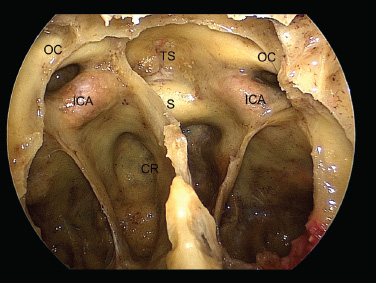
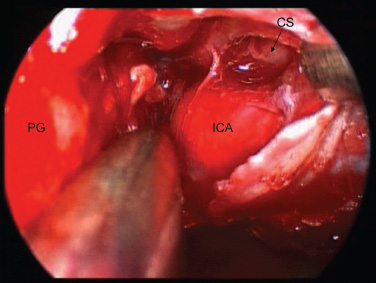
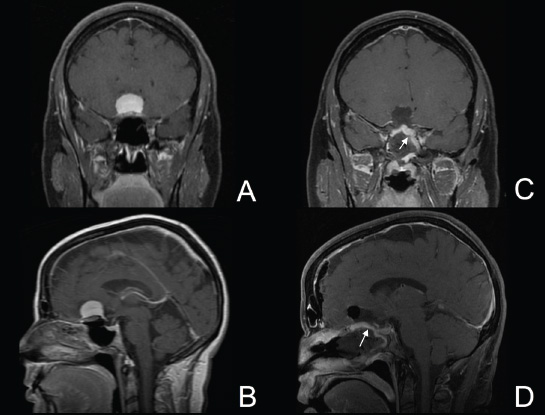
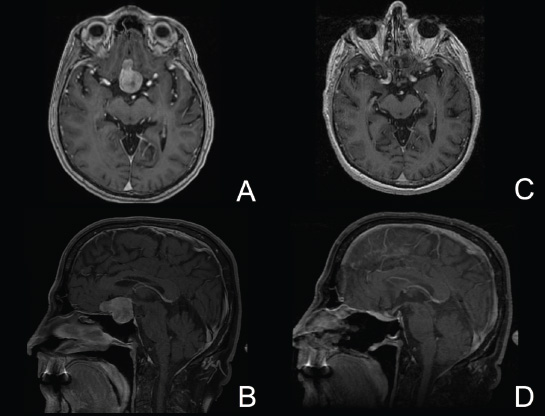
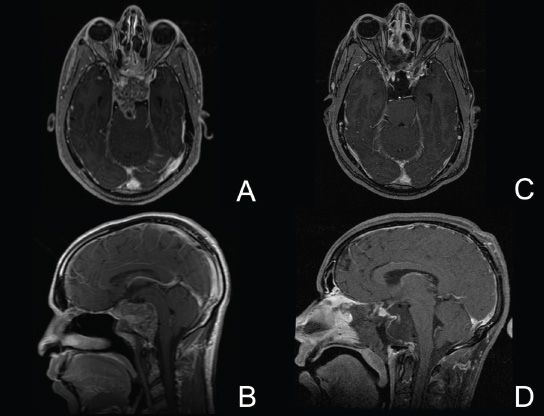
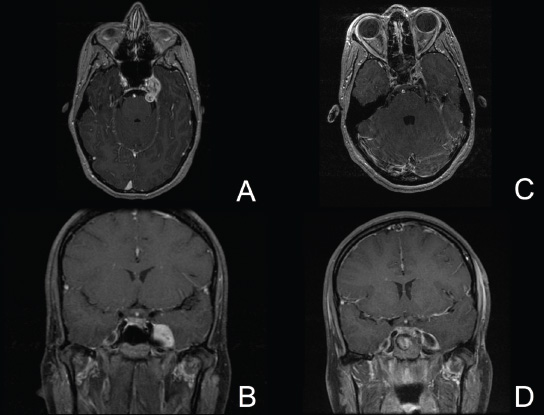
11 Transsphenoidal Approaches to the Sellar and Parasellar Area The sinuses have been used as a corridor to access the pituitary fossa for over 100 years. From the beginning, surgeons appreciated the advantage of approaching the target from below and having direct access to the sella without manipulating the brain.1 The technology at that time was very limited, however, and most surgeons did not feel it safe enough to perform major neurosurgical procedures through narrow nasal pathways. Harvey Cushing is probably the greatest example because he abandoned the transsphenoidal approach in 1927 owing in part to an inability to properly illuminate the surgical field.2,3 Although Norman Dott, who had learned the technique from Cushing, employed a lighted speculum, it was only after the contributions of Gerard Guiot, who added fluoroscopy, and Jules Hardy, who added the operating microscope, that the transsphenoidal route became popular and was disseminated around the world.1 One of the limitations of the transsphenoidal approach performed with the microscope is the relative restriction of parasellar illumination and visualization. Although the sella can be well visualized, areas such as the cavernous sinus and anterior fossa region can be completely out of sight. With that in mind, some neurosurgeons started to add the endoscope to the scene in parallel with the microscope to augment visualization in these particular areas and so improve tumor resection and outcomes. Gerard Guiot was the first neurosurgeon to use the endoscope in the transsphenoidal route in the 1960s, but at that time the technology was still fairly primitive.1 In the late 1970s, Apuzzo and colleagues,4 as well as Bushe and Halves,5,6 demonstrated the use of the endoscope as an adjunctive tool to remove pituitary lesions. Endoscopically “assisted” transsphenoidal microsurgery has since been reported by various authors, emphasizing its importance for tumors that extend beyond the sella.7–12 Surgeons progressively realized that the combination of speculum and endoscope is not ideal because one limits the other. Roger Jankowski and co-workers reported the first series of three cases in which an endoscopic transsphenoidal approach to the sella was used in 1992.13 Ricardo Carrau and colleagues pursued a purely direct endoscopic endonasal approach to the pituitary fossa and described their experience in 1996,14 followed by their first 50-patient series in 1997.14–16 Paolo Cappabianca, Enricode Divitiis, Luigi Cavallo, and colleagues developed appropriate surgical instrumentation and became a world reference for endoscopic pituitary surgery.17–27 At the same time that various surgeons were developing and demonstrating the feasibility of gaining access beyond the sella by using the transsphenoidal route, particularly to the midline anterior skull base with the microscope as the primary visualization tool,7,8,28–32 Amin Kassam, Ricardo Carrau, Carl Snyderman, and colleagues defined and standardized modules of approaches to the skull base, expanded from the sella, while using the endoscope as the sole tool for illumination and visualization.1,33,34 Although it is still controversial whether the endoscope brings real advantages to dealing with sellar disease in relation to the microscope, the use of endoscopic endonasal techniques to reach the areas beyond the sella has become well accepted.11,35–43 The endonasal corridor is a median approach that is in the same axis as the sella and therefore represents an ideal route to reach the sella with no brain manipulation. Historically, craniotomies were the general approach used to deal with sellar disease. However, this concept was completely altered once Jules Hardy brought the operating microscope to the transsphenoidal operation.44 Any transcranial approach requires some level of brain retraction and yields indirect visualization of the pituitary fossa, which is hidden by the planum sphenoidale.45 Currently, fewer than 5% of patients with a pituitary adenoma have a craniotomy as an alternative approach for resection, and it is always related to an extrasellar tumor component.45 There is a consensus that the sellar portion of tumor is better treated by a transsphenoidal route in an approach with the microscope, endoscope, or both.45–50 The transsphenoidal approach has had some variations over the years. The initial description by Schloffer required a paranasal incision followed by a complete transethmoidal exposure to reach the sphenoid sinus and sella.51 With the evolution of the technique, the approach has become progressively less invasive. The procedure is performed with the patient under general anesthesia. The patient is positioned typically in a semirecumbent angle with the head tilted to the left. There are three current variations of the transsphenoidal approach: sublabial, hemitransfixion, and direct endonasal, or “pushover.” From the time Cushing was using the technique until recently, the transsphenoidal approach was invariably performed as a sublabial procedure. Indeed, it is still performed that way in many centers around the world.47,52 An incision is made under the superior lip inside the mouth, and the mucoperiosteum is elevated from the bone at the piriform aperture. The plane between the mucoperichondrium and the mucoperiosteum posteriorly is encountered and the mucosa is separated from the septum on both sides. The septum is retracted, often to the left side, and a speculum is positioned and advanced until the rostrum of the sphenoid is well exposed in the center of the field. An anterior sphenoidectomy is performed with a rongeur. The sphenoid sinus is visualized, and the position of the sella is confirmed with lateral fluoroscopy or some other type of navigation.53 The sella is opened either with a drill or with a chisel. Once the bone of the sellar face is removed and the dura is exposed, a knife, usually an 11-blade scalpel, is used to incise the dura. The sella is explored and the tumor can be removed, usually with ring curettes. The sublabial approach has the advantage of creating a broad corridor, which can be as wide as the piriform aperture.47 The disadvantage of the sublabial technique is related to potential complications of the approach. There is a higher incidence of anterior septal perforation with the sublabial approach. The superior anterior teeth can develop various grades of numbness, and saddle nose deformation due to anterior septum manipulation can also occur. The other commonly used variations of the transsphenoidal route are endonasal procedures performed with the microscope. The transnasal hemitransfixion technique requires an incision in the septal mucosa ~1 cm behind the columella. The speculum is inserted directly in the nostril, and the plane between the mucoperiosteum and the septum is elevated posteriorly as in the sublabial approach. The rest of the procedure continues in a fashion similar to the sublabial approach. The other variation is the direct endonasal (“pushover”) technique. In this variant, the speculum is introduced earlier in the case, and the nasal pathway is followed directly until the posterior aspect of the septum. The mucosa is not elevated from the septum anteriorly. The middle turbinate is retracted laterally, and the posterior septum is dislocated to the opposite side. The mucosa is then disrupted very posteriorly at the bony septum area. The submucosal plane is elevated and the rostrum is exposed. The rest of the procedure follows the same technique as the sublabial one. The advantage of the transnasal transsphenoidal approach is that it avoids manipulation of the anterior teeth and saddle nose deformities. On the other hand, it generates a narrower corridor, and the specula used need to be adapted for small nostrils. If the speculum is forced open during the procedure with the intent of creating better exposure, a nasal skin tear may occur. The other disadvantage is related to the fact that the speculum comes obliquely from one of the nostrils. This can generate a false impression of the midline, and the surgeon can inadvertently cross to the other side in the depths without appropriate awareness of the situation. This can be the scenario for a catastrophic complication because the surgeon can open the cavernous sinus directly in front of the internal carotid artery thinking that that is the midline.54,55 Totally endoscopic transsphenoidal surgery started in the 1990s as a collaboration between otolaryngologists and neurosurgeons, with the goal of developing a less disruptive approach to the nasal structures.1,13–16,23,56 It consists of a direct endonasal approach to the back of the nasal cavity, followed by identification of the sphenoid ostium. The anterior wall of the sphenoid sinus is opened, and the sinus can be visualized. Although there was an initial trend to perform this approach through only one nostril, currently most centers performing this procedure follow the binostril-bimanual technique, which allows much more freedom of movement.33 A posterior septectomy is performed, communicating both nostrils posteriorly and creating a single working cavity. The intrasphenoid septa are drilled, allowing broad visualization of all the sphenoid walls. At this point, the surgeon is able to determine with precision the skull base landmarks, particularly in well-pneumatized sinuses, which avoids the absolute need for fluoroscopy or image guidance (Fig. 11.1). Nonetheless, if the surgeons have easy access to image guidance, it is probably wise to use it for every endoscopic case because it is hard to predict in which cases it may be helpful to confirm a certain structure intraoperatively. Of course, cases of nonpneumatized sphenoid sinus clearly benefit from intraoperative image guidance. Such cases are simple to foresee based on preoperative computed tomography. However, cases with unexpected vascular complications or tumor-distorted anatomy can also benefit from intraoperative image guidance, even when there is a wide-open sphenoid sinus. The face of the sella is then identified, and the bone is removed to expose the dura. The superior and inferior intercavernous sinuses and the face of the cavernous sinuses bilaterally are exposed. This relatively wide exposure coupled with an ample dural opening allows an unencumbered intrasellar dissection. The dura of the sella is incised with a sharp blade that incises both layers of the dura but does not transgress the pituitary capsule. The dura is elevated from the sellar face, exposing the gland or tumor. An extracapsular resection of the tumor is always attempted, particularly in hyperfunctioning adenomas, to secure a complete resection. Even in cases in which the pituitary gland needs to be explored, in magnetic resonance imaging–negative cases of Cushing disease, for instance, an extracapsular resection of the hidden adenoma is attempted to permit a thorough resection of the functional adenoma and its margins because the pseudocapsule is indeed compact normal gland46,57 (Fig. 11.2). Occasionally, the medial wall of the cavernous sinus needs to be resected to increase the chance of remission in more invasive adenomas. This affords an advantage of the endoscopic technique over the microscopic because the visualization offered by the endoscope laterally is far superior (Fig. 11.3). Two surgeons perform the entire procedure. In our personal experience, because there are no data to support a significant difference in the literature, we believe that the visualization allowed by the endoscope is far superior to that from the operating microscope. Furthermore, the illumination and image captured by the endoscope are closer to the target, whereas with the microscope, the imaging is from a greater distance. This is particularly important because the surgeon’s hands can block the view during microscopic surgery. The wide field of visualization provided by the endoscope allows dissection in the parasellar area, which is crucial to resect invasive pituitary adenomas from the cavernous sinus and to deal with extensive suprasellar projections of tumor, always under direct visualization and without the use of blind curettage (Fig. 11.3). The most common indications for this approach are pituitary adenomas, intrasellar craniopharyngiomas, and Rathke cleft cysts. The use of microscope to reach areas around the sella is possible from a transsphenoidal approach. Because the light source is external, however, there are limitations for visualization of areas that are not in line with the approach. The surgeon often is obliged, in this scenario, to extend the size of the corridor by using a sublabial incision, which can be augmented laterally by a medial maxillectomy or a LeForte maxillary disarticulation.58,59 The other aspect of limitation is related to the focal plane of the microscope, which becomes very constricted as the dissection is performed more deeply. To compensate for this limitation, the surgeon is required to move the microscope often to see areas of the skull base that are not located in the narrow microscopic field. Fig. 11.3 Intraoperative photograph during a resection of a pituitary adenoma that was invading the left cavernous sinus. Note that the pituitary gland (PG) is protected on the right. The left medial wall of the cavernous sinus was opened by resection of the tumor that was invading the cavernous sinus (CS) and encasing the internal carotid artery (ICA). Once a tumor has been removed, the venous bleeding can be profuse, and one can see the roof of the cavernous sinus from below. Based on the advantages of the endoscopic illumination and wide visualization, it becomes natural to use the endoscope to reach lesions located in the parasellar space. It is ideal when the sellar space is the primary source of the disease, as with pituitary adenomas. However, other lesions, like craniopharyngiomas, meningiomas, chordomas, chondrosarcomas, and schwannomas, may be present in these locations and may be treated by the endoscopic expanded endonasal approaches as well.40,41,60–62 It is important to have dynamic endoscopic visualization to compensate for the lack of three-dimensional imaging obtained from a static endoscopic view. Very often, a suprasellar tumor is the extension of a disease that started inside the sella, particularly in pituitary adenomas and some craniopharyngiomas. In these cases, most of the time, a sellar approach is sufficient to evacuate the tumor completely, starting with the sellar component and then following the tumor superiorly. Often, the superior tumor descends naturally as the sellar disease is removed. For these cases, a microscopic transsphenoidal approach is a feasible alternative; however, it becomes difficult to determine if there is any residual tumor superiorly at the end of the dissection. One alternative would be the use of interoperative MRI, or the insertion of an angled endoscope for investigation. Nevertheless, because the corridor is not prepared for an endoscopic approach, the surgeon often is able to see residua but ends up having difficulty reaching and removing such residua under direct visualization and frequently uses blind dissection at the corners with instruments like angled ring curettes, which carry some risk, even in experienced hands. To reach tumors that originate or extend to the suprasellar space from the sella, we prefer an endoscopic endonasal transtuberculum and/or transplanum approach. This approach is defined by the removal of the tuberculum sellae and, depending on the anterior extension of the tumor, the planum sphenoidale. The optic canals mark the lateral limits for this approach. The suprasellar region is well reached through resection of the tuberculum sellae. For most of the macroadenomas that extend to the suprasellar space, a simple bony removal of the tuberculum sellae can be enough to allow the superior component to descend during surgery. Thus, the resection is inside out, and the diaphragma sellae and suprasellar arachnoid are often preserved, avoiding exposure of cerebrospinal fluid (CSF) spaces. On the other hand, tumors like craniopharyngiomas (Fig. 11.4), tuberculum sellae meningiomas (Fig. 11.5), and some rare pituitary adenomas (Fig. 11.6) require a subarachnoid dissection to free them from critical suprasellar structures (Fig. 11.7). The critical anatomic landmark for this approach is the medial optic carotid recess, which is the lateral aspect of the tuberculum sellae. It must be removed to allow adequate exposure of the suprasellar compartment. Once the bone is removed, the dura is opened above the superior intercavernous sinus, which is ligated only when there is disease that requires arachnoid dissection involving the sella and suprasellar space. The dural opening can occur from one internal carotid artery to the other with direct safe access to the medial aspect of the carotid cave and the optic canals. The opening is extended anteriorly in case the tumor is located above the planum sphenoidale. Some pituitary macroadenomas can extend posteriorly over the dorsum sellae, reaching the retrosellar space. These lesions are virtually impossible to resect through a standard transsphenoidal microscopic approach. The alternatives are either a retromastoid posterior fossa approach or an endoscopic endonasal transclival approach. An endonasal transclival approach can be performed in segmental portions (upper, middle, or lower thirds) or as complete removal of the clivus (panclivectomy). The upper third is related to the dorsum sellae in the midline and the posterior clinoids in the paramedian region. The posterior wall of the sella can be removed via a transsellar approach when the tumor has created intrasellar space, or after a pituitary transposition and ligation of the posterior intercavernous sinus.63 The retrosellar bone can also be resected extradurally via a subsellar corridor after removal of the sellar bone and superior retraction of the sellar dura.63 After transposition, the retrodorsal dura containing the basilar plexus is exposed. Dural opening with basilar plexus control provides access to the retrodorsal area and the interpeduncular cistern, surrounded laterally by the posterior communicating arteries and third cranial nerves and posteriorly by the basilar artery with its terminal branches. Fig. 11.4 (A,B) MRI with contrast of a 32-year-old man with a craniopharyngioma who presented with visual loss. The tumor has large components, both solid and cystic. (A) Coronal view. (B) Sagittal view. (C,D) Postoperative MRI with contrast 24 hours after an expanded endonasal transtuberculum and transplanum approach demonstrating complete resection of the craniopharyngioma. (C) Coronal view. (D) Sagittal view. Note the presence of an enhancing nasoseptal flap that was used for reconstruction (black arrow) and the preserved pituitary stalk. Along with the indications for resection of macroadenomas invading the posterior fossa and the clivus, a transclival approach is frequently used for the resection of extradural and intradural diseases, such as chordomas and chondrosarcomas (Fig. 11.8). It is also used to access purely intradural disease anterior to the brainstem, such as meningiomas and craniopharyngiomas. The middle clivus can be directly accessed at the posterior aspect of the sphenoid sinus, and its resection is limited laterally by both internal carotid arteries ascending in the paraclival areas. If the bone drilling continues inferiorly, the lower third of the clivus is restricted laterally by the fossa of Rosenmüller and the torus tubarius. Once intradural, a middle clivectomy gives access to the prepontine cistern and basilar artery, guarded laterally by the sixth cranial nerves. The inferior third gives access to the premedullary cistern, with the vertebral arteries posteriorly and the hypoglossal nerves limiting the approach laterally. A panclivectomy can extend all the way from the dorsum sellae and posterior clinoids down to the basion at the foramen magnum. Fig. 11.6 MRI with contrast of a 56-year-old man with a pituitary macroadenoma with extension to the anterior fossa who presented with profound bitemporal hemianopsia. (A) Axial view. (B) Sagittal view. (C,D) Postoperative MRI with contrast at 3-month follow-up after an expanded endonasal transtuberculum and transplanum approach demonstrating complete adenoma resection. (C) Axial view. (D) Sagittal view. Although pituitary macroadenomas often push the cavernous sinus wall laterally, respecting its limits, they may truly invade that space by crossing the plane of the medial cavernous sinus wall. There is debate in the literature regarding the pattern of this invasion. It is not clear if the invasiveness is a consequence of tumor cell characteristics or if it is related to the fact that some people may have a dehiscence of the medial wall of the cavernous sinus that facilitates adenoma penetration.64 The reality is that any pituitary adenoma that disseminates into the cavernous sinus becomes a challenging entity to be cured, independently of the treatment strategy. Based on the potential morbidity, a surgical procedure, endonasal or craniotomy, will rarely be indicated for an invasive adenoma located inside the cavernous sinus. Radiosurgery and/or medical treatment, particularly in functional adenomas, are generally the most prudent options in these situations. However, there are circumstances in which a cavernous sinus approach is a reasonable option, such as when the patient develops cavernous sinus syndrome with florid cranial nerve neuropathy and decompression is indicated, which can be achieved with an endoscopic endonasal approach or a craniotomy with middle fossa peeling. Another reasonable indication is a patient with a functional pituitary adenoma that is limited to the medial compartment of the cavernous sinus. In this scenario, an endoscopic endonasal approach can be performed to resect the medial cavernous sinus wall and remove the disease located medial to the cavernous internal carotid artery (Fig. 11.3). Other tumors can be present in these areas, such as meningiomas and schwannomas. When the disease is located lateral to the internal carotid artery in the middle fossa, filling the lateral compartment of the cavernous sinus and the Meckel cave, then a craniotomy with middle fossa skull base exposure becomes a reasonable surgical approach. However, when cavernous sinus meningiomas are resected, independently of the approach, the morbidity can be extremely high since they can invade cranial nerves.65 Trigeminal schwannomas are usually not invasive and can be approached endonasally (Fig. 11.9) or by craniotomy.62 To reach the middle fossa through an endonasal corridor, a transpterygoid approach is performed. Initially, a maxillary antrostomy is developed, exposing the posterior wall of the maxillary sinus. The sphenopalatine artery is identified, and its branches are coagulated and ligated. The posterior wall of the maxillary sinus is removed, and the soft contents of the sphenopalatine fossa are retracted laterally. The vidian foramen and foramen rotundum are identified posteriorly in the sphenoid bone and preserved when possible. The lateral sphenoid recess can be completely exposed once the pterygoid base of the sphenoid plates is drilled. This exposure allows direct access to the middle fossa. Fig. 11.8 MRI with contrast of a 28-year-old man with a recurrent clival chordoma who presented with right sixth cranial nerve palsy. (A) Axial view. (B) Sagittal view. (C,D) Postoperative MRI with contrast at 24 hours after an expanded endonasal transclival approach with intradural and extradural dissection demonstrating thorough chordoma resection. (C) Axial view. (D) Sagittal view. Note the preservation of the pituitary gland and stalk. The quadrangular space is the front door to the Meckel cave.22,62 This space is outlined by the horizontal petrous internal carotid artery inferiorly, the ascending vertical cavernous/paraclival internal carotid artery medially, the sixth cranial nerve superiorly in the cavernous sinus, and the maxillary division of the trigeminal nerve (V2) laterally. The vidian nerve is followed posteriorly up to the level of the lacerum segment of internal carotid artery. Once the internal carotid artery is identified, it can be skeletonized if needed, depending on the pathology. The bone of the medial temporal fossa is drilled and the periosteum exposed and opened at the quadrangular space, as described above. Tumors commonly encountered in this region are invasive adenoid cystic carcinomas, juvenile nasal angiofibromas, meningiomas, schwannomas, and invasive pituitary adenomas. The lateral compartment of the cavernous sinus is rarely approached because of the presence of cranial nerves coming and going to the superior orbital fissure. As we mentioned above, this area is explored surgically almost exclusively when the patient already has cranial nerve (III, IV, VI) deficits. Following the principles of reconstruction in open skull base surgery, we use vascularized tissue to rebuild the skull base defect. Hadad and Bassagasteguy, from Argentina, and colleagues developed a nasoseptal flap supplied by the posterior nasoseptal arteries, which are branches of the sphenopalatine artery.66 This nasoseptal mucosal flap has been our preferred reconstruction technique.67 The flap can be harvested initially in cases in which CSF exposure is likely, or as a rescue flap at the end of the procedure in cases in which there is an unsuccessful attempt to avoid an intraoperative CSF leak. In general, it is harvested on the side that requires less lateral exposure, contralateral to the lesion.67 During this reconstruction, the flap needs to be in contact with the denuded bone for proper defect closing, so all the sinus mucosa is removed. Besides the nasoseptal flap, we use an inlay subdural graft of collagen matrix. Rarely, in cases in which the nasoseptal flap does not cover the entire defect, an additional onlay fascial graft and/or abdominal free fat is used. It is imperative to avoid any foreign body or nonvascularized tissue between the flap and the surrounding edges of the defect. When a nasoseptal flap is not available, then other sources of vascularized tissue exist. Excellent alternatives can be encountered in the nasal cavity, such as an inferior turbinate flap, optimized for clival defects, or middle turbinate flaps for anterior fossa small defects.1,68 In situations in which a vascularized flap is not available in the nasal cavity, such as after multiple surgical procedures or radiation, then healthy vascularized tissue can be elevated from outside and rotated into the nasal cavity to cover the defect. Examples of extranasal flaps that can be rotated into the nasal cavity are the transpterygoid temporoparietal fascia flap, based on the superficial temporal artery,69 and a pericranial flap vascularized by the supraorbital artery, which can be elevated endoscopically and transferred to the nasal cavity through an opening in the nasal bone.70 Biological glue helps to fix the flap in place (but should not be overused), and nasal sponge packing or the balloon of a 12F Foley catheter is inserted to press the Hadad-Bassagasteguy flap against the defect. Inflation of the Foley balloon should be under microscopic or endoscopic observation to avoid overdistension and inadvertent compression of intracranial structures or flap vascular pedicle compromise. The microscopic transsphenoidal approach is the standard surgical technique for the resection of sellar lesions. However, its main limitation is related to the lack of appropriate visualization of areas distant to the sella. For those locations, the endoscopic endonasal approach is a great substitute based on the inherent characteristics of the endoscope, which allows broad-angle illumination and visualization. Although the endoscopic technique offers superior advantages over microscopic transsphenoidal surgery in dealing with parasellar lesions, it is not clear if the same is true for purely intrasellar lesions. However, the wide field given by the endoscope offers a comprehensive exposure of skull base landmarks, which allows a better surgical orientation and consequently a safer procedure.
 The Sella
The Sella
Microscopic Transsphenoidal Approach
Sublabial
Endonasal
Endoscopic Endonasal Approach
 The Parasellar Areas
The Parasellar Areas
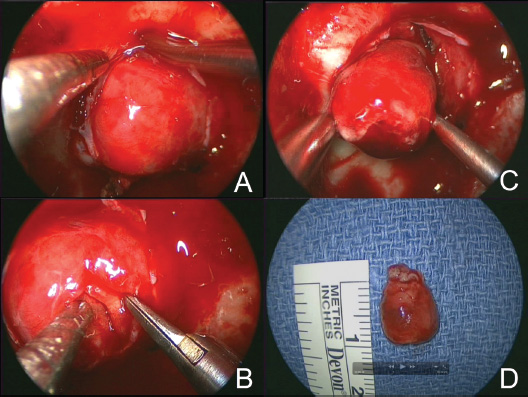
Suprasellar and Anterior Fossa
Retrosellar Space and Clivus
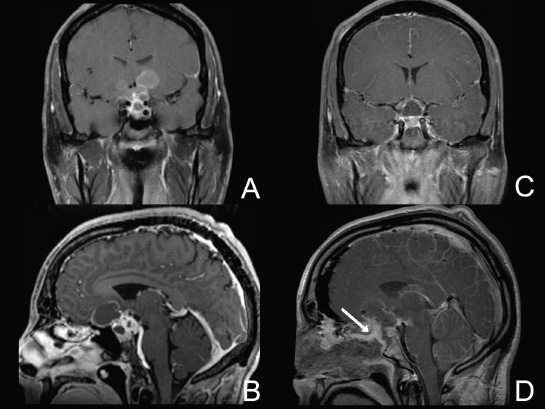
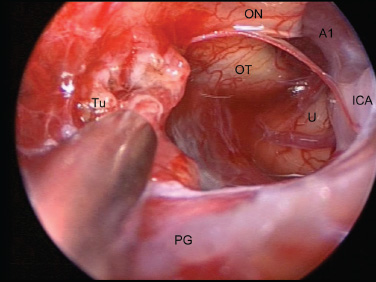
Cavernous Sinus and the Meckel Cave
 The Reconstruction
The Reconstruction
 Conclusion
Conclusion
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree