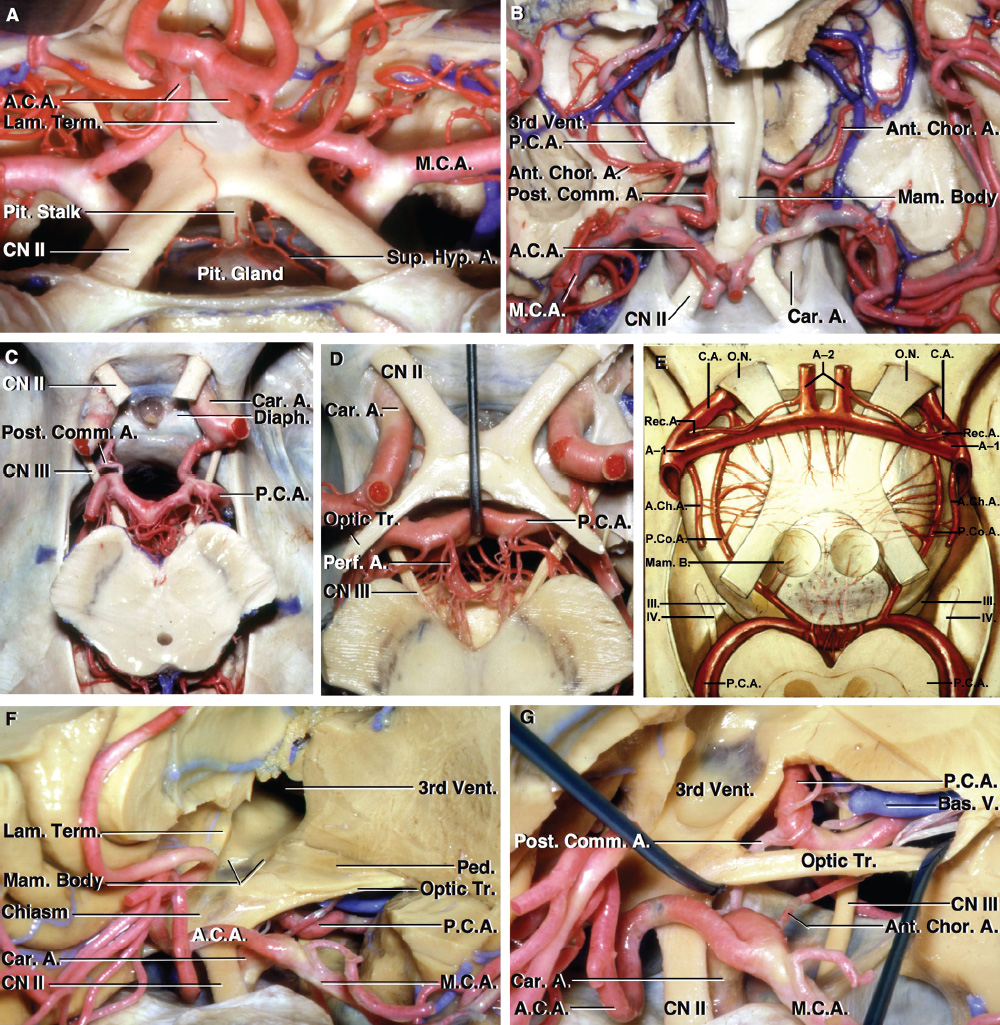
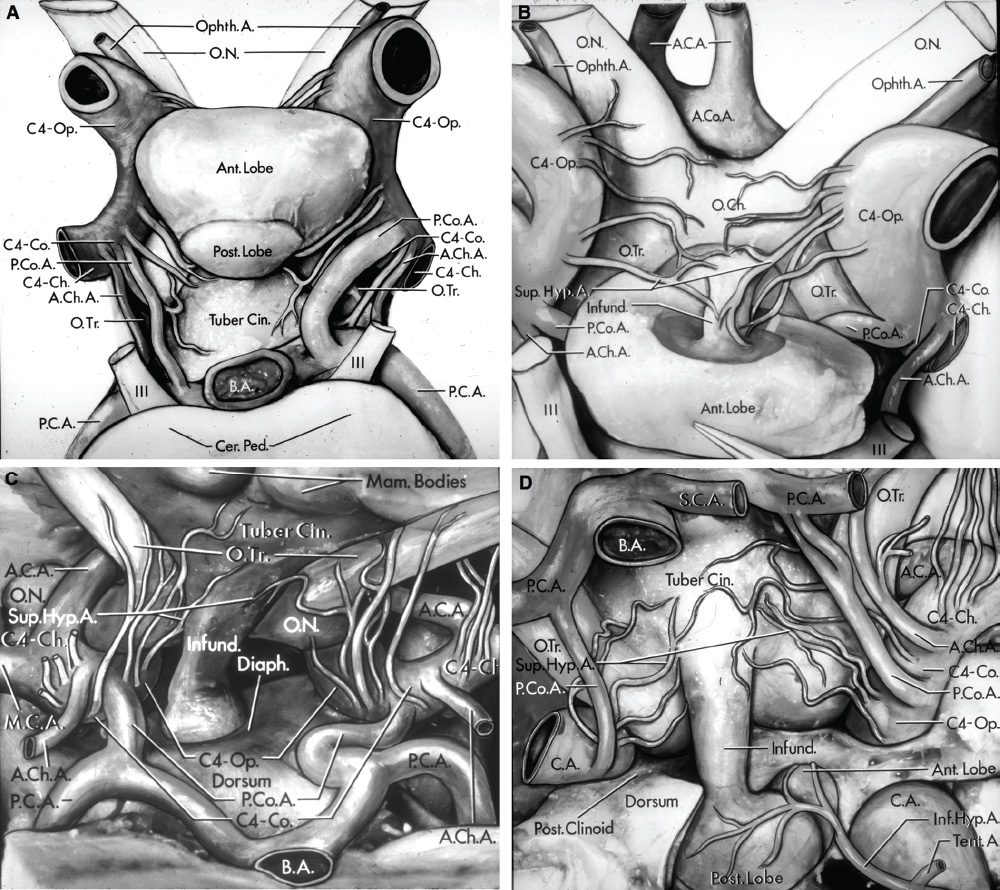
2 Anatomy of the Sellar and Parasellar Region This chapter focuses on the anatomic basis of the microsurgical and endoscopic approaches to the sellar and parasellar regions. The pituitary gland and sella are located in the cranial base below the center of the brain (Fig. 2.1). Access to the sella is limited from above by the optic nerves and chiasm and the circle of Willis, from the sides by the cavernous sinuses and internal carotid arteries, and from behind by the brainstem and basilar artery. Because of the vital structures blocking superior, lateral, and posterior access to the sella, the preferred surgical route to most sellar tumors is from below, through the nasal cavity and sphenoid sinus. These subcranial approaches are being expanded with the aid of the endoscope to include other areas bordering the sella, including the cavernous sinus, Meckel’s cave, and the adjacent parts of the anterior, middle, and posterior cranial fossae. This section focuses on the sella and sphenoid sinus and their subcranial relationships. The sella can be reached by several routes through the nasal cavity.1,2 The nasal cavity, wider below than above, is bounded above by the anterior cranial fossa, laterally by the ethmoid and maxillary sinuses and orbits, and below by the hard palate (Figs. 2.2 and 2.3). This cavity is divided sagittally by the nasal septum, which is formed anteriorly and superiorly by the perpendicular plate of the ethmoid and inferiorly and posteriorly by the vomer; an anterior bony deficiency is occupied by septal cartilage. The nasal cavity opens anteriorly onto the face through the anterior nasal aperture and posteriorly into the nasopharynx by way of the posterior nasal apertures. Each posterior nasal aperture, measuring ~25 mm vertically and 13 mm transversely, is bordered above by the anterior aspect of the sphenoid body, below by the posterior margin of the hard palate formed by the horizontal plate of the palatine bones, medially by the part of the nasal septum formed by the vomer, and laterally by the medial pterygoid plate and perpendicular plate of the palatine bone. Fig. 2.1 The pituitary gland and its relationships. (A) Anterior view of pituitary gland. The gland is located below the optic nerves and chiasm and between the cavernous segments of the internal carotid arteries. The right optic nerve has been elevated to expose the pituitary stalk. The superior hypophyseal arteries arise from the medial side of the supraclinoid portion of the internal carotid arteries and pass medially to the pituitary stalk and optic chiasm. (B) Superior view of pituitary gland. In this case, the diaphragm was largely absent, so that the subarachnoid space extended across and was separated from the top of the anterior lobe only by arachnoid membrane. The right half of the dorsum sellae has been removed to expose the posterior lobe, which was hidden under the dorsum. The inferior hypophyseal artery travels medially from the intracavernous carotid to the posterior lobe. (C,D) Superior and inferior surfaces of a gland in which the anterior and posterior lobes form a relatively ovoid structure. The pars tuberalis wraps partially around the stalk. (E) The anterior and posterior lobes have been separated. The stalk joins the anterosuperior surface of the posterior lobe and is partially surrounded by the pars tuberalis. (F) Midsagittal section of the sella extending through the anterior and posterior lobes and sphenoid sinus. The intracavernous carotid produces prominences in the lateral wall of the sphenoid sinus below and anterior to the gland. The sella extends forward to the anterior edge of the intracavernous carotid only if greatly expanded by tumor. The intercavernous sinuses course along the anterior and posterior margins of the diaphragm. The basilar sinus, located on the back of the dorsum, is the largest connection across the midline between the posterior edge of the paired cavernous sinuses. The inferior hypophyseal artery arises from the posterior bend of the intracavernous carotid and is directed medially toward the posterior lobe. (G) Anterior view of another gland. The optic nerves and chiasm have been elevated to expose the pituitary stalk and the superior hypophyseal and ophthalmic arteries. The superior hypophyseal arteries arise from the medial side of the supraclinoid carotid and pass to the chiasm and stalk. (H) Another gland and sella viewed superiorly. The diaphragm partially covers the upper surface of the gland, but the opening in the diaphragm is larger than the pituitary stalk. The posterior lobe (not shown) was entirely hidden below the dorsum sellae. An intercavernous sinus passes across the upper anterior surface of the gland. (I) Superior view of another gland exposed below a large natural opening in the diaphragm. Fig. 2.2 Stepwise dissection of the nasal pathway along which the transsphenoidal surgery is directed. (A) Sagittal section to the left of the midline and nasal septum. The nasal septum is formed anteriorly by the septal cartilage, above by the perpendicular plate of the ethmoid, and below and posteriorly by the vomer. The posterior inferior part of the septum is supplied by the branches of the sphenopalatine artery, a terminal branch of the maxillary artery. The upper part of the septum, below the cribriform plate, is supplied by the branches of the ethmoidal arteries, which arise from the ophthalmic artery. A septum divides the sphenoid sinus near the midline. The optic chiasm, optic and oculomotor nerves, third ventricle, and pituitary stalk are located above the pituitary gland. The gyrus rectus of the frontal lobe is located above the cribriform plate and olfactory tract. (B) Midsagittal section of the sphenoid sinus and pituitary gland. Prominences overlie the optic canal, internal carotid artery, superior orbital fissure, and maxillary nerve in the wall of the sphenoid sinus. The opticocarotid recess extends laterally between the optic nerve, internal carotid artery, and the prominence medial to the superior orbital fissure, and it extends into the optic strut, which separates the optic canal from the superior orbital fissure. The serpiginous prominence overlying the internal carotid artery is located anterior to and below the pituitary gland. (C) The lateral wall of the nasal cavity is constituted below by the nasal surface of the maxilla and above by the nasal surface of the ethmoid sinuses. The inferior concha (turbinate) is an independent bone that articulates with the nasal surface of the maxilla and perpendicular plate of the palatine bone. The middle and superior conchae are appendages of the ethmoid bone. The lacrimal duct opens below the anterior part of the inferior concha. The inferior, middle, and superior nasal meati are located below their respective conchae. The superior meatus is located between the middle and superior conchae. The sphenoethmoidal recess, a narrow cleft located above the superior concha, separates the superior concha from the anterior surface of the sphenoid sinus and is the site of the ostium communicating the sphenoid sinus and nasal cavity. The eustachian tube opens into the nasopharynx behind the medial pterygoid plate and below the sphenoid sinus. (D) The concha has been removed. The maxillary and frontal sinuses drain into the middle meatus. The lacrimal duct opens below the inferior turbinate into the inferior meatus. The ethmoid bullae are rounded prominences overlying the ethmoidal air cells. The anterior ethmoidal air cells drain into the superior meatus. The posterior ethmoidal air cells and the sphenoid sinus drain into the sphenoethmoidal recess. (E) The medial wall of the maxillary sinus has been removed to expose the sinus roof, which forms the orbital floor. The infraorbital sulcus and canal, which transmit the infraorbital nerve, are situated in the floor of the orbit, forming a prominence in the roof of the maxillary sinus. The anterior and posterior ethmoidal arteries arise from the ophthalmic artery and pass through the anterior and posterior ethmoidal canals to reach the floor of the anterior fossa beside the cribriform plate, where they again penetrate the bone to reach the walls of the nasal cavity. The vidian canal is the site of passage of the vidian nerve to the pterygopalatine ganglion. The vidian nerve is formed by parasympathetic fibers from the greater petrosal nerve and sympathetic fibers from the deep petrosal branch of the carotid plexus. The frontal sinus is situated at the upper anterior part of the medial wall. (F) Removing the lateral wall of the ethmoid air cells, which forms the medial wall of the orbit, exposes the periorbita. The medial wall of the nasolacrimal duct has been removed to expose the interior of the duct. Fig. 2.3 (A–D) Comparison of osseous and mucosal structures in the nasal septum and conchae. (E–F) Osseous relationships along the transsphenoidal and endonasal approaches to the sella. (A) The structures anterior to the left orbital apex and the portion of the maxilla above the alveolar process have been removed to expose the nasal septum, which is formed posteriorly by the vomer, above by the perpendicular plate of the ethmoid, and anteriorly by the septal cartilage. (B) The nasal septum and anterior wall of the sphenoid sinus have been removed. This exposes the superior, middle, and inferior conchae and a midline septum within the sphenoid sinus. The ethmoid air cells are exposed in the medial wall of the right orbit. The part of the sphenoid sinus medial to and below the orbital apex has been opened. (C) The left half of facial skeleton, including the left half of the maxilla and orbit, has been removed to expose the left side of the nasal septum, which is formed above by the perpendicular plate of the ethmoid bone and below by the vomer. The palate is formed anteriorly by the maxilla and posteriorly by the horizontal plate of the palatine bone. (D) The nasal septum has been removed. The inferior concha is a separate bone, which protrudes into the nasal cavity from the maxilla. The middle and superior conchae are appendages of the ethmoid bone. The maxillary ostium is located between the perpendicular plate of the palatine bone behind, the ethmoid superiorly, and the medial maxillary wall below. The maxillary and frontal sinuses and the anterior ethmoid air cells drain into the middle meatus and the posterior ethmoid air cells drain into the superior meatus. (E) The anterior nasal aperture is formed above by the nasal bones, and laterally and below by the maxilla. The anterior part of the osseous nasal septum is formed above by the perpendicular ethmoid plate and below by the vomer. The inferior concha, a separate bone, and the middle concha, an appendage of the ethmoid bone, are visible through the aperture. (F) Posterior view of the posterior nasal aperture. The floor of the posterior aperture is formed by the horizontal plate of the palatine bone. The lateral margin is formed by the medial plate of the pterygoid process and is joined anteriorly by the perpendicular plate of the palatine bone, which forms the part of the lateral nasal wall between the maxilla and the medial pterygoid plate. Posteriorly, the middle concha is much more prominent than the inferior concha and often must be displaced laterally in the transsphenoidal approach to the sphenoid sinus and sella. The vomer extends from the upper surface of the hard palate to the body of the sphenoid bone and separates the paired nasal cavities at the posterior aperture. The lateral nasal wall has three medially directed projections—the superior, middle, and inferior nasal conchae—below which are the corresponding superior, middle, and inferior nasal meati (Figs. 2.2 and 2.3). The paired sphenoethmoidal recesses, located above and behind the superior nasal conchae, are the site of the paired sphenoid ostia, which communicate between the nasal cavity and the sphenoid sinus. The upper half of the lateral nasal wall, composed anteriorly to posteriorly of the frontal process of the maxilla, lacrimal bone, and ethmoid bone with its air cells, separate the nasal cavity from the orbit. The nasolacrimal groove and canal, the site of the lacrimal sac and nasolacrimal duct, respectively, pass downward in front of the anterior end of the middle nasal concha and open into the inferior nasal meatus. The frontoethmoidal suture, located at the junction of the roof and medial orbital wall, is situated at the level of the roof of the nasal cavity and the cribriform plate. The anterior and posterior ethmoidal foramina, through which the anterior and posterior ethmoidal arteries and nerves enter the ethmoidal canals, are located on the medial orbital wall at or just above the frontoethmoidal suture. These arteries and nerves exit the orbit by passing through the ethmoidal canals to enter the anterior cranial fossa at the lateral edge of the cribriform plate. The anterior ethmoidal artery, a terminal branch of the ophthalmic artery, supplies the mucosa of the anterior and middle ethmoid sinuses, the dura covering the cribriform plate and planum sphenoidale, and the upper part of the nasal spectrum. It gives rise to the anterior falcine artery intracranially. The posterior ethmoidal artery, usually smaller than the anterior ethmoidal artery and absent in up to 30% of orbits, feeds the mucosa of the posterior ethmoid sinus, adjacent part of the nasal spectrum, and dura of the planum sphenoidale. The average distance between the anterior lacrimal crest of the frontal process of the maxilla and the anterior ethmoidal foramen is 22 to 24 mm; between the anterior and posterior ethmoidal foramina, 12 to 15 mm; and between the posterior ethmoidal foramen and the optic canal, 3 to 7 mm.2 In transfacial procedures, these arteries may be divided between the periorbita and the medial orbital wall. Care should be taken to prevent damaging the optic nerve, which is sometimes located immediately behind the posterior ethmoidal foramen. The lower part of the lateral nasal wall is formed anteriorly to posteriorly by the maxilla, the perpendicular plate of the palatine bone, and the medial pterygoid plate. The eustachian tube opens into the nasopharynx along the posterior edge of the medial pterygoid plate. The middle nasal concha, an appendage of the ethmoid bone, attaches to the lateral nasal wall at the level of the orbit floor and roof of the maxillary sinus. Thus, the medial wall of the maxillary sinus is bounded medially by the middle and inferior nasal meati and the inferior nasal concha (Figs. 2.2 and 2.3). The maxillary sinus communicates with the middle nasal meatus through an opening located in the medial sinus wall just below the sinus roof. The pterygopalatine fossa, situated just outside the lateral wall of the nasal cavity, is positioned between the posterior wall of the maxillary sinus anteriorly, the pterygoid process posteriorly, and the perpendicular plate of the palatine bone medially (Figs. 2.2, 2.3, and 2.4). The pterygopalatine fossa contains the pterygopalatine ganglion, which receives the vidian nerve (nerve of the pterygoid canal), the segment of the maxillary nerve and its branches located just anterior to the foramen rotundum, and the internal maxillary artery and its terminal branches. This fossa communicates laterally with the infratemporal fossa through the pterygomaxillary fissure and medially with the nasal cavity via the sphenopalatine foramen, through which pass the corresponding nerve and vessels, which provide the predominant supply to the turbinates and nasal septum. The maxillary artery exits the infratemporal fossa to enter the pterygopalatine fossa by passing through the pterygomaxillary fissure. The greater and lesser palatine arteries and nerves arise from the maxillary artery and nerve and descend in the greater and lesser palatine canals, which are separated medially from the nasal cavity by the perpendicular plate of the palatine bone. The sphenoid bone is located in the center of the cranial base3–5 (Figs. 2.3 and 2.5). Because of the intimate contact of the body of the sphenoid bone with the nasal cavity below and the pituitary gland above, the transsphenoidal route is the operative approach of choice for most pituitary tumors. The neural relationships of the sphenoid bone are among the most complex of any bone: the olfactory tracts, gyrus rectus, and posterior part of the frontal lobe rest against the smooth upper surface of the lesser wing; the temporal lobe rests against the inner surface of the greater wing; the pons and mesencephalon lie posterior to the clival portion; and the optic chiasm lies posterior to the chiasmatic sulcus. Additionally, the second through sixth cranial nerves are intimately related to the sphenoid bone, and all exit the skull through the optic canal, superior orbital fissure, or foramen rotundum or ovale, all of which are located in the sphenoid bone (Fig. 2.6). Fig. 2.4 Transnasal route to the sphenoid sinus and sella. (A) The cross-section extends across the nasal cavity, the superior and middle turbinates, the maxillary sinuses, the orbits near the apex, and the ethmoid sinuses in front of the sphenoid sinus. The zygomatic and infraorbital nerves arise from the maxillary nerve in the pterygopalatine fossa, which is located behind the posterior maxillary wall. The nasal septum is formed above by the perpendicular ethmoid plate, below by the vomer, and anteriorly by the cartilaginous septum. (B) The concha and posterior ethmoid air cells have been removed to expose the vomer and the anterior wall of the sphenoid sinus and the sphenoid ostia. The nasolacrimal duct descends along the lateral wall of the nasal cavity. (C) Enlarged view. The perpendicular ethmoid plate joins the anterior sphenoid face, and the vomer extends upward to join the inferior sphenoid wall, both in the midline. The posterior ethmoid air cells are located anterior to the lateral part of the sphenoid face and overlap the superolateral margins of the sphenoid ostia. (D) The anterior face of the sphenoid has been removed to expose the multiseptate sphenoid sinus and the anterior wall of the sella. The bony prominences over the optic canals are situated in the superolateral margins of the sinus. (E) The anterior wall of the sella and the lateral wall of the sphenoid sinus have been removed to expose the petrous and cavernous segments of the carotid artery and the pituitary gland. The posterior wall of the maxillary sinus has been removed to expose the maxillary nerve and artery and the pterygopalatine ganglion in the pterygopalatine fossa. The branches of the maxillary artery penetrate the lateral wall of the nasal cavity to course along the sphenoid face. The maxillary nerve sends communicating rami to the sphenopalatine ganglion. The vidian nerve enters the posterior aspect of the sphenopalatine ganglion. The pituitary gland is surrounded by the cavernous sinuses laterally and an anterior intercavernous sinus above. (F) The optic nerves have been elevated to show the suprasellar area and the relationships between the orbital apex, optic canals, nasal cavity, pterygopalatine fossa, and the petrous and intracavernous segments of the internal carotid artery. The superior hypophyseal arteries pass to the lower margin of the optic chiasm and the pituitary stalk. Fig. 2.5 Types of sphenoid bone. Anterior views. (A) Conchal type of sphenoid bone. (B) Bone with presellar type of sphenoid sinus. (C) Bone with sellar type of sphenoid sinus and well-defined sphenoid ostia. (D) Bone with sellar type of sphenoid sinus with poorly defined sphenoid ostia and obliquely oriented sphenoidal septa. The sphenoid bone has many important arterial and venous relationships: the carotid arteries form a groove on each side of the sphenoid bone and often form a serpiginous prominence in the lateral wall of the sphenoid sinus; the basilar artery rests against its posterior surface; the circle of Willis is located above its central portion; and the middle cerebral artery courses parallel to the sphenoid ridge of the lesser wing. The cavernous sinuses rest against the sphenoid bone, and intercavernous venous connections line the walls of the pituitary fossa and dorsum sellae. In the anterior view, the sphenoid bone resembles a bat with wings outstretched (Fig. 2.5). It has a central portion called the body; two lesser wings, which spread outward from the superolateral part of the body; two greater wings, which spread upward from the lower part of the body; and two pterygoid processes, whose medial and lateral pterygoid plates are directed downward from the body. The body of the sphenoid bone is more or less cubical and contains the sphenoid sinus. The superior orbital fissure, through which the oculomotor, trochlear, abducens, and ophthalmic nerves pass, is formed on its inferior and lateral margins by the greater wing and on its superior margin by the lesser wing. The lesser wing forms the posterior part of the roof of each orbit, and the greater wing forms a large part of the lateral wall of the orbit, the floor of the middle fossa, and the roof of the infratemporal fossa. The optic canals are situated above and are separated from the superomedial margin of the superior orbital fissure by the optic strut, a bridge of bone that extends from the lower margin of the base of the anterior clinoid process to the body of the sphenoid. The narrowest part of the optic canal is closer to the orbital than to the intracranial end. The optic canals average 5 mm in length and are of a conical configuration, tapering to a narrow waist near the orbit end. The sphenoid ostia communicate the nasal cavity with the sinus. The infratemporal crest on the inferior surface of the greater wing is positioned above the junction of the temporal and infratemporal fossae. The lateral pterygoid muscles arise from the lower surface of the greater wing between the infratemporal crest and the lateral pterygoid plate. The area lateral to the infratemporal crest gives origin to the temporalis muscle. The pterygoid (vidian) canal courses posteriorly to anteriorly through the junction of the pterygoid process and the sphenoid body and may be exposed under the mucosa lining the sinus floor by a bony dehiscence. Fig. 2.6 Superior view of sellar region. (A) The sella is located between the cavernous sinuses. The diaphragm, which usually separates the sella from the suprasellar cisterns, is absent in this case. The oculomotor nerves enter the roof of the cavernous sinus, where there is a narrow cistern around the nerve. The oculomotor triangle, the triangular patch of dura through which the oculomotor nerve enters the dura in the cavernous sinus roof, is positioned between the anterior and posterior clinoid processes and the petrous apex. The roof of the cavernous sinus extends forward under the anterior clinoid process. (B) The dura covering the anterior clinoid process and optic canal has been removed. The outer layer of dura in the lateral wall of the cavernous sinus has been removed to expose the thin inner layer of the lateral sinus wall and the lateral surface of Meckel’s cave. The falciform ligament, the dural fold extending above the optic nerve proximal to the entrance of the nerve into the optic canal, extends from the anterior clinoid to the tuberculum. (C) The inner layer of the lateral wall of the cavernous sinus has been removed to expose the nerves coursing in the wall of the cavernous sinus and middle fossa. The dura covering the dorsum sellae, basilar sinus, and posterior clinoid process has been removed. The oculomotor nerve passes forward lateral to the posterior clinoid and below the anterior clinoid. An abnormal bony projection extends laterally from the right posterior clinoid below the oculomotor nerve toward the petrous apex. The basilar sinus crosses the back of the dorsum and upper clivus and communicates widely with the posterior edge of the paired cavernous sinuses. The abducens nerve passes through the lower margin of the basilar sinus. An anterior intercavernous sinus passes along the anterior margin of the sella. (D) The anterior clinoid process has been removed to expose the clinoid segment of the internal carotid artery, defined by the upper and lower dural rings. The upper ring is formed by the dura extending medially from the upper surface of the anterior clinoid. The lower dural ring is formed by the dura, which extends medially from the lower margin of the anterior clinoid and separates the lower clinoid margin from the oculomotor nerve. (E) Posterosuperior view of the sella. The dorsum and posterior clinoid have been removed to expose the posterior lobe of the pituitary, which was hidden below the dorsum. The abducens nerve passes through Dorello’s canal, which is roofed by the petrosphenoid ligament. The trigeminal nerve has been reflected forward to expose the petrolingual ligament, which extends above the internal carotid artery just proximal to the entry of the artery into the cavernous sinus. (F) Enlarged view. The carotid artery protrudes medially to deform the lateral surface of the anterior lobe of the pituitary gland. A tongue of anterior lobe extends laterally above the intercavernous carotid. The inferior hypophyseal branch of the meningohypophyseal artery passes medially to reach the posterior lobe. In the superior view, the pituitary fossa occupies the central part of the body and is bounded anteriorly by the tuberculum sellae and posteriorly by the dorsum sellae (Figs. 2.1 and 2.6). The chiasmatic groove (sulcus), a shallow depression between the paired optic foramina, is bounded posteriorly by the tuberculum sellae and anteriorly by the planum sphenoidale. The frontal lobes and the olfactory tracts rest against the smooth upper surface of the lesser wings and the planum sphenoidale. The posterior margin of the lesser wing forms a free edge, the sphenoid ridge, which projects into the sylvian fissure to separate the frontal and temporal lobes. The anterior clinoid processes are located at the medial end of the lesser wings, the middle clinoid processes are lateral to the tuberculum sellae, and the posterior clinoid processes are situated at the superolateral margin of the dorsum sellae. The dorsum sellae is continuous below with the clivus. The upper part of the clivus is formed by the sphenoid bone and the lower part by the occipital bone. The depth of the sella turcica is the greatest distance between the floor and a perpendicular line connecting the tuberculum and dorsum. Sellar length is defined as the greatest anteroposterior diameter of the pituitary fossa, which may occur at the level of the tuberculum sellae or below. Sellar width is defined as the width of the horizontal plateau of the sellar floor between the carotid sulci. The volume is calculated by applying the simplified mathematical formula for the volume of an ellipsoid: volume (mm3) = 0.5 (length × width × depth [mm])/1000. The upper limit of normal depth is 13 mm; length, 17 mm; width, 15 mm; and volume, 1100 mm3.6 The superior surface of each greater wing is concave upward and filled by the pole of each temporal lobe. The foramen rotundum, foramen ovale, and foramen spinosum, anteriorly to posteriorly, are located near the junction of the body and greater wing. When viewed from the inferior aspect, the vomer, a separate bone forming the lower part of the osseous nasal septum, frequently remains attached to the lower surface of the anterior half of the body of the sphenoid. The pterion and the “keyhole” are two important anatomic landmarks in the region of the greater wing in the lateral view. The pterion is located over the upper part of the greater wing and approximates the site of the lateral end of the sphenoid ridge. The “keyhole” is located behind the junction of the temporal line and the zygomatic process of the frontal bone several centimeters anterior to the pterion. A burr hole placed over the pterion will be located near the lateral end of the sphenoid ridge. A burr hole placed at the keyhole will expose the periorbita in its lower part, the frontal dura in its upper part, and the orbital roof in its midportion. The placement of the keyhole burr hole is reviewed elsewhere.7 The sphenoid sinus is positioned in the sphenoid body between the paired cavernous sinuses, internal carotid arteries, and optic, extraocular, and trigeminal nerves. In addition, the sinus sits between the pituitary gland and the nasal cavity. The sphenoid sinus varies considerably in size and shape and in the degree of pneumatization8,9 (Figs. 2.5, 2.7, and 2.8). It is present as minute cavities at birth, but its main development takes place after puberty. In early life, it extends backward into the presellar area and subsequently expands into the area below and behind the sella turcica, reaching full size during adolescence. As the sinus enlarges, it may partially encircle the optic and vidian canals and extend into the roots of the pterygoid processes, greater wings of the sphenoid bone, anterior clinoid processes, and clival part of the occipital bone. With advancing age, the sinus frequently undergoes further enlargement associated with absorption of its bony walls. Occasionally, there are gaps in its bone, with the mucous membrane lying directly against the dura mater. There are three types of sphenoid sinus in adults: conchal, presellar, and sellar, based on the extent of sinus pneumatized (Fig. 2.5). In the conchal type, the sphenoid body below the sella is a solid block of bone without an air cavity. In the presellar type of sinus, the air cavity does not penetrate beyond a vertical plane parallel to the anterior sellar wall. In the sellar type of sphenoid sinus, the most common type, the air cavity extends into the sphenoid body below the sella and as far posteriorly as the clivus. In our previous study in adult cadavers, this sinus was of the presellar type in 24% and of the sellar type in 76%.6 The conchal type is most common in children before the age of 12 years, at which time pneumatization begins within the sphenoid sinus. In the conchal type, which is infrequent in adults, the thickness of bone separating the sella from the sphenoid sinus is at least 10 mm. The depth of the sphenoid sinus is defined as the distance from the ostium of the sphenoid sinus to the closest part of the sella (Fig. 2.8). In the adult, the average depth is 17 mm (range, 12–23 mm).8 This measurement defines the length of the path within the sinus through which instruments must be passed to reach the sellar wall and is important when instruments are selected for transsphenoidal surgery. The speculum most commonly used for transsphenoidal surgery is 9 cm in length, and its tip should be placed anterior to the sphenoid sinus. In reaching the floor of the sella turcica, the depth of the sphenoid sinus (≥ 2 cm) is added to the 9-cm length of the speculum. Thus, after traversing a distance of 11 to 12 cm, the dissecting instruments must enter the sella turcica and be able to reach above the sella if a suprasellar tumor is present. The distance may be greater in the presence of acromegaly; therefore, it is important that transsphenoidal instruments have shafts at least 12 cm in length. The fact that important neural and vascular structures are exposed either in the lateral sinus wall, directly lateral to the sella, or above the diaphragma sellae, especially if the latter is defective, has led this writer to prefer blunt rather than sharp ring curettes for dissection and tumor removal. Another measurement important in transsphenoidal surgery is the thickness of the anterior sellar wall and sellar floor. In the sellar type of sinus, the thickness of the anterior sellar wall ranged from 0.1 to 0.7 mm (mean, 0.4 mm), compared with 0.3 to 1.5 mm (mean, 0.7 mm) for the presellar type. The thickness of bone covering the sinus was defined at the planum sphenoidale, tuberculum sellae, anterior sellar wall, sellar floor, and clivus. The thickest bone was found at the clivus and tuberculum sellae and the thinnest along the anterior sellar wall.4,6 Fig. 2.7 (A–D) Inferior view of the sellar region and surrounding skull base. (A) The right half of the floor of the sphenoid sinus has been removed to expose the sellar floor and the part of the sphenoid sinus below the planum and tuberculum. On the left side of the specimen, the eustachian tube, pterygoid process, and posterior part of the maxillary sinus have been preserved. On the right side, the medial portion of the eustachian tube and the pterygoid process have been removed. This exposes the right mandibular nerve exiting the foramen ovale and the maxillary nerve exiting the foramen rotundum and passing forward as the infraorbital nerve. The pterygopalatine ganglion is located in the pterygopalatine fossa behind the maxillary sinus in the lateral wall of the nasal cavity. The right pterygoid process has been removed to expose the vidian canal, in which the vidian nerve travels to reach the pterygopalatine ganglion. The bone below the petrous carotid has been removed up to the point where the artery turns upward to enter the posterior part of the cavernous sinus. (B) Part of the vomer, perpendicular ethmoid plate, and floor of the sphenoid sinus have been removed to expose the cavernous sinus, intracavernous carotid, and pituitary gland. The floors of the optic canals have been removed to expose the ophthalmic arteries coursing below the optic nerves. The cavernous sinus surrounds the intracavernous carotid. An anterior intercavernous sinus crosses the anterior margin of the gland. Some of the upper clivus has been removed to expose the basilar sinus, which sits on the back of the dorsum and is the largest connection between the cavernous sinuses. (C) The venous spaces around the pituitary gland have been cleared to expose the petrous and intracavernous carotid segments. (D) Enlarged view of the pituitary gland, intracavernous carotid, and optic nerves and ophthalmic arteries. The inferior hypophyseal arteries pass to the posterior lobe. The superior hypophyseal arteries arise in the chiasmatic cistern and pass medially to reach the stalk and chiasm. The septa within the sphenoid sinus vary greatly in size, shape, thickness, location, completeness, and relation to the sellar floor (Fig. 2.9). The cavities within the sinus are seldom symmetric from side to side and are often subdivided by irregular minor septa. The septa are often located offthe midline as they cross the floor of the sella. In our previous study, a single major septum separated the sinus into two large cavities in only 68% of specimens, and even in these cases the septa were often located offthe midline or deflected to one side.6 The most common type of sphenoid sinus has multiple small cavities in the large paired sinuses. The smaller cavities may be separated by septa oriented in all directions. Computed tomography (CT) or magnetic resonance imaging (MRI) of the sella provides the definition of the relationship of the septa to the floor of the sella needed for transsphenoidal surgery. Major septa may be found as far as 8 mm offthe midline.6 The septa are not to be used as a guide to the midline but may be used as landmarks based on where preoperative CT and MRI show them to be located in relation to the sella and the tumor. The internal carotid artery rests directly against the lateral surface of the body of the sphenoid bone, and its course is marked by a groove in the bone, the carotid sulcus, which defines the course of the intracavernous portion of the carotid artery. As the sinus expands and its walls are resorbed, the carotid sulcus produces a prominence within the sinus wall below the floor and along the anterior margin of the sella6,8 (Figs. 2.6, 2.8, and 2.10). This prominence is most pronounced with maximal pneumatization of the sphenoid and varies from a small focal bulge to a serpiginous elevation marking the full course of the carotid artery along the lateral sinus wall. The intrasinus carotid prominence can be divided into three parts: the retrosellar, infrasellar, and presellar segments. The first part, the retrosellar segment, is located in the posterolateral part of the sinus. This segment of the prominence is present only in well-pneumatized sellar-type sinuses in which the air cavity extends laterally in the area below the dorsum. The second part, the infrasellar segment, is located below the sellar floor. The third part, the presellar segment, is located anterolateral to the anterior sellar wall. Of the 50 specimens we examined, 98% had presellar, 80% had infrasellar, and 78% had retrosellar prominences.1,6 Any part of the prominence may be present and the others absent. If all three parts are present and connected, they form a serpiginous bulge marking the full course of the carotid artery along the lateral wall of the sinus. In the normal sinus, the presellar part courses anterolateral to the anterior sellar wall. The anterior sellar wall bulges forward of the carotid prominence only when the sella is greatly expanded by tumor. Fig. 2.8 Nasal pathway to the sphenoid sinus. Stepwise dissections showing the structures that form the lateral limit of the transnasal route to the sphenoid sinus and sella. (A) Sagittal section to the right of midline. The nasal septum, along which the transsphenoidal approach is directed, is formed above by the perpendicular plate of the ethmoid, anteriorly by the nasal septal cartilage, and below by the vomer. The vomer articulates with the anterior-inferior part of the sphenoid body, and the perpendicular plate articulates with the anterior face. The sphenoid sinus is located in the body of the sphenoid bone. (B) The sagittal section has been extended to the right of midline. The nasal conchae and meati and the eustachian tubes are in the lateral margin of the exposure. (C) A portion of the middle and inferior turbinates has been removed. The ostia of the maxillary and frontal sinuses open into the middle meatus, located below the middle turbinate. The nasolacrimal duct opens below the lower turbinate into the inferior meatus. Rosenmuller’s fossa is located behind the eustachian tube. (D) The mucosa in the lateral margin of the nasal cavity and the posterior part of the inferior and middle turbinates have been removed to expose the pterygoid process and posterior maxillary wall, which form the posterior and anterior boundaries of the pterygopalatine fossa, respectively. The eustachian tube opens into the nasopharynx at the posterior edge of the pterygoid process. The terminal branches of the maxillary artery pass through the pterygopalatine fossa, located between the posterior maxillary wall and the pterygoid process, and give rise to the sphenopalatine artery, which enters the posterior superior part of the nasal cavity by passing through the sphenopalatine foramen. The medial wall of the pterygopalatine fossa is formed by the perpendicular plate of the palatine bone. (E) The medial wall of the maxillary sinus has been opened to expose the infraorbital nerve, which arises in the pterygopalatine fossa and passes forward in the sinus roof. The maxillary nerve passes through the foramen rotundum to enter the pterygopalatine fossa, where it gives rise to the infraorbital, zygomatic, and greater palatine nerves, plus communicating rami to the pterygopalatine ganglion. (F) Enlarged view. The bone and dura covering the optic canal in the superolateral part of the sphenoid sinus have been opened to expose the optic nerve and ophthalmic artery in the optic canal. The junction of the petrous and cavernous carotid limits the exposure below the level of the sella. Terminal branches of the maxillary artery intermingle with the neural structures in the pterygopalatine fossa and exit the fossa to supply structures within and bordering the nasal cavity. Fig. 2.9 Septa in the sphenoid sinus. The heavy broken line on the central diagram shows the plane of the section of each specimen from which the drawings were taken, and the large arrow shows the direction of view. The planum is above, the dorsum and clivus are below, and the sella is in an intermediate position on each diagram. The heavy dark lines on the drawings show the location of the septa in the sphenoid sinus. A wide variety of septa separate the sinus into cavities that vary in size and shape and are seldom symmetric from side to side. Only the presellar part of the carotid prominence is present in a presellar type of sphenoid sinus, and it is this part that is also most frequently present in the sellar type of sinus. The corresponding arterial segments are slightly longer than the segments of the prominence because of tortuosity of the artery. This tortuosity, although present, is limited by the dural walls of the cavernous sinus, particularly if the artery is encircled by a ring of bone formed by the union of the anterior and middle clinoid processes. Serial coronal scans through the cavernous sinus show that the artery does not always nestle into the bony carotid sulcus on the intracranial surface of the sphenoid bone, but is separated from it by an extension of the cavernous sinus. Fig. 2.10 Anterior view of a coronal section in front of the sphenoid sinus, through the nasal cavity, orbits, and ethmoid and maxillary sinuses. (A) The upper part of the nasal cavity is separated from the orbits by the ethmoid sinuses. The lower part of the nasal cavity is bounded laterally by the maxillary sinuses. The middle concha projects medially from the lateral nasal wall at the junction of the maxillary and ethmoid sinuses. The posterior ethmoid air cells are located in front of the lateral part of the face of the sphenoid sinus. (B) The middle and inferior nasal conchae on the left side and the nasal septum and the posterior ethmoid sinuses on both sides have been removed to exposed the posterior nasopharyngeal wall, the anterior aspect of the sphenoid body, and the sphenoid ostia. The posterior ethmoid air cells overlap the lateral margin of the sphenoid ostia. (C) The anterior wall of the sphenoid sinus has been opened, and the sphenoid septum has been removed to expose the anterior sellar wall in the midline and the prominences over the optic canals and carotid arteries in the lateral walls of this well-pneumatized sphenoid sinus. The medial part of the posterior wall of the left maxillary sinus has been removed to expose branches of the maxillary artery in the pterygopalatine fossa. The opticocarotid recesses extend laterally between the prominences over the carotid arteries and optic nerves. (D) The pituitary gland, intracavernous carotids, optic nerves, ophthalmic arteries, and cavernous sinuses have been exposed by removing the bone of the sinus wall. The inferolateral trunk passes above and lateral to the abducens nerve. The shortest distance between the paired carotid arteries is usually located just below the tuberculum sellae. A capsular artery arises from the intracavernous carotid and passes upward and medially. (E) Oblique view. The bony prominences overlying the optic canal, superior orbital fissure, intracavernous carotid artery, and maxillary nerve are exposed in the lateral wall of the sphenoid sinus. The bony depression between the carotid prominence and the optic canal, the opticocarotid recess, extends into the medial end of the optic strut. The broad, round prominence below the opticocarotid recess is produced by the structures passing through the superior orbital fissure. (F) Oblique view. The pituitary gland, intracavernous carotid artery, ophthalmic artery, and optic, ophthalmic, maxillary, oculomotor, and abducens nerves have been exposed. The abducens nerve courses medial to the ophthalmic nerve. The bone separating the artery and the sphenoid sinus is thinner over the anterior than over the posterior parts of the carotid prominence and is thinnest over the part of the artery just below the tuberculum sellae. A layer of bone less than 0.5 mm thick separates the artery and sinus in nearly 90% of sinuses, and areas of absence of bone between the artery and the sinus are present in nearly 10%.8 Only the dura covering the intracranial surface of the sphenoid bone and the sinus mucosa separate the air cavity and carotid arteries if there is a dehiscence of bone along the carotid prominences. The proximity of the carotid prominences to the midline is important in pituitary surgery. The transverse separation between the carotid prominences of each side was measured at the level of the tuberculum sellae, anterior sellar wall, sellar floor, dorsum sellae, and clivus. The shortest distance between the prominences was located just below the tuberculum in 72%, at the level of the sellar floor in 20%, and adjacent the clivus in 8% of our specimens8 (Figs. 2.4, 2.7, and 2.10). The optic canals protrude into the superolateral portion of the sinus, and there are areas where no bone separates the optic sheath and sinus mucosa (Figs. 2.2, 2.4, 2.8, 2.10, and 2.11). The superior orbital fissure produces a smooth, wide prominence in the midlateral sinus wall below the optic canal, and the maxillary nerve underlies a prominence in the inferolateral part. A bone thickness of 0.5 mm or less separates 80% of optic nerves from the sinus. Care must be taken to avoid damage to the nerves in the transsphenoidal approach if a dehiscence of the bone covering exposes them in the sinus.10,11 A pneumatized diverticulum of the sinus, the opticocarotid recess, often extends laterally into the optic strut between the prominences along the optic canal, carotid artery, and superior orbital fissure (Figs. 2.2, 2.10, and 2.11). This pneumatization may extend through the optic strut into the anterior clinoid process, thus creating a channel through which cerebrospinal fluid (CSF) can leak into the sinus after an anterior clinoidectomy, with resulting CSF rhinorrhea. There is frequently a prominence in the lateral sinus wall overlying the maxillary branch of the trigeminal nerve just proximal to the extracranial end of the foramen rotundum, especially if the sinus is well pneumatized. There also may be areas where no bone separates the nerve from the sinus mucosa, and the presence of a bone thickness of less than 0.5 mm separating the nerve from the sinus is common. The length of maxillary division bulging into the sinus ranges from 7.0 to 15.0 mm (mean, 10.9 mm).8 The sphenoid sinus may also extend laterally below the maxillary nerve into the medial part of the greater sphenoid wing and partially surround the foramen ovale. The prominences overlying the optic canal, superior orbital fissure, and maxillary nerve located in the lateral part of the presellar portion of the sphenoid sinus are not normally visible with the operating microscope; however, they are easily identified with the use of straight and angled endoscopes. Removing the mucosa and bone from the lateral wall of the sinus exposes the dura mater covering the medial surface of the cavernous sinus and optic canals (Figs. 2.10 and 2.11). Opening this dura may expose the carotid artery and the nerves passing through the optic canal, superior orbital fissure, cavernous sinus, and foramen rotundum. The abducens nerve is located between the lateral side of the carotid artery and the medial side of the first trigeminal division. The second and third trigeminal divisions are seen in the lower margin of the opening through the lateral wall of sphenoid sinus. In half of the cases, the optic and trigeminal nerves and the carotid arteries have areas where bone 0.5 mm or less in thickness separates them from the mucosa of the sphenoid sinus, and in a few cases the bone separating these structures from the sinus is absent6,12 (Figs. 2.2, 2.8, and 2.10). The absence of such bony protection within the walls of the sinus may explain some of the cases of cranial nerve deficits and carotid artery injury after transsphenoidal operations.10 Placing the speculum in the sinus, as has been advocated, also increases the risk for damaging other structures in the wall of the sinus, including the maxillary nerves and the nerves passing through the superior orbital fissure.13 Vigorous curettage of the walls of the sphenoid sinus can also cause damage to the nerves passing through the optic canal, superior orbital fissure, and foramen rotundum and to the carotid arteries. The carotid arteries always project anterior to the plane of the anterior sellar wall unless the sella has been greatly expanded anteriorly by tumor. The bone overlying the carotid arteries at the lateral edges of the anterior sellar wall is often thinner than the bone anterior to the pituitary gland. The bulge of the sellar floor is usually identifiable, unless the sinus is of a presellar or conchal type, in which case the sellar bulge may not be apparent. However, the floor of the sella turcica should be directly ahead of the long axis of the transsphenoidal exposure if the blades of the speculum have been positioned correctly, with the vertical crest on the face of the sphenoid positioned between the tips of the speculum blades (Fig. 2.12). The prominences overlying the carotid arteries are frequently exposed at the lateral edge of the anterior sellar wall and are not to be confused with the prominence overlying a tumor (Figs. 2.2, 2.8, and 2.10). Excessive spreading of the bivalve speculum has been reported to cause a fracture of the medial orbital wall with visual loss.14 The distance between the optic nerves at the coronal plane through the anterior wall of the sphenoid sinus averages 2.93 cm (range, 2.71–3.09 cm).15 This distance, measured 5 and 10 mm behind the anterior sinus wall and inside the sinus, decreases to an average of 2.62 cm (range, 2.47–2.71 cm) and 1.58 cm (range, 1.39–1.74 cm), respectively. Care should be taken in opening the speculum at the anterior wall of the sphenoid sinus beyond 2.5 cm. This distance between the optic nerves may narrow to less than 1.5 cm if the speculum is advanced 10 mm into the sinus. It is important to remember that the blades of the speculum are pushing several millimeters of soft and osseous tissue from the turbinates and nasal wall laterally ahead of the tips of the blades. The transsphenoidal speculum usually encounters the firm resistance of the middle turbinates and the lateral nasal wall as it is opened, so that the extent of opening the blades is limited. Displacement of the speculum contralaterally by the middle turbinate places the tip of the blades near the contralateral optic nerve and may be associated with optic nerve injury with lesser degrees of speculum opening. This natural resistance, which tends to limit speculum opening, may be absent in some cases of reduced bone strength due to extensive erosion of bone by tumor, reoperation after extensive bone removal, and softening of osteoporotic bone associated with Cushing disease and long-standing steroid use. In these cases, the speculum should be opened gently. Fig. 2.11 Stepwise dissection examining the relationship of the sphenoid sinus to the pituitary fossa, cavernous sinus, and sellar region. (A) Anterior view into a sphenoid sinus with the mucosa removed to show the relationships of the structures that can be exposed by the transsphenoidal approach. The structures in the exposure include the major sphenoidal septum, anterior sellar wall, and prominences over the carotid arteries and optic canals. The tuberculum sellae and planum sphenoidale are located above the anterior sellar wall. The opticocarotid recess extends laterally between the carotid artery and optic canal. (B) The bone in the walls of the sphenoid sinus has been removed while the dura is preserved. The optic nerves, intracavernous carotids, and pituitary gland are seen through the dura. The anterior bend of the intracavernous carotid bulges forward inside the dura immediately below the optic canals. The basilar sinus, which forms the largest connection between the paired cavernous sinuses, is situated behind the clivus and dorsum sellae. The inferior hypophyseal artery passes to the capsule of the posterior lobe. The optic nerve and ophthalmic artery can be seen through the optic sheath. (C) The dura forming the medial and lower walls of the cavernous sinuses has been removed. Intercavernous sinuses connect the paired cavernous sinuses across the midline. The dura in the floor of the optic canals has been opened to expose the ophthalmic arteries and optic nerves. The basilar sinus sits on the dorsum sellae and clivus and interconnects the posterior end of the paired cavernous sinuses. (D) The venous space has been cleared to expose the intracavernous carotid and the anterior and posterior pituitary lobes. The inferior hypophyseal arteries arise from the meningohypophyseal branch of the intracavernous carotid and pass to the capsule of the posterior lobe. Sympathetic nerves ascend on the carotid arteries. The abducens nerve passes through the cavernous sinus on the lateral side of the internal carotid artery and medial to the ophthalmic nerve. (E) Oblique view of the intracavernous carotid showing the inferior hypophyseal artery passing to the capsule of the posterior lobe. (F) The dura has been removed to expose the intradural structures. The anterior cerebral arteries are situated above and the pituitary gland is situated below the optic chiasm. The basilar apex is located behind the gland. The oculomotor nerve passes forward between the posterior cerebral and superior cerebellar arteries. Fig. 2.12 Endonasal approaches to the sphenoid sinus. (A) The Rhoton endonasal transsphenoidal speculum has been inserted along the route of the endonasal transsphenoidal approach directed through one nostril, between the conchae laterally and the nasal septum medially. (B) The broken line shows the area where the posterior septum is separated from the face of the sphenoid sinus. (C) Superior view of the nasal cavity (left side). The speculum is advanced in one nostril between the septum and turbinates to the sphenoid face. The mucosa is opened on the sphenoid face to the left of the septum (arrow), and the mucosa is elevated and the posterior septum separated from the face of the sphenoid (red arrow, right side). The speculum blades have been advanced submucosally along the face of the sphenoid sinus. The speculum is positioned so that the crest on the face of the sphenoid is in the midline between the tips of the blades of the speculum. (D) Upper left, lateral view of the endonasal exposure of the sphenoid sinus. Lower right, a small osteotome is used to open the face of the sphenoid. No bone is removed from the septum, and the bone taken from the face of the sphenoid is preserved to serve as a stent for closing the anterior sellar wall. (E) Variations of transsphenoidal specula. The speculum on the left has blades that are tapered so that the opening at the nostril is greater than the exposure obtained at the sphenoid face. The Rhoton speculum on the right was designed for endonasal transsphenoidal surgery. It has parallel blades that open so that the exposure at the sphenoid face is slightly greater than the opening of the speculum at the anterior nasal aperture. (F) The upper right shows the thicker, wider blades on the traditional transsphenoidal speculum. The lower left shows the Rhoton modified endonasal speculum with smaller, thinner blades that open like the parallel blades shown in (E). Several routes through the nasal cavity have been used to reach the sphenoid sinus (Fig. 2.12). The sublabial approach is directed under the lip and submucosally along the nasal septum to the sphenoid sinus. The transseptal approach avoids the oral cavity because it is directed through a small incision along one side of the columella and submucosally along the septum. The direct endonasal approach, used in recent years by the author, is directed through one nostril, between the conchae laterally and the nasal septum medially, and does not require an incision in the nose before the anterior face of the sphenoid is reached and opened16 (Fig. 2.12). No incision is needed in the anterior part of the nasal cavity, and nasal packing is uncommonly needed at the end of the procedure. In the direct endonasal approach, a handheld nasal speculum inserted into one nostril between the conchae and nasal septum is opened to compress the conchae and septum sufficiently that the endonasal transsphenoidal speculum can be advanced through one nostril to the sphenoid face. Removal of the conchae is not required. The junction of the crest in the sphenoid face at the attachment of the septum is the most reliable anatomic landmark for maintaining the exposure in the midline. The sphenoid ostia are situated on each side of the perpendicular ethmoid plate, which forms the upper part of the nasal septum and marks the upper limit of the sinus opening for most pituitary tumors. The sinus mucosa should be preserved if possible because the ciliary action of the normal mucosa aids in clearing secretions from the sinus. A sinus devoid of mucosa lacks normal drainage, becomes easily infected, and may be the source of a patient experiencing a foul odor following surgery. The diaphragma sellae forms the roof of the sella turcica (Fig. 2.1). It covers the pituitary gland, except for a small central opening in its center, which transmits the pituitary stalk. The diaphragma is more rectangular than circular, tends to be convex or concave rather than flat, and is thinner around the infundibulum and somewhat thicker at the periphery. It frequently is a thin, tenuous structure that would not be an adequate barrier for protecting the suprasellar structures during a transsphenoidal operation. In a prior anatomic study, Renn and Rhoton17 found that the diaphragma was at least as thick as one layer of dura in 38% of cases, and in these cases it furnishes an adequate barrier during transsphenoidal hypophysectomy. In the remaining 62%, the diaphragma was extremely thin over some portion of the pituitary gland. It was concave when viewed from above in 54% of the specimens, convex in 4%, and flat in 42%. Even when flat, it lies below the plane of the upper surface of the anterior clinoid process, so that a medially projecting supradiaphragmatic lesion, such as an aneurysm, may appear on neuroradiologic studies to be located below the anterior clinoid and within the cavernous sinus when it is above the diaphragm in the subarachnoid space. The opening in the center of the diaphragm is large compared with the size of the pituitary stalk. The diaphragmatic opening was 5 mm or greater in 56% of our cases, and in these, it would not have formed a barrier during transsphenoidal pituitary surgery. The opening was round in 54% of the cases and elliptic, with the short diameter of the ellipse oriented in an anteroposterior direction, in 46%. A deficiency of the diaphragma sellae is assumed to be a precondition to the formation of an empty sella. An outpouching of the arachnoid protruded through the central opening in the diaphragma in about half of the sellae. This outpouching, if opened, represents a potential source of postoperative CSF leakage.10 When exposed from above by opening the diaphragma, the superior surface of the posterior lobe of the pituitary gland is lighter in color than the anterior lobe (Figs. 2.1 and 2.6). The anterior lobe wraps around the lower part of the pituitary stalk to form the pars tuberalis.4,17 The posterior lobe is softer, almost gelatinous, and more densely adherent to the posterior sellar wall. The anterior lobe is firmer and more easily separated from the sellar walls. The width of the gland is equal to or greater than its depth or length in most patients. Its inferior surface usually conforms to the shape of the sellar floor, but its lateral and superior margins vary in shape because these walls are composed of soft tissue rather than bone. If there is a large opening in the diaphragma, the gland tends to be concave superiorly in the area around the stalk. The superior surface may become triangular as a result of being compressed laterally and posteriorly by the carotid arteries. As the anterior lobe is separated from the posterior lobe, there is a tendency for the pars tuberalis to be retained with the posterior lobe. Small intermediate lobe cysts may be encountered during separation of the anterior and posterior lobes. The distance separating the medial margin of the carotid artery and the lateral surface of the pituitary gland is an important consideration in transsphenoidal surgery (Figs. 2.6, 2.7, and 2.10). There is often a separation between the lateral surface of the gland and the carotid artery. In the cases in which the artery did not indent the gland, the distance between the gland and artery varied from 1 to 7 mm (average, 2.3 mm); however, in about one in four cases, the artery will indent the medial wall of the cavernous sinus and the gland6,18 (Fig. 2.6F). In these cases, the gland loses its spherical shape and conforms to the wall of the artery, often developing protrusions above or below the artery that may be confused with extensions of tumor. Intrasellar tumors are subjected to the same forces, which prevent them from being spherical, and the increased pressure within a tumor increases the degree to which the tumor insinuates into surrounding crevices and tissue planes. Separation of these extensions from the main mass of a tumor during surgery may explain cases in which the tumor and elevated pituitary hormone levels persist or recur after adenoma removal. The proximity of the carotid arteries to the midline is extremely important in pituitary surgery. In a previous study, the shortest distance between the two carotid arteries was found in the supraclinoid area in 82% of the cases, in the cavernous sinus along the lateral sellar margin in 14%, and in the sphenoid sinus in 4%.6 Arterial bleeding during transsphenoidal surgery has been reported as due to carotid artery injury, but it may also have arisen from a tear in an arterial branch of the carotid, such as the inferior hypophyseal artery, or by avulsion of a small capsular artery from the carotid artery.10 It is best to avoid using a pointed knife blade to open the dura in the corners of the sellar opening because of the proximity of the carotid arteries to these corners. It is best to begin the dural opening with a short vertical midline incision in the dura, after which a small, blunt, right-angled ring curette is inserted through the small vertical dural opening and the dura is separated from the anterior surface of the gland or tumor. After the dura has been freed, a 45-degree-angle alligator scissor, rather than a knife, is selected to open the dura in an x-shaped cut from corner to corner because a pointed knife may damage the carotid arteries in the far lateral corners of the exposure. Venous sinuses that interconnect the paired cavernous sinuses may be found in the margins of the diaphragma and around the gland6 (Figs. 2.1, 2.6, 2.11, and 2.13). The intercavernous connections within the sella are named on the basis of their relationship to the pituitary gland; the anterior intercavernous sinuses pass anterior to the hypophysis, and the posterior intercavernous sinuses pass behind the gland. Actually, these intercavernous connections can occur at any site along the anterior, inferior, or posterior surface of the gland, or all connections between the two sides may be absent. The anterior intercavernous sinus may cover the whole anterior wall of the sella. The anterior sinus is usually larger than the posterior sinus, but either or both may be absent. If the anterior and posterior connections coexist, the whole structure constitutes the “circular sinus.” Entering an anterior intercavernous connection that extends downward in front of the gland during transsphenoidal operation may produce brisk bleeding. However, this usually stops with temporary compression of the channel with hemostatic foam or with light bipolar coagulation, which serves to glue the walls of the channel together. Fig. 2.13 Six sagittal sections of the sellar region showing variations in the intercavernous venous connections within the dura. The variations shown include combinations of anterior, posterior, and inferior intercavernous connections and the frequent presence of a basilar sinus posterior to the dorsum. Either the anterior (lower center) or posterior (lower left) intercavernous connections or both (top center) may be absent. The anterior intercavernous sinus may extend along the whole anterior margin of the gland (lower left). The basilar sinus may be absent (lower right). A large intercavernous venous connection called the basilar sinus passes posterior to the dorsum sellae and upper clivus, connecting the posterior aspect of both cavernous sinuses (Figs. 2.6, 2.7, and 2.11). The basilar sinus is the largest and most constant intercavernous connection across the midline. The superior and inferior petrosal sinuses join the basilar sinus. The abducens nerve often enters the posterior part of the cavernous sinus by passing through the basilar sinus or the junction of the inferior petrosal and basilar sinus. There are several variations in the extensions of pneumatization of the sellar type of sphenoid sinus that may facilitate entry into areas bordering the sphenoid sinus19,20 (Figs. 2.14 and 2.15). These extensions or recesses act as “windows” opening from the sinus in different areas of the skull base that may facilitate minimally invasive access to lesions involving the cavernous sinus, Meckel’s cave, middle cranial fossa, planum sphenoidale, suprasellar region, and clivus. These variations in the sellar type of sinus have been classified into six basic types. (1) Sphenoid body type: the pneumatization does not progress beyond the body of sphenoid bone. (2) Lateral type: the sinus extends lateral to a line connecting the medial edge of the anterior opening of vidian canal and the extracranial end of the foramen rotundum (VR line). (3) Clival type: the posterior wall of the sphenoid sinus extends beyond the vertical coronal plane of the posterior wall of pituitary fossa. (4) Lesser wing type: the pneumatization extends into the lesser sphenoid wing and possibly into the anterior clinoid process. (5) Anterior type: the anterior wall of the sinus extends anterolaterally beyond the vertical coronal plane of the sinus side of the sphenoid crest. (6) Combined type: more than one type of extension appears in the same sinus. Among the sellar type of sinuses, the combined type was the most common (59.2%), and the lesser wing type was the least common. The lateral type, present in 46% of lateral sinus walls, extends into either the greater wing or pterygoid process, or both. It is referred to as a greater wing type if it extends only into the greater wing, as a pterygoid type if it extends into only the pterygoid process, and a full lateral type if it extends into both the greater wing and pterygoid process. In the endoscopic view from inside the sphenoid sinus, the lateral recess is a quadrangular area bordered by four prominences in the sinus wall: the maxillary nerve superiorly, the vidian nerve inferiorly, the petrous segment of the carotid artery posteriorly, and the part of the sinus wall between the foramen rotundum and vidian canal anteriorly. A prominence in the lateral sinus wall overlying cranial nerve V3 can be seen when the lateral recess extends to the foramen ovale. A clival type of sinus was identified when it had a clival recess in which the posterior wall of the sphenoid sinus extended posteriorly beyond the vertical coronal plane of the posterior wall of the pituitary fossa. A clival recess, present in 68% of the sinuses, may extend superiorly into the dorsum sellae and/or inferiorly to the basilar part of the occipital bone. Three types of clival recesses were found: dorsum type, which extends above the horizontal plane of the floor of the pituitary and into the dorsum sellae; subdorsum type, which lies between the horizontal plane of the floor of pituitary fossa and the horizontal plane passing through the anterior opening of vidian canals; and occipital type, which extends inferiorly below the horizontal plane crossing the anterior opening of the paired vidian canals. Of the clival-type sinuses, 24% were of the dorsum type, 63% subdorsum type, 2% occipital type, and 9% combined dorsum-occipital type. The subdorsum type is seen directly ahead as the sinus is entered, whereas the dorsum type may be partially hidden behind the pituitary fossa and the clival type may be partially hidden below the floor of the sphenoid sinus. Approximately, 10% of the sellar-type sinuses had pneumatization of the anterior clinoid process, referred to as lesser wing type. Every specimen with pneumatization of the anterior clinoid process also had optic strut aeration, but optic strut aeration did not always extend into the anterior clinoid process. An anterior recess originating from the lateral portion of anterior wall of the sphenoid sinus was identified when the recess extended anteriorly beyond a line directed from side to side along the sinus side of the sphenoid crest on the axial CT. An anterior recess, found in anterior-type sinuses, present either unilaterally or bilaterally in 25% of sinuses, extends in an inferolateral direction and is separated from the maxillary sinus by a thin bony plate. Fig. 2.14 Lateral extensions of the sphenoid sinuses. Anterior view of the sphenoid sinus illustrating the pneumatized extensions of the sphenoid sinus. Structures surrounding the sinus, such as the sella, carotid artery, and the optic, vidian, and the trigeminal divisions, may underlie prominences and recesses inside the sinus. The lateral extensions of the sinus extend beyond the VR line directed along the medial edge of the anterior end of the vidian canal and the extracranial end of the foramen rotundum. (A) Body type of sphenoid sinus. The pneumatization is confined to the body of the sphenoid bone and does not extend beyond the VR line. (B) Lesser wing type. The sinus pneumatizes through the optic strut (arrow) and into the anterior clinoid process. (C–E) Lateral type. The lateral wall of the sinus extends laterally beyond the VR line, which crosses the medial edge of the foramen rotundum and vidian canal. Three lateral subtypes were found: greater wing, pterygoid, and full lateral types. (C) Greater wing type. The pneumatization extends laterally between the foramen rotundum and vidian canal into the greater wing. (D) Pterygoid type. The pneumatization extends laterally between the foramen rotundum and vidian canal and inferiorly into the pterygoid process. (E) Full lateral type. The sinus extends laterally into both the greater wing and the pterygoid process. The parasellar region includes the cavernous sinuses and adjacent part of the middle cranial fossae.21,22 The paired cavernous sinuses have dural walls that surround a venous space through which a segment of the internal carotid artery courses. Each sinus extends from the superior orbital fissure in front to the area lateral to the dorsum sellae behind (Figs. 2.16 and 2.17). Its anterior edge is attached to the margins of the superior orbital fissure, and its posterior wall is located between the dorsum sellae medially and the ostium of Meckel’s cave laterally. The oculomotor, trochlear, and ophthalmic nerves course in the lateral wall. The abducens nerve courses on the medial side of the ophthalmic nerve between it and the internal carotid artery. The lateral wall faces the temporal lobe, the roof faces the basal cisterns, the medial wall faces the sella, pituitary gland, and body of the sphenoid bone, and the lower edge is located below the horizontal portion of the intracavernous segment of the internal carotid artery. The sinus is the site of a venous confluence that receives the terminal end of multiple veins draining the orbit, sylvian fissure, and middle and anterior fossae, and it has free communication with the basilar, superior and inferior petrosal, and intercavernous sinuses. Over all, it is shaped like a boat, with its narrow keel located at the superior orbital fissure and its broader bow (posterior wall) located lateral to the dorsum sellae above the petrous apex. It has four walls: a roof and lateral, medial, and posterior walls. The deck or roof of the sinus, which is narrow anteriorly and wide posteriorly, faces upward. The lower edge, formed at the junction of the medial and lateral walls below the intracavernous segment of the internal carotid artery, gives the sinus a triangular shape in cross-section (Fig. 2.17). The roof is formed by the dura lining the lower margin of the anterior clinoid process anteriorly and the patch of dura, called the oculomotor triangle, between the anterior and posterior clinoid processes and the petrous apex through which the oculomotor nerve penetrates the sinus roof. The medial edge of the oculomotor triangle is formed by the interclinoid dural fold, which extends from the anterior to the posterior clinoid process; the lateral margin by the anterior petroclinoid fold, which extends from the anterior clinoid process to the petrous apex; and the posterior margin by the posterior petroclinoid fold, which extends from the posterior clinoid process to the petrous apex. The lateral wall extends from the medial edge of Meckel’s cave posteriorly to the lateral margin of the nerves passing through the superior orbital fissure anteriorly, and from the anterior petroclinoid dural fold above to the lower margin of the carotid sulcus below. The carotid sulcus is the groove on the lateral aspect of the body of the sphenoid along which the internal carotid artery courses (Fig. 2.18). The sheet of dura forming the posterior part of the lateral wall of the sinus also forms the upper third of the medial wall of Meckel’s cave, which is located lateral to and is separated from the posterior part of the cavernous sinus by their shared dural wall. The medial wall is formed by the dura that constitutes the lateral wall of the sella turcica and covers the lateral surface of the body of the sphenoid bone. The medial wall extends from the lateral edge of the dorsum sellae posteriorly to the medial edge of the superior orbital fissure anteriorly, and from the interclinoid dural fold above to the lower margin of the carotid sulcus below. Anteriorly, the lower edge of the sinus, where the medial and lateral walls meet, is located just below where the ophthalmic nerve courses in the lateral sinus wall, and proceeding posteriorly it is located medial to the junction of the upper and middle thirds of the gasserian ganglion; finally, at the posterior part it slopes upward medial to the upper part of Meckel’s cave (Fig. 2.16). Behind the site where the ophthalmic nerve arises from the trigeminal ganglion, the lower edge of the medial and lateral walls of the sinus come together at the lateral edge of the carotid sulcus on the medial side of the upper part of Meckel’s cave. Only the upper part of the medial wall of Meckel’s cave and the upper part of the gasserian ganglion are located directly lateral to the cavernous sinus; thus, the lower two-thirds of Meckel’s cave is located below and lateral to the posterior part of the cavernous sinus in the medial part of the middle fossa (Fig. 2.16). Meckel’s cave extends forward from the posterior fossa, where its ostium is located between the medial part of the petrous ridge below, the superior petrosal sinus above, and the lateral edge of the cavernous sinus medially. The subarachnoid space extends forward within Meckel’s cave to approximately the level of the midportion of the gasserian ganglion. The terminal part of the petrous carotid exits the carotid canal and passes below the trigeminal nerve and the petrolingual ligament, where it turns upward to enter the posterior part of the cavernous sinus. It is only after the artery exits the region of the foramen lacerum and turns upward after traveling below the petrolingual ligament to reach the carotid sulcus on the lateral surface of the sphenoid body that it becomes enclosed in the dural envelope of the cavernous sinus (Fig. 2.16). Fig. 2.15 Clival extensions of the sphenoid sinus. Sagittal section. (A) Body type of sinus without a clival extension. In the body type of sinus, the posterior wall of the sphenoid sinus does not extend beyond a vertical line directed along the posterior wall of the pituitary fossa (line 1). (B–E) Clival type of sinuses. The posterior wall of the sphenoid sinus extends beyond the vertical coronal plane of the posterior wall of the pituitary fossa (line 1). There are four types of clival extensions: subdorsum, dorsum, occipital, and combined dorsum-occipital types. (B) Subdorsum type. The sinus extends posterior to a line directed along the posterior wall of the sella but not into the dorsum sellae or into the clivus below the level of the vidian canal. (C) Occipital type. The expansion of the sinus behind the posterior wall of the sella (line 1) extends inferiorly below the level of the horizontal plane directed along the upper edge of the paired vidian canals (line 3). (D) Dorsum type. The sinus extends above the line directed along the floor of the sella (line 2) and into the dorsum sellae. (E) Combined dorsum-occipital type. The sinus extends superiorly into the dorsum and downward below the horizontal plane directed along the upper edge of the vidian canals. (F) Superior view of a cross-section extending through an anterior type of sphenoid sinus. The sinus has an anterolateral protrusion that extends anterior to a transverse line crossing the sphenoid sinus side of the sphenoid crest to form an anterior recess facing the maxillary sinus. The sphenomaxillary plate separates the sphenoid and maxillary sinuses in this anterior type of recess. This type of sinus extends anteriorly above the sphenopalatine artery and foramen. (G) Superior view of an oblique axial section showing the angle between the midline plane at the anterior nasal spine and the most lateral point of the sphenoid sinus, which averaged 16.7 degrees (range, 10.1–32.1 degrees). The angle from the midline plane to the sphenopalatine foramen averaged 12.2 degrees (range, 7.0–18.6 degrees). The sphenopalatine artery passes through the sphenopalatine foramen to enter the lateral wall of the nasal cavity. Fig. 2.16 Stepwise dissection of the right cavernous sinus. (A) The lateral wall of the cavernous sinus extends downward from the tentorial edge and blends into the dura covering Meckel’s cave and the middle fossa. The oculomotor and trochlear nerves enter the roof of the cavernous sinus. The carotid artery exits the cavernous sinus on the medial side of the anterior clinoid process. (B) The outer layer of dura has been peeled away from the lateral wall of the cavernous sinus and Meckel’s cave. This exposes the oculomotor and trochlear nerves entering the roof of the cavernous sinus and passing forward through the superior orbital fissure. The thin layer covering Meckel’s cave consists in part of the arachnoid membrane extending forward from the posterior fossa and surrounding trigeminal nerve to the level of the trigeminal ganglion. The superior petrosal sinus passes above the ostium of Meckel’s cave and joins the posterior part of the cavernous sinus. (C) The oculomotor nerve enters a short cistern in the sinus roof (red arrow) and does not become incorporated into the lateral wall until it reaches the lower margin of the anterior clinoid process (yellow arrow). The arachnoid covering of Meckel’s cave, which extends forward around the posterior trigeminal root to the level of the midportion of the ganglion, has been removed. The cavernous sinus extends from the superior orbital fissure to the petrous apex. It is located medial to the upper third of the gasserian ganglion. The pericavernous venous plexus surrounds the maxillary and mandibular nerves in the region of the foramen rotundum and foramen ovale. (D) The remaining dura covering the lateral wall has been removed. The oculomotor, trochlear, and ophthalmic nerves pass forward to converge on the superior orbital fissure. The segment of the superior petrosal sinus above the posterior trigeminal root has been removed. (E) The posterior trigeminal root has been reflected forward to expose the posterior part of the lower margin of the cavernous sinus (yellow arrow) in the area medial to the trigeminal impression on the petrous apex, in which Meckel’s cave sits. The superior ophthalmic vein exits the orbit through the superior orbital fissure and passes posteriorly below the ophthalmic nerve to enter the cavernous sinus. (F) The trigeminal nerve and its three divisions have been reflected forward to expose the venous spaces of the cavernous sinus. The lower margin of the cavernous sinus (broken line) is located at the site where the internal carotid artery exits the carotid canal by passing below the petrolingual ligament. The venous spaces in the cavernous sinus communicate posteriorly with the inferior and superior petrosal and basilar sinuses. In addition, the cavernous sinus communicates with the superior ophthalmic veins and the venous plexus around the maxillary and mandibular nerves and the pituitary gland. Stepwise dissection of the right cavernous sinus. (G) The venous plexus surrounding the nerves has been removed to expose the trigeminal divisions and the nerves coursing in the wall of the cavernous sinus. (H) The ophthalmic nerve has been depressed to expose the abducens nerve, which passes under the petrosphenoid ligament roofing Dorello’s canal and courses medial to the ophthalmic nerve. The abducens nerve crosses laterally below the ophthalmic nerve as it passes through the superior orbital fissure. (I) The anterior clinoid process has been removed. The optic strut separates the optic canal and superior orbital fissure. The dura extending medially offthe upper surface of the anterior clinoid forms the upper dural ring around the internal carotid artery, and the dura lining the lower margin of the clinoid extends medially to form the lower dural ring. The clinoid segment of the carotid artery, located between the upper and lower rings, is enclosed in the dura sheath, referred to as the carotid collar. (J) The trigeminal nerve has been folded downward to expose the petrolingual ligament, which extends above the internal carotid artery, just proximal to where the artery enters the cavernous sinus. The abducens nerve passes around the internal carotid artery and courses medial to the ophthalmic nerve in the lower part of the cavernous sinus. The margins of the cavernous sinus are shown with a broken line. The cavernous sinus does not extend laterally into the area of the trigeminal impression where Meckel’s cave sits. (K) Enlarged view. The optic nerve has been elevated to expose the ophthalmic artery coursing within the optic sheath. At the orbital apex, the artery penetrates the optic sheath and enters the orbital apex on the lateral side of the optic nerve. Removal of additional optic strut exposes the mucosa lining the sphenoid sinus on the medial side of the optic strut. (L) The bone between the first and second and the second and third trigeminal divisions has been drilled to expose the lateral wing of the sphenoid sinus. The vidian nerve, which passes forward to enter the sphenopalatine ganglion in the pterygopalatine fossa, is exposed between the second and third trigeminal divisions. Stepwise dissection of the right cavernous sinus. (M) The trigeminal nerve has been reflected forward to expose the opening into the lateral wing of the sphenoid sinus. The vidian nerve, formed by the union of the greater and deep petrosal nerves, courses forward in the vidian canal to reach the pterygopalatine fossa. (N) Enlarged view of the petrolingual and petrosphenoid ligaments. The petrosphenoid ligament extends from the lower part of the lateral margin of the dorsum sellae above the abducens nerve to the petrous apex. The lower margin of the posterior wall of the cavernous sinus is located at the lower margin of Dorello’s canal. Anteriorly, the lower margin of the cavernous sinus is located at the level at which the internal carotid artery exits the area below the petrolingual ligament and enters the posterior part of the cavernous sinus. (O) The exposure has been extended down to the infratemporal and pterygopalatine fossae. The infratemporal fossa contains branches of the mandibular nerve and maxillary artery, the pterygoid muscles, and the pterygoid venous plexus. The maxillary nerve passes through the foramen rotundum to enter the pterygopalatine fossa. (P) Enlarged view. The pterygopalatine fossa is located between the posterior maxillary wall and the pterygoid process. The vidian nerve penetrates the upper part of the pterygoid process and the area below the foramen rotundum to enter pterygopalatine fossa. The maxillary nerve gives rise to the zygomatic, infraorbital, and posterosuperior alveolar nerves, and branches and rami to the pterygopalatine ganglion. Fig. 2.17 Stepwise dissection of the roof of the cavernous sinus. (A) Superior view. The dura covering the upper surface of the right anterior clinoid process, optic canal, and adjacent part of the planum has been removed. The roof of the cavernous sinus is formed anteriorly by the dura lining the lower margin of the anterior clinoid and posteriorly by the dura covering the oculomotor triangle, located between the anterior and posterior petroclinoidal and intraclinoidal dural folds. The oculomotor nerve enters the roof of the cavernous sinus through the oculomotor triangle. (B) The anterior clinoid and roof of the optic canal have been removed. The optic nerve has been elevated to expose the ophthalmic artery entering the optic foramen. Removing the anterior clinoid exposes the floor of the clinoidal triangle located between the optic and oculomotor nerves. The dura separating the lower surface of the clinoid from the oculomotor nerve and extending medially around the carotid artery, referred to as the carotid-oculomotor membrane, forms the floor of the clinoidal triangle and the anterior part of the roof of the cavernous sinus. The dura extending medially offthe upper surface of the clinoid forms the upper dural ring, and the carotid-oculomotor membrane extending medially from the lower surface of the clinoid forms the lower dural ring. (C) The dura in the floor of the clinoidal triangle and roof of the oculomotor triangle, which together form the roof of the cavernous sinus, have been removed to expose the upper part of the cavernous sinus. The dura has been elevated from the lateral wall of the cavernous sinus and middle fossa floor to expose the nerves coursing in the lateral wall of the cavernous sinus. (D) The sinus has been cleared to expose the clinoid segment of the internal carotid artery in the clinoidal triangle and the posterior bend of the intercavernous carotid below the oculomotor triangle. The anterior part of the roof is formed by the dura that separates the anterior clinoid and oculomotor nerve and that extends medially to form the lower dural ring. The posterior part of the roof consists of the dura forming the oculomotor triangle. Fig. 2.18 Osseous relationships of the cavernous sinus and carotid collar. (A) Superior view. The osseous structures, which nearly encircle the clinoid segment of the internal carotid artery, include the anterior clinoid laterally, the optic strut anteriorly, and the carotid sulcus medially. The carotid sulcus begins lateral to the dorsum sellae at the intracranial end of the carotid canal, extends forward just below the sellar floor, and turns upward along the posterior surface of the optic strut. The anterior clinoid process projects backward from the lesser wing of the sphenoid bone, often overlapping the lateral edge of the carotid sulcus. The anterior root of the lesser sphenoid wing extends medially to form the roof of the optic canal. The posterior root of the lesser wing, referred to as the optic strut, extends from the inferomedial aspect of the anterior clinoid to the sphenoid body. The bony collar around the carotid artery, formed by the anterior clinoid, optic strut, and carotid sulcus, is inclined downward as it slopes medially from the upper surface of the anterior clinoid to the carotid sulcus. Another small prominence, the middle clinoid process, situated on the medial side of the carotid sulcus at the level of the tip of the anterior clinoid process, projects upward and laterally. In some cases, there is an osseous bridge extending from the tip of the middle clinoid to the tip of the anterior clinoid. In well-pneumatized sphenoid bones, the carotid sulcus is seen as a prominence in the lateral wall of the sphenoid sinus just below the floor of the sella. (B) Posterior view of the optic strut, optic canal, and superior orbital fissure. The optic strut separates the optic canal and superior orbital fissure and forms the floor of the optic canal and the superomedial part of the roof of the superior orbital fissure. The posterior surface of the strut is shaped to accommodate the anterior wall of the clinoid segment. The artery courses along and may form a groove in the medial half of the lower aspect of the anterior clinoid before turning upward along the medial edge of the clinoid. The air cells in the sphenoid sinus may extend into the optic strut and anterior clinoid. In this case, the sphenoid sinus has pneumatized to a degree that bone is absent over the anterior part of the carotid sulcus, just medial to where the optic strut attaches to the body of the sphenoid bone. The maxillary strut is the bridge of bone separating the superior orbital fissure from the foramen rotundum. (C) Oblique posterior view of the right optic strut. The lateral part of the bony collar around the clinoid segment is formed by the anterior clinoid, and the anterior part is formed by the posterior surface of the optic strut and the part of the carotid sulcus located medial to the anterior clinoid process. The posterior surface of the optic strut is wider medially adjacent to the carotid sulcus than it is laterally at the site of attachment to the anterior clinoid process. The optic strut slopes downward from its lateral end so that the medial part of the bony collar is located below the level of the part of the collar joining the anterior clinoid. The inferomedial aspect of the right anterior clinoid has a groove formed by the artery. (D) Superior view of a specimen with bilateral caroticoclinoidal foramina and interclinoidal osseous bridges. An osseous bridge connects the tips of the anterior and middle clinoid processes bilaterally, thus creating a bony ring around the artery, called a caroticoclinoidal foramen, on each side. There is also an interclinoidal osseous bridge connecting the anterior and posterior clinoid processes on both sides. (E) Superior view of another specimen, in which the lesser sphenoid wings and the base of the anterior clinoids and roof of the optic canals have been removed. The remaining part of the anterior clinoid is held in place by its attachment to the optic strut. The medial side of the anterior clinoid is grooved to accommodate the clinoid segment. (F) Enlarged view of the left half of (E). The posterior face of the optic strut is shaped to accommodate the anterior surface, and the medial aspect of the anterior clinoid is grooved to accommodate the lateral surface of the clinoid segment. The tip of the anterior clinoid process is the site of a small bony projection directed toward the middle clinoid process, with the anterior and middle clinoids nearly completing a ring around the clinoid segment at the level of the cavernous sinus roof. The sphenoid sinus may pneumatize through the optic strut into the anterior clinoid. The carotid sulcus is the shallow groove on the lateral aspect of the body of the sphenoid bone along which the internal carotid courses in the cavernous sinus. The intracavernous carotid sits against and is separated from the carotid sulcus by the dura of the medial sinus wall (Fig. 2.18). The carotid sulcus begins below and lateral to the dorsum sellae at the intracranial end of the carotid canal, turns forward to form a groove in the body of the sphenoid immediately below the lateral edge of the floor of the sella, and turns upward to end medial to the anterior clinoid process. The segment of the internal carotid artery that courses along the medial side of the clinoid is referred to as the clinoid segment. The carotid sulcus, in well-pneumatized sphenoid bones, forms a serpiginous prominence that can be seen in the lateral wall of the sphenoid sinus below and anterior to the pituitary fossa. The bone in the lateral wall of the sphenoid sinus may be thin or even absent in some areas, allowing the artery to be observed through the sinus wall. The anterior clinoid process projects posteriorly from the lesser wing of the sphenoid bone above the anterior part of the roof of the sinus (Fig. 2.18). The base of the clinoid has three sites of continuity with the adjacent part of the sphenoid bone. The base is attached anteriorly at the medial edge of the sphenoid ridge, formed by the lesser sphenoid wing, and medially to the anterior and posterior roots of the lesser wing. The anterior root of the lesser wing extends medially from the base of the anterior clinoid to the body of the sphenoid bone and forms the roof of the optic canal. The posterior root of the lesser wing, called the optic strut, extends medially below the optic nerve to the sphenoid body and forms the floor of the optic canal. The base of the anterior clinoid forms the lateral margin of the optic canal. The segment of the internal carotid artery that courses along the medial aspect of, and is exposed by removal of the anterior clinoid, is referred to as the clinoid segment. The clinoid segment courses below the medial half of the lower margin of the clinoid, where it forms a grooves in the bone before coursing upward along the medial edge of the clinoid (Fig. 2.18F). The medial edge of the clinoid, just behind the base, is frequently the site of a shallow, rounded indention that accommodates the lateral surface of the clinoid segment. The posterior tip of the clinoid often projects posteriorly lateral to the lateral part of the clinoid segment. The anterior clinoid is the site of attachment of the anteromedial part of the tentorium and the anterior petroclinoid and interclinoid dural folds. Another dural fold, the falciform ligament, extends from the base of the clinoid across the roof of the optic canal to the planum sphenoidale. The chiasmatic sulcus is a shallow trough on the upper surface of the sphenoid bone between the intracranial end of the optic canals. The tuberculum sellae is located in the midline along the ridge forming the posterior margin of the chiasmatic sulcus. The anterior clinoid has a dense surface of cortical bone and a weak diploe of cancellous bone that is sometimes crossed by small venous channels that communicate with the cavernous sinus and the diploic veins of the orbital roof. The air cells in the sphenoid sinus may also extend through the optic strut into the anterior clinoid. There is another small prominence, the middle clinoid process, that projects upward on the medial side of the terminal part of the carotid sulcus and medial to the tip of the anterior clinoid process (Fig. 2.18). An osseous bridge may extend from the tip of the middle clinoid to the tip of the anterior clinoid, thus converting the roof of the anterior part of the cavernous sinus into a bony ring or foramen, referred to as a caroticoclinoidal foramen, through which the internal carotid artery passes (Fig. 2.18D). This type of variant may infrequently occur bilaterally.23 There may also be interclinoidal osseous bridges that extend from the anterior to the posterior clinoid unilaterally or bilaterally (Fig. 2.18D). Such bridges make it difficult to remove the anterior clinoid process. The optic strut (posterior root of the lesser wing) is a small bridge of bone that extends from the inferomedial aspect of the base of the anterior clinoid process to the body of the sphenoid just in front of the carotid sulcus24 (Figs. 2.16 and 2.18). The strut, from its junction with the clinoid, slopes gently downward as it approaches the body of the sphenoid. The strut separates the optic canal and superior orbital fissure. The superior surface of the strut, which slopes downward and forward from its intracranial edge, forms the floor of the optic canal. The inferior surface of the optic strut forms the medial part of the roof of the superior orbital fissure and the anterior part of the roof of the cavernous sinus. The strut sits at the junction of the orbital apex anteriorly with the superior orbital fissure and optic canal posteriorly. The anterior edge of the strut is a narrow ridge located at the junction of its superior and inferior surfaces. The posterior face of the optic strut, which faces slightly downward, is shaped to accommodate the anterior surface of the anterior bend of the intracavernous carotid, which rests against the posterior surface of the optic strut as it ascends on the medial side of the anterior clinoid process. The site at which the strut blends into the sphenoid body is marked in the sphenoid sinus by a recess, the opticocarotid recess, that extends laterally from the superolateral part of the sphenoid sinus between the prominences in the sinus wall overlying the carotid sulcus and optic canal. This recess may extend deeply into the strut, so that the strut is partially or completely aerated by a lateral extension of the sphenoid sinus. The aeration may extend through the strut into the anterior clinoid process. Venous channels connecting the cavernous sinus with diploic veins of the orbital roof and anterior clinoid process may extend into or through the optic strut. The dural relationships of the anterior clinoidal process are especially important in planning surgical approaches to the area (Figs. 2.16, 2.17, 2.18, and 2.19). The dura extends medially from the upper surface of the anterior clinoid to surround the carotid artery and form a dural ring, referred to as the upper or distal ring, at the upper margin of the clinoid segment of the carotid.25 The dura forming the lateral part of the upper ring extends forward and medially below the optic nerve to line the upper surface of the optic strut and posteriorly at the level of the upper part of the carotid sulcus to form the medial part of the upper ring. Further medially, the dura forming the upper ring blends into the diaphragma sellae. The dura extending medially above the optic nerve from the clinoid process to line the anterior root of the lesser wing and attaching to the posterior edge of the planum sphenoidale is located at the horizontal level of the upper surface of the clinoid. However, the dura that extends medially offthe upper surface of the clinoid to line the upper surface of the optic strut and form the upper dural ring slopes downward as it proceeds medially, so that the medial part of the upper dural ring actually lies at the level of the lower rather than the upper surface of the anterior clinoid and optic canal. Fig. 2.19 Triangles in the region of the cavernous sinus and middle fossa formed by the convergence and divergence of the cranial nerves. (A,B) Lateral aspect of brainstem and posterior fossa showing the brainstem origin of the cranial nerves, which form the margins of the cavernous sinus and middle fossa triangles. The tentorial edge was preserved in (A) and removed in (B). There are four cavernous sinus triangles, four middle fossa triangles, and two paraclival triangles. The cavernous sinus triangles are the clinoidal, oculomotor, supratrochlear, and infratrochlear triangles. The clinoidal triangle, exposed by removing the anterior clinoid process, is situated in the interval between the optic and oculomotor nerves. The optic strut is in the anterior part, the clinoid segment is in the midportion, and the thin roof of the cavernous sinus is in the posterior part of this triangle. The oculomotor triangle is the triangular patch of dura through which the oculomotor nerve enters the roof of the cavernous sinus. The posterior margin of this triangle is formed by the posterior petroclinoid dural fold, which extends from the petrous apex to the posterior clinoid process. The lateral margin is formed by the anterior petroclinoid dural fold, which extends from the petrous apex to the anterior clinoid process. The medial margin is formed by the intraclinoid dural fold, which extends from the anterior to the posterior clinoid. The supratrochlear triangle is situated between the lower surface of the oculomotor nerve and the upper surface of the trochlear nerve, and a line joining the points of entrance of these nerves into the dura is its third margin. This triangle is very narrow. The infratrochlear triangle (Parkinson’s triangle) is located between the lower margin of the trochlear nerve and the upper margin of the ophthalmic nerve, and a third margin is formed by a line connecting the point of entry of the trochlear nerve into the dura to the site where the trigeminal nerve enters Meckel’s cave. The posterior bend of the carotid artery and the origin of the meningohypophyseal trunk are located in this triangle. The middle fossa triangles are the anteromedial, anterolateral, posterolateral, and posteromedial triangles. The anteromedial triangle is situated between the lower margin of the ophthalmic and the upper margin of the maxillary nerves, and a third edge is formed by a line connecting the point where the ophthalmic nerve passes through the superior orbital fissure and the maxillary nerve passes through the foramen rotundum. Removing bone in the medial wall of this triangle will create an opening into the sphenoid sinus. The anterolateral triangle is located between the lower surface of the maxillary nerve, the upper surface of the mandibular nerve, and a line connecting the foramen ovale and rotundum. Opening the bone in the medial wall of this triangle exposes the sphenoid sinus. The posterolateral triangle (Glasscock’s triangle) is formed on the anterolateral side by the lateral surface of the mandibular nerve distal to the point at which the greater petrosal nerve crosses below the lateral surface of the trigeminal nerve; on the posterolateral side it is formed by the anterior margin of the greater petrosal nerve. This triangle encompasses the floor of the middle cranial fossa between these two structures. The middle meningeal artery passes through the foramen spinosum in this triangle. Opening the floor of the middle fossa in this triangle exposes the infratemporal fossa. The posteromedial triangle (Kawase’s triangle) is located between the greater petrosal nerve and the lateral edge of the trigeminal nerve behind the point where the greater petrosal nerve passes below the lateral edge of the trigeminal nerve, and a line along the connecting hiatus fallopii to the dural ostium of Meckel’s cave. The petrous carotid crosses the anterior margin of this triangle. The cochlea is located below the floor of the middle fossa in the lateral apex of the triangle. Drilling the bony floor of the triangle in the area behind the internal carotid artery and medial to the cochlea exposes the lateral edge of the clivus. The paraclival triangles are the inferomedial and inferolateral triangles. The inferolateral paraclival triangle is located on the posterior surface of the clivus and temporal bone. The medial margin is formed by a line connecting the dural entry sites of the trochlear and abducens nerves; the upper margin extends from the dural entrance of the trochlear nerve to the point at which the first petrosal vein lateral to Meckel’s cave joins the superior petrosal sinus (removed), and the lower margin is formed by a line connecting the point at which the abducens nerve enters the dura to the site at which the first petrosal vein, lateral to the trigeminal nerve, joins the superior petrosal sinus. The porus, through which the posterior trigeminal root enters Meckel’s cave, is situated in the center of the inferolateral paraclival triangle. The inferomedial paraclival triangle is formed above by a line extending from the posterior clinoid process to the dural entrance of the trochlear nerve, laterally by a line connecting the dural entrances of the trochlear and abducens nerves, and medially by a line extending from the dural entrance of the abducens nerve to the posterior clinoid process. The dura in this triangle forms the posterior wall of the cavernous sinus. Triangles in the region of the cavernous sinus and middle fossa formed by the convergence and divergence of the cranial nerves. (C) Lateral view of the parasellar area and the oculomotor triangle. The temporal lobe has been elevated to expose the oculomotor and trochlear nerves as they enter the roof of the cavernous sinus. The oculomotor triangle is the triangular patch of dura through which the oculomotor nerve enters the roof of the cavernous sinus. The optic tract passes backward on the medial side of the uncus. (D) Enlarged view of the clinoidal, oculomotor, supratrochlear, and infratrochlear cavernous sinus triangles. The optic strut is exposed in the anterior part of the clinoidal triangle, the clinoid segment is exposed in the midportion, and the roof of the cavernous sinus is exposed in the posterior part. The upper margin of the clinoid segment is surrounded by the upper dural ring, which is formed by the dura extending medially from the upper surface of the anterior clinoid. The lower margin of the clinoid segment is defined by the lower dural ring, which is formed by the dura extending medially from the lower margin of the anterior clinoid. The dura on the lower margin of the anterior clinoid, referred to as the carotid-oculomotor membrane, separates the lower surface of the anterior clinoid from the upper surface of the oculomotor nerve and extends medially to form the lower dural ring. The posterior bend of the internal carotid artery and the origin of the meningohypophyseal trunk, which gives rise to the tentorial and dorsal meningeal arteries, are exposed in the infratrochlear triangle. The abducens nerve passes through Dorello’s canal and between the lateral surface of the intracavernous carotid and the medial side of the ophthalmic nerve. The inferolateral trunk arises from the horizontal segment of the intracavernous carotid and passes above the abducens nerve. (E,F) Side-by-side comparison of the medial and lateral aspects of the cavernous sinus. (E) Lateral view of cavernous sinus. (F) View, through the sphenoid sinus, of the medial side of the cavernous sinus. The optic nerve is exposed at the upper margin of the clinoidal triangle and above the optic strut. On the sphenoid sinus side of the specimen, the optic canal is seen above the opticocarotid recess, which leads into the optic strut. The clinoid segment rests against the posterior aspect of the optic strut in both views. In the lateral view, the superior orbital fissure, through which the ophthalmic, trochlear, and abducens nerves pass, is seen below the optic strut. In the view through the sphenoid sinus, the medial edge of the superior orbital fissure produces a wide, rounded prominence below the optic strut, and the maxillary nerve produces a prominence in the lower part of the sphenoid sinus just distal to the foramen rotundum. The lateral wing of the sphenoid sinus extends laterally under the maxillary nerve into the medial part of the floor of the middle fossa. Opening the middle fossa floor in the anteromedial and anterolateral triangles exposes the sphenoid sinus. (G) Posterior view of the inferolateral triangle. The medial edge of the inferolateral triangle extends between the dural entrances of cranial nerves IV and V. The inferior limb extends from cranial nerve VI to where the first vein lateral to Meckel’s cave joins the superior petrosal sinus and the superior limb extends from that vein to the dural entrance of cranial nerve IV. The ostium of Meckel’s cave is located within the inferolateral triangle. (H) Posterior view of the inferomedial triangles. The medial limb of the inferomedial triangle extends from the posterior clinoid to the dural entrance to the abducens nerve. The lateral limb extends between the dural entrances of nerves IV and VI, and the superior limb extends from nerve IV to the posterior clinoid. On the right side, there is an abnormal projection of the posterior clinoid process that extends below the oculomotor nerve toward the petrous apex. The layer of dura that lines the lower margin of the anterior clinoid and extends medially to form the lower or proximal dural ring is called the carotid-oculomotor membrane because it separates the lower margin of the clinoid from the oculomotor nerve and extends medially around the carotid artery (Figs. 2.16 and 2.19). This membrane extends medially and forward to line the lower surface of the optic strut. The segment of the internal carotid artery located between the upper and lower dural rings, which is exposed by removing the anterior clinoid process, is referred to as the clinoid segment. It may be necessary to divide the dural rings to mobilize the carotid artery in the management of tumors and aneurysms arising at the level of the roof of the cavernous sinus. The location of the nerves in the sinus wall or sinus, superiorly to inferiorly, are the third nerve followed by the trochlear, ophthalmic, and abducens nerves18,26,27 (Figs. 2.16, 2.17, and 2.19). The oculomotor, trochlear, and ophthalmic nerves course in the inner part of the lateral sinus wall. The abducens courses medial to the ophthalmic nerve and is adherent to the lateral surface of the intracavernous carotid medially, but it also is adherent laterally to the medial surface of the ophthalmic nerve and the inner part of the lateral sinus wall. The oculomotor nerve pierces the roof of the cavernous sinus near the center of the oculomotor triangle, and the fourth nerve enters the dura at the posterolateral edge of the triangle. A short length of both the trochlear and oculomotor nerves is surrounded by a dural and arachnoid cuff, to create the oculomotor and trochlear cisterns, as they pass through the roof of the cavernous sinus and below the anterior clinoid process. Both nerves are situated medial to, and slightly beneath, the level of the free edge of the tentorium at their point of entry. The oculomotor nerve enters the cavernous sinus slightly lateral and anterior to the dorsum sellae, almost directly above the origin of the meningohypophyseal trunk from the intracavernous carotid, and courses along the lower margin of the anterior clinoid and the carotid-oculomotor membrane. The oculomotor nerve pierces the sinus roof between 2 and 7 mm posterior to the initial supraclinoid segment of the carotid artery; the average separation is 5 mm.18 The trochlear nerve enters the roof of the sinus posterolateral to the third nerve and courses below the oculomotor nerve in the posterior part of the lateral wall. Anteriorly, below the base of the anterior clinoid process, it passes upward along the lateral surface of the oculomotor nerve. From there, the trochlear nerve passes medially between the oculomotor nerve and dura lining the lower margin of the anterior clinoid and optic strut to reach the medial part of the orbit and the superior oblique muscle. The ophthalmic nerve is the smallest of the three trigeminal divisions. It is inclined upward as it passes forward near the medial surface of the dura forming the lower part of the lateral wall of the cavernous sinus to reach the superior orbital fissure. It is flattened in the wall of the cavernous sinus, but at the superior orbital fissure it takes on an oval configuration. The ophthalmic nerve splits into the lacrimal, frontal, and nasociliary nerves as it approaches the superior orbital fissure. The superior petrosal sinus passes above the posterior root of the trigeminal root to form the upper margin of the ostium of Meckel’s cave, the dural and subarachnoid cavern that communicates with the subarachnoid space in the posterior fossa (Fig. 2.16). The cave extends forward around the posterior trigeminal root to the midportion of the ganglion. The motor root of the trigeminal nerve courses on the medial side of the sensory fibers at the level of Meckel’s cave. The abducens nerve pierces the dura forming the lower part of the posterior wall of the sinus at the upper border of the petrous apex and enters a dural cave, referred to as Dorello’s canal; here it passes below the petrosphenoid ligament (Gruber’s ligament), which extends from the lower part of the lateral edge of the dorsum sellae to the petrous apex, to enter the cavernous sinus (Figs. 2.6 and 2.16). The nerve bends laterally around the proximal portion of the intercavernous carotid and gently ascends as it passes forward inside the cavernous sinus medial to the ophthalmic nerve and on the lateral side of the internal carotid artery and below and medial to the nasociliary nerve. It has the most medial site of entry of the nerves coursing in the sinus wall and maintains that position in its course through the sinus. The nerve usually enters the sinus as a single bundle but may persist as two bundles in the subarachnoid space. After entering the sinus, it may split into as many as five rootlets as it courses between the internal carotid artery and ophthalmic nerve. In a study of 50 sinuses, the intracavernous segment of the nerve consisted of a single rootlet in 34 specimens, two in 13, three in two, and five in one.18 Sympathetic fiber bundles large enough to be recognized without a surgical microscope travel on the surface of the carotid as it emerges from the foramen lacerum. Some of the bundles join the sixth nerve within the sinus before ultimately being distributed to the first trigeminal division, which sends sympathetic fibers that reach the pupillodilator through the long ciliary nerves and pass through the ciliary ganglion.28,29 Some sympathetic fibers pass directly from the carotid plexus to the ciliary ganglion, and others may travel along the ophthalmic artery to the globe.30 The parasellar region is the site of several significant triangular relationships formed by the convergence and divergence of the cranial nerves in the region of the cavernous sinus and middle fossa. The four triangles in the cavernous sinus, four in the middle fossa lateral to the cavernous sinus, and two in the paraclival area are helpful in understanding and planning approaches to the cavernous sinus (Fig. 2.19). The cavernous sinus triangles are formed by the optic, oculomotor, trochlear, and ophthalmic nerves converging on the optic canal and superior orbital fissure. The middle fossa triangles are formed by the trigeminal divisions diverging as they pass from the gasserian ganglion to reach their foramina.27 The internal carotid artery exits the foramen lacerum lateral to the posterior clinoid process, where it passes under the petrolingual ligament and turns abruptly forward to course along the carotid sulcus and lateral part of the body of the sphenoid (Figs. 2.16, 2.17, 2.18, and 2.19). It passes forward in a horizontal direction for ~2 cm and terminates by passing upward along the medial side to the anterior clinoid process and the posterior surface of the optic strut, where it penetrates the roof of the cavernous sinus. The clinoid segment of the carotid artery is tightly surrounded by the anterior clinoid process laterally, the optic strut anteriorly, and the carotid sulcus medially, with only a narrow space left between the bone and the artery. The dura lining the surface of these osseous structures facing the clinoid segment forms the carotid collar around the clinoid segment. The intracavernous carotid is relatively fixed by the bony ring, but despite this, large extensions of pituitary tumor may produce lateral displacement of the artery. Just proximal to the cavernous sinus in the foramen lacerum, the artery lies beneath the trigeminal nerve.18 In surgical approaches to the trigeminal nerve directed through the middle cranial fossa, there is a tendency to assume that the carotid artery is distant from the trigeminal nerve. However, nearly 85% of carotid arteries are exposed under some portion of Meckel’s cave and the trigeminal nerve, with only dura, and no bone, separating the nerve from the artery18 (Figs. 2.16 and 2.19). In the remainder, the bone separating the nerve and artery is often paper-thin. The region without bone over the carotid often extends to the lateral edge of the trigeminal nerve, and in more than one-third, the bone covering the carotid is defective lateral to the edge of the third division. The maximum length of artery exposed lateral to the nerve was 7 mm in our study. The branches of the intracavernous carotid are the meningohypophyseal trunk, the largest branch, present in 100% of our specimens; the artery of the inferior cavernous sinus, present in 84%; and McConnell’s capsular arteries, present in 28%21,33 (Figs. 2.1, 2.11, and 2.19). Less frequent branches of the intracavernous carotid were the ophthalmic artery (8%) and the dorsal meningeal artery (6%).18 The meningohypophyseal trunk, the most proximal intracavernous branch, arises at or just before the apex of the first curve of the intracavernous carotid, where it turns forward after leaving the foramen lacerum (Fig. 2.19). The meningohypophyseal trunk typically gives rise to three branches: (1) the tentorial artery, also called the artery of Bernasconi-Cassinari,34 which courses lateral to the tentorium; (2) the inferior hypophyseal artery, which travels medially to supply the posterior pituitary capsule; and (3) the dorsal meningeal artery, which enters the dura of the posterior sinus wall and supplies the clival dura and cranial nerve VI. The artery of the inferior cavernous sinus, also called the inferolateral trunk, may infrequently arise from the meningohypophyseal trunk.18 The tentorial artery, the most constant branch of the meningohypophyseal trunk, present in 100% of instances, passes forward to the roof of the cavernous sinus and then posterolaterally along the free edge of the tentorium. The dorsal meningeal artery arises from the meningohypophyseal trunk in 90% of cavernous sinuses. It passes posteriorly through the cavernous sinus with the abducens nerve to reach the dura over the dorsum and clivus. The inferior hypophyseal artery, the least frequent of the three common branches of the meningohypophyseal trunk, arises from the meningohypophyseal trunk in 80% of cavernous sinuses.18,26 It passes medial to the posterior pituitary capsule and lobe and anastomoses with its mate of the opposite side after supplying the dura of the sellar floor. The dorsal meningeal and inferior hypophyseal arteries that do not arise from the meningohypophyseal artery usually arise directly from the intracavernous carotid. The inferolateral trunk (artery of the inferior cavernous sinus) arises from the lateral side of the midportion of the horizontal segment of the intracavernous carotid ~5 to 8 mm distal to the origin of the meningohypophyseal trunk. It arises directly from the carotid artery in 84% of cavernous sinuses and from the meningohypophyseal artery in another 6%.18,26 It passes above or below the sixth nerve and downward medial to the first trigeminal division to supply the dura of the inferior lateral wall of the cavernous sinus and the area of the foramen rotundum and foramen ovale. McConnell’s capsular arteries arise from the medial side of the carotid artery and pass to the capsule of the gland or the dura lining the anterior wall and floor of the sella. They are frequently absent, found in approximately a quarter of cavernous sinuses.18 The ophthalmic artery commonly arises just above the upper ring from the medial half of the anterior wall of the internal carotid artery, but it may also arise in the cavernous sinus, in which case it usually passes through the superior orbital fissure. It may rarely arise from the middle meningeal artery.35 This section deals with neural, arterial, and venous relationships in the suprasellar and third ventricular regions that are important in the management of tumors that extend upward from the sella into these areas. Tumors arising in the sella often extend upward into the suprasellar cisterns to compress the optic nerves and chiasm and floor of the third ventricle, and to involve the circle of Willis36 (Fig. 2.20). The area involved by those tumors arising in the sella corresponds to the anterior incisural space located between the free edges of the tentorium and the front of the midbrain. The anterior incisural space corresponds roughly to the suprasellar area. From the front of the midbrain, it extends obliquely forward and upward around the optic chiasm to the subcallosal area. It opens laterally into the sylvian fissure and posteriorly between the uncus and the brainstem. The part of the anterior incisural space located below the optic chiasm has posterior and posterolateral walls.37,38 The posterior wall is formed by the cerebral peduncles and walls of the interpeduncular cistern. The posterolateral wall is formed by the anterior one-third of the uncus, which extends medially above the free edge of the tentorium and oculomotor nerve. The infundibulum of the pituitary gland crosses the anterior incisural space to reach the opening in the diaphragma sellae. The part of the anterior incisural space situated above the optic chiasm is limited superiorly by the rostrum of the corpus callosum, posteriorly by the lamina terminalis, and laterally by the part of the medial surfaces of the frontal lobes located below the frontal horns. The anterior incisural space opens laterally into the part of the sylvian fissure situated below the anterior perforated substance. The anterior limb of the internal capsule, the head of the caudate nucleus, and the anterior part of the lentiform nucleus are located above the anterior perforated substance. The interpeduncular cistern, which sits in the posterior part of the anterior incisural space between the cerebral peduncles and the dorsum sellae, communicates anteriorly with the chiasmatic cistern, which is located below the optic chiasm. The interpeduncular and chiasmatic cisterns are separated by Liliequist’s membrane, an arachnoidal sheet extending from the dorsum sellae to the mamillary bodies. The chiasmatic cistern communicates around the optic chiasm with the cisterna laminae terminalis, which lies anterior to the lamina terminalis. The optic and oculomotor nerves and the posterior part of the olfactory tracts pass through the suprasellar region and anterior incisural space (Figs. 2.16, 2.17, 2.19, and 2.20). Each olfactory tract runs posteriorly and splits just above the anterior clinoid process to form the medial and lateral olfactory striae, which course along the anterior margin of the anterior perforated substance. The optic nerves and chiasm and the anterior part of the optic tracts cross the anterior incisural space. The optic nerves emerge from the optic canals medial to the anterior clinoid processes and are directed posteriorly, superiorly, and medially toward the optic chiasm. From the chiasm, the optic tracts continue in a posterolateral direction around the cerebral peduncles toward the lateral geniculate bodies. The optic nerve proximal to its entrance into the optic canal is covered by a reflected leaf of dura mater, the falciform process, which extends medially from the anterior clinoid process across the top of the optic nerve. The length of nerve covered only by the dura of the falciform process at the intracranial end of the optic canal may vary from less than 1 mm to as much as 1 cm.6 Coagulation of the dura above the optic nerve just proximal to the optic canal on the assumption that bone separates the dura mater from the nerve can lead to nerve injury. Compression of the optic nerve against the sharp edge of the falciform process may result in a visual field deficit even if the compressing lesion does not damage the nerve enough to cause visual loss.6,8 The optic chiasm is situated at the junction of the anterior wall and floor of the third ventricle (Figs. 2.20 and 2.21). The anterior cerebral and anterior communicating arteries, the lamina terminalis, and the third ventricle are located above the chiasm. The tuber cinereum and the infundibulum are posterior to, the internal carotid arteries are lateral to, and the diaphragma sellae and pituitary gland are below the optic chiasm. The suprachiasmatic recess of the third ventricle is located between the chiasm and lamina terminalis. The infundibular recess extends into the base of the pituitary stalk behind the optic chiasm. The relationship of the chiasm to the sella is an important determinant of the ease with which the pituitary fossa can be exposed by the transfrontal surgical route (Fig. 2.21). The normal chiasm overlies the diaphragma sellae and the pituitary gland, the prefixed chiasm overlies the tuberculum, and the postfixed chiasm overlies the dorsum. In ~70% of cases, the chiasm is in the normal position. Of the remaining 30%, about half are “prefixed” and half “postfixed.”6 Fig. 2.20 Neural relationships in the suprasellar area. (A) Midsagittal section of the sella, pituitary gland, sphenoid sinus, and third ventricle. The anterior part of the third ventricle is located above the sella. The columns of the fornix descend along the superior and anterior margins of the foramen of Monro to reach the mamillary bodies. The optic chiasm and stalk are located above the sella. The internal cerebral veins course in the roof of the third ventricle. (B) Enlarged view. The suprachiasmatic recess of the third ventricle is located between the lamina terminalis and the chiasm. The infundibular recess extends into the stalk in the area behind the chiasm. The lamina terminalis extends upward and is continuous with the rostrum of the corpus callosum. The thin part of the third ventricular floor between the chiasm and the mamillary bodies is suitable for a third ventriculostomy. The anterior commissure crosses the wall of the third ventricle in front of the columns of the fornix. The massa intermedia crosses the midportion of the third ventricle. (C) The anterior part of the hemisphere has been removed to expose the lateral ventricles and suprasellar area. The optic nerve and chiasm are located above the sella. The chiasm cistern is located below the optic chiasm and opens upward between the optic nerves to the area in front of the lamina terminalis. The anterior commissure crosses the anterior wall of the third ventricle above the lamina terminalis. The anterior part of the third ventricle is located above the sella. The body of the lateral ventricle is situated above the third ventricle. The columns of the fornix form the superior and anterior margins of the foramen of Monro. The olfactory tracts pass above the optic nerves, and the optic tracts pass above the oculomotor nerves. (D) The cross-section on the right hemisphere has been extended backward to the midportion of the temporal horn and thalamus. This exposes the oculomotor nerve arising on the medial surface of the cerebral peduncle and passing below the floor of the third ventricle and lateral to the sella. The crural cistern is located between the uncus and the cerebral peduncle. The mamillary bodies are positioned in the floor of the third ventricle. The posterior commissure sits above the aqueduct. (E) The full length of the floor of the third ventricle has been exposed by removing the thalami bilaterally. The mamillary bodies are located at the junction of the anterior and middle thirds of the floor. The floor behind the mamillary bodies is formed by the upper midbrain. The floor in front of the mamillary bodies is very thin and serves as a suitable site for third ventriculostomy. (F) Anterior-superior view. The upper part of the left thalamus has been removed to expose the optic tract, which extends backward above the oculomotor nerve in the lateral part of the suprasellar area to reach the lateral geniculate body. The chiasm and posterior part of the optic nerves are located directly above the sella. The anterior cerebral arteries pass above the chiasm. The left anterior cerebral artery is hypoplastic. Fig. 2.21 Sagittal sections and superior views of the sellar region showing the optic nerve and chiasm and the carotid artery. The prefixed chiasm is located above the tuberculum. The normal chiasm is located above the diaphragma. The postfixed chiasm is situated above the dorsum. A prominent tuberculum sellae may restrict access to the sella even in the presence of a normal chiasm. The tuberculum may vary from almost flat to protruding upward as much as 3 mm, and it may project posteriorly to the margin of a normal chiasm6 (Fig. 2.18). A prefixed chiasm, a normal chiasm with a small area between the tuberculum and the chiasm, and a superior protruding tuberculum sellae do not limit exposure by the transsphenoidal approach, but they do limit the access to the suprasellar area provided by the transcranial approach. There are several methods of gaining access to the suprasellar area when these variants are present. One is to expose the sphenoid sinus from above by opening through the tuberculum and planum sphenoidale, thus converting the approach to a transfrontal-transsphenoidal exposure. If the chiasm is prefixed and the tumor is seen through a thin, stretched anterior wall of the third ventricle, the lamina terminalis may be opened to expose the tumor, but this exposure is infrequently used for pituitary adenomas and more commonly for craniopharyngiomas and gliomas involving the third ventricle. If the space between the carotid artery and the optic nerve has been enlarged by a lateral or parasellar extension of tumor, the tumor may be removed through this space.17 An understanding of the relationships of the carotid artery, optic nerve, and anterior clinoid process is fundamental to all surgical approaches to the sellar and parasellar areas (Figs. 2.6, 2.16, and 2.17). The carotid artery and the optic nerve are medial to the anterior clinoid process. The artery exits the cavernous sinus beneath and slightly lateral to the optic nerve. The optic nerve pursues a posteromedial course toward the chiasm, and the carotid artery pursues a posterolateral course toward its bifurcation below the anterior perforated substance. The oculomotor nerve arises in the interpeduncular cistern from the midbrain on the medial side of the cerebral peduncle and courses between the posterior cerebral and superior cerebellar arteries (Fig. 2.19). The oculomotor nerve courses in the lateral wall of the interpeduncular cistern and forms the pillars to which the lateral edge of Liliequist’s membrane attaches. Liliequist’s membrane extends upward from the arachnoid membrane covering the dorsum sellae and separates the chiasmatic and interpeduncular cisterns. The uncus of the temporal lobe is situated lateral to the oculomotor nerve. The oculomotor nerve pierces the roof of the cavernous sinus and slopes downward in the superolateral corner of the cavernous sinus. The trochlear nerve is the longest and thinnest cranial nerve (Figs. 2.6, 2.16, and 2.19). The trochlear nerve arises from the midbrain below the inferior colliculus and passes around the brainstem near the junction of the midbrain and pons to reach the lower margin of the tentorial edge. The trochlear nerve pierces the medial edge of the tentorium and enters the roof of the cavernous sinus just behind the anterior tentorial attachment. The abducens nerve arises at the lower margin of the pons and passes above or below or is split into two bundles by the anterior inferior cerebellar artery (Figs. 2.16 and 2.19). It passes upward in the prepontine cistern, then turns forward at the upper border of the petrous apex, where it pierces the dura and passes below the petrosphenoid (Gruber’s) ligament to enter the posterior part of the cavernous sinus. The trigeminal nerve arises in the posterior fossa from the midpons. The posterior root passes above the petrous apex to enter Meckel’s cave, a cavern in the subarachnoid space located lateral to the cavernous sinus. Meckel’s cave extends forward to the level of the trigeminal ganglion. The arterial relationships in the suprasellar area are among the most complex in the head because this area contains all the components of the circle of Willis (Figs. 2.22 and 2.23). Numerous arteries, including the internal carotid and basilar arteries and the circle of Willis and its branches, may be stretched around tumors in this area. The posterior part of the circle of Willis and the apex of the basilar artery are located in the anterior incisural space below the floor of the third ventricle; the anterior part of the circle of Willis and the anterior cerebral and anterior communicating arteries are intimately related to the anterior wall of the third ventricle; both the anterior and posterior cerebral arteries send branches into the roof of the third ventricle; the internal carotid, anterior choroidal, anterior and posterior cerebral, and anterior and posterior communicating arteries give rise to perforating branches that reach the walls of the third ventricle and anterior incisural space; and all the arterial components of the circle of Willis and the adjacent segments of the carotid and basilar arteries and their perforating branches may be stretched around suprasellar extensions of pituitary tumors.1 The internal carotid artery exits the cavernous sinus along the medial surface of the anterior clinoid process to reach the anterior incisural space (Figs. 2.16, 2.17, and 2.19). After entering this space it courses posteriorly, superiorly, and laterally to reach the site of its bifurcation below the anterior perforated substance. It is first below and then lateral to the optic nerve and chiasm. It sends perforating branches to the optic nerve, chiasm, and tract and to the floor of the third ventricle. These branches pass across the interval between the internal carotid artery and the optic nerve and may be an obstacle to the operative approaches directed through the triangular space between the internal carotid artery, the optic nerve, and the anterior cerebral artery. The internal carotid artery also gives offthe superior hypophyseal artery, which runs medially below the floor of the third ventricle to reach the tuber cinereum and joins its mate of the opposite side to form a ring around the infundibulum. The ophthalmic artery, the first branch of the internal carotid artery above the cavernous sinus, usually arises and enters the optic canal below the optic nerve (Figs. 2.16, 2.17, and 2.23). Its origin and proximal segment may be visible below the optic nerve without retraction of the nerve, although elevation of the optic nerve away from the carotid artery is usually required to see the segment proximal to the optic foramen. The artery arises from the supraclinoid segment of the carotid artery in most cases, but in some cases, it arises within the cavernous sinus or rarely as a branch of the middle meningeal artery.6,18,26,39 The posterior communicating artery arises from the posterior wall of the internal carotid artery and courses posteromedially below the optic tracts and the floor of the third ventricle to join the posterior cerebral artery (Figs. 2.22 and 2.23). Its branches penetrate the floor of the third ventricle between the optic chiasm and the cerebral peduncle and reach the thalamus, hypothalamus, subthalamus, and internal capsule. Its posterior course varies depending on whether the artery provides the major supply to the distal posterior cerebral artery. If it is normal, with the posterior cerebral artery arising predominantly from the basilar artery, it is directed posteromedially above the oculomotor nerve toward the interpeduncular fossa. If the posterior cerebral artery has a fetal type of configuration, in which it arises predominantly from the carotid artery, the posterior communicating artery is directed posterolaterally above or lateral to the oculomotor nerve. Fig. 2.22 Vascular relationships in the suprasellar area. (A) The anterior cerebral arteries course above the optic chiasm and in front of the lamina terminalis. The carotid arteries exit the cavernous sinus and pass upward in the lateral margins of the suprasellar area. The superior hypophyseal arteries cross the chiasmatic cistern to reach the lower margin of the chiasm and pituitary stalk. (B) Superior view of the suprasellar region. The floor of the third ventricle has been exposed from the suprachiasmatic recess anteriorly to the level of the aqueduct posteriorly. The anterior cerebral arteries pass above the chiasm. The posterior communicating arteries pass backward below the floor of the third ventricle. The basilar artery bifurcates into the posterior cerebral arteries below the floor of the third ventricle. (C) Superior view of the suprasellar region. The carotid arteries course along the lateral margin of the chiasmatic cistern. The basilar bifurcation is located above and behind the sella. The posterior communicating arteries travel backward across the dorsum to join the posterior cerebral arteries. The posterior communicating arteries usually course above and medial to the oculomotor nerves. (D) The optic chiasm is positioned above the diaphragm and sella. The optic tracts extend backward and laterally above the posterior cerebral arteries and oculomotor nerves toward the lateral geniculate bodies. The basilar bifurcation has been retracted forward to show the perforating arteries entering the midbrain, which can be damaged in the transsphenoidal approach if the posterior wall of the capsule of a pituitary adenoma is opened. (E) Diagrammatic view of the arteries in the suprasellar area, which can be stretched over the margin of a large tumor with suprasellar extension. All the components of the circle of Willis and their perforating branches can be stretched over the dome of these tumors. (F) Superolateral view of the left optic nerve, optic chiasm, and left optic tract and the floor of the third ventricle. The optic tract extends backward from the optic chiasm, around the upper edge of the cerebral peduncle, and above the posterior cerebral artery. The anterior cerebral arteries pass in front of the lamina terminalis and around the corpus callosum. (G) Some of the anterior part of the left cerebral peduncle has been removed while the optic tract is preserved. The posterior cerebral artery and terminal part of the posterior communicating artery can be seen through the interval between the floor of the third ventricle and the optic tract. Fig. 2.23 Relationships in the sellar and suprasellar areas. (A) Inferior view. The supraclinoid portion of the carotid artery is divided into three segments based on the site of origin of its major branches; the ophthalmic segment extends from the origin of the ophthalmic artery to the origin of the posterior communicating artery, the communicating segment extends from the origin of the posterior communicating artery to the origin of the anterior choroidal artery, and the choroidal segment extends from the origin of the anterior choroidal artery to the bifurcation of the carotid artery. The optic nerves are above the ophthalmic arteries. The optic chiasm and optic tracts are above the anterior and posterior lobes of the pituitary gland. The tuber cinereum is anterior to the apex of the basilar artery. The posterior cerebral arteries pass around the cerebral peduncles above the oculomotor nerves. The perforating branches arising from the ophthalmic segment pass to the anterior lobe, optic nerve and chiasm, and the anterior part of the tuber cinereum. A single perforating branch arises from the communicating segment on each side and passes upward to the optic tract and floor of the third ventricle. (B) The pituitary gland has been reflected backward to show the superior hypophyseal arteries passing from the ophthalmic segments to the infundibulum. The anterior cerebral and anterior communicating arteries pass above the optic chiasm. (C) Posterior view. The basilar artery and brainstem have been divided and the floor of the third ventricle elevated to provide this posterior view of the arteries in the suprasellar area. The tuber cinereum and mamillary bodies are exposed between the optic tracts. (D) The right half of the dorsum and the right posterior clinoid process have been removed to expose the anterior and posterior lobes of the pituitary gland. The basilar, posterior cerebral, and superior cerebellar arteries have been elevated to expose the pituitary stalk and floor of the third ventricle. The inferior hypophyseal and tentorial arteries arise from the carotid artery. The anterior choroidal artery arises from the posterior surface of the internal carotid artery above the origin of the posterior communicating artery (Figs. 2.22 and 2.23). It is directed posterolaterally below the optic tract between the uncus and cerebral peduncle. It passes through the choroidal fissure behind the uncus to supply the choroid plexus in the temporal horn, sending branches into the optic tract and posterior part of the third ventricular floor that reach the optic radiations, globus pallidus, internal capsule, midbrain, and thalamus. The anterior cerebral artery arises from the internal carotid artery below the anterior perforated substance and courses anteromedially above the optic nerve and chiasm to reach the interhemispheric fissure, where it is joined to the opposite anterior cerebral artery by the anterior communicating artery (Figs. 2.22 and 2.23). The junction of the anterior communicating artery with the right and left A1 segments is usually above the chiasm rather than above the optic nerves. The shorter A1 segments are stretched tightly over the chiasm, and the larger ones pass anteriorly over the nerves. Visual loss caused by elevation of the chiasm against these arteries may occur before visual loss caused by direct compression of the tumor on the visual pathways. The arteries with a more forward course are often tortuous and elongated, and some may course forward and rest on the tuberculum sellae or planum sphenoidale. The anterior cerebral and anterior communicating arteries give rise to perforating branches that terminate in the whole anterior wall of the third ventricle and reach the adjacent parts of the hypothalamus, fornix, septum pellucidum, and striatum. The recurrent branch of the anterior cerebral artery, frequently encountered in the area, arises from the anterior cerebral artery in the region of the anterior communicating artery, courses laterally above the bifurcation of the internal carotid artery, and enters the anterior perforated substance. The bifurcation of the basilar artery into the posterior cerebral arteries is located in the posterior part of the suprasellar area below the posterior half of the floor of the third ventricle (Figs. 2.11, 2.22, and 2.23). A high basilar bifurcation may indent the ventricular floor. The posterior cerebral artery courses laterally around the cerebral peduncle and above the oculomotor nerve, and then passes between the uncus and the cerebral peduncle to reach the ambient and quadrigeminal cisterns. Its branches reach the floor, roof, and posterior and lateral walls of the third ventricle. The thalamoperforating arteries are a pair of larger perforating branches that arise from the proximal part of the posterior cerebral artery in the suprasellar region and enter the brain through the posterior part of the floor and lateral walls of the third ventricle. The author is aware of several cases in which damage to the thalamoperforating branches occurred during transsphenoidal surgery after the posterosuperior part of the tumor capsule was opened, with resulting coma and death. Veins do not pose a formidable obstacle to operative approaches to the suprasellar area and lower part of the third ventricle, as they do in the region of the roof and posterior third ventricle, because the veins in the suprasellar region are small. The suprasellar area is drained, almost totally, by tributaries of the basal vein. The basal veins are formed by the union of veins draining the suprasellar area and proceed posteriorly between the midbrain and the temporal lobes to empty into the internal cerebral or great vein. The internal cerebral veins course in the roof of the third ventricle and are only infrequently involved in sellar tumors. They originate just behind the foramen of Monro and course posteriorly within the velum interpositum. They join above or posterior to the pineal body to form the great vein. The treatment of pathologic entities in the parasellar and sellar regions requires exquisite knowledge of neuroanatomy. The anatomy of the normal state is complex. Variations in anatomy related to pathologic conditions make this region even more challenging.
 Subcranial Relationships
Subcranial Relationships
Nasal Cavity
Sphenoid Bone
Sphenoid Sinus
Diaphragma Sellae
Pituitary Gland
Pituitary Gland and Carotid Artery
Intercavernous Venous Connections
Extensions of the Sellar Type of Sphenoid Sinus
 The Parasellar Region
The Parasellar Region
Anterior and Middle Clinoid Processes
Optic Strut
Dural Relationships
Neural Relationships
Parasellar Triangles
Cavernous Sinus Triangles
Middle Fossa Triangles
Paraclival Triangles
Arterial Relationships
 Suprasellar and Third Ventricular Region
Suprasellar and Third Ventricular Region
Ventricular and Cisternal Relationships
Cranial Nerves
Arterial Relationships
Venous Relationships
 Conclusion
Conclusion
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree